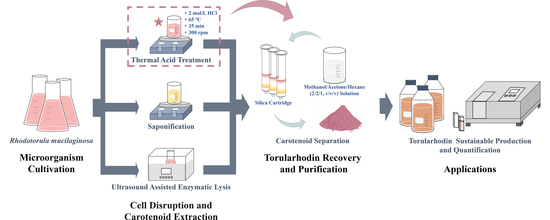
Strategies for Recovery, Purification and Quantification of Torularhodin Produced by Rhodotorula mucilaginosa Using Different Carbon Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism Cultivation
2.2. Characterization of Yeast Cells
2.3. Cell Disruption and Carotenoid Extraction
2.3.1. Sample Preparation
2.3.2. Thermal Acid Treatment
2.3.3. Saponification
2.3.4. Ultrasound-Assisted Enzymatic Lysis
2.4. Carotenoid Separation and Analysis
2.5. Determination of the Absorption Coefficient () of Torularhodin
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Characterization of Yeast Cells
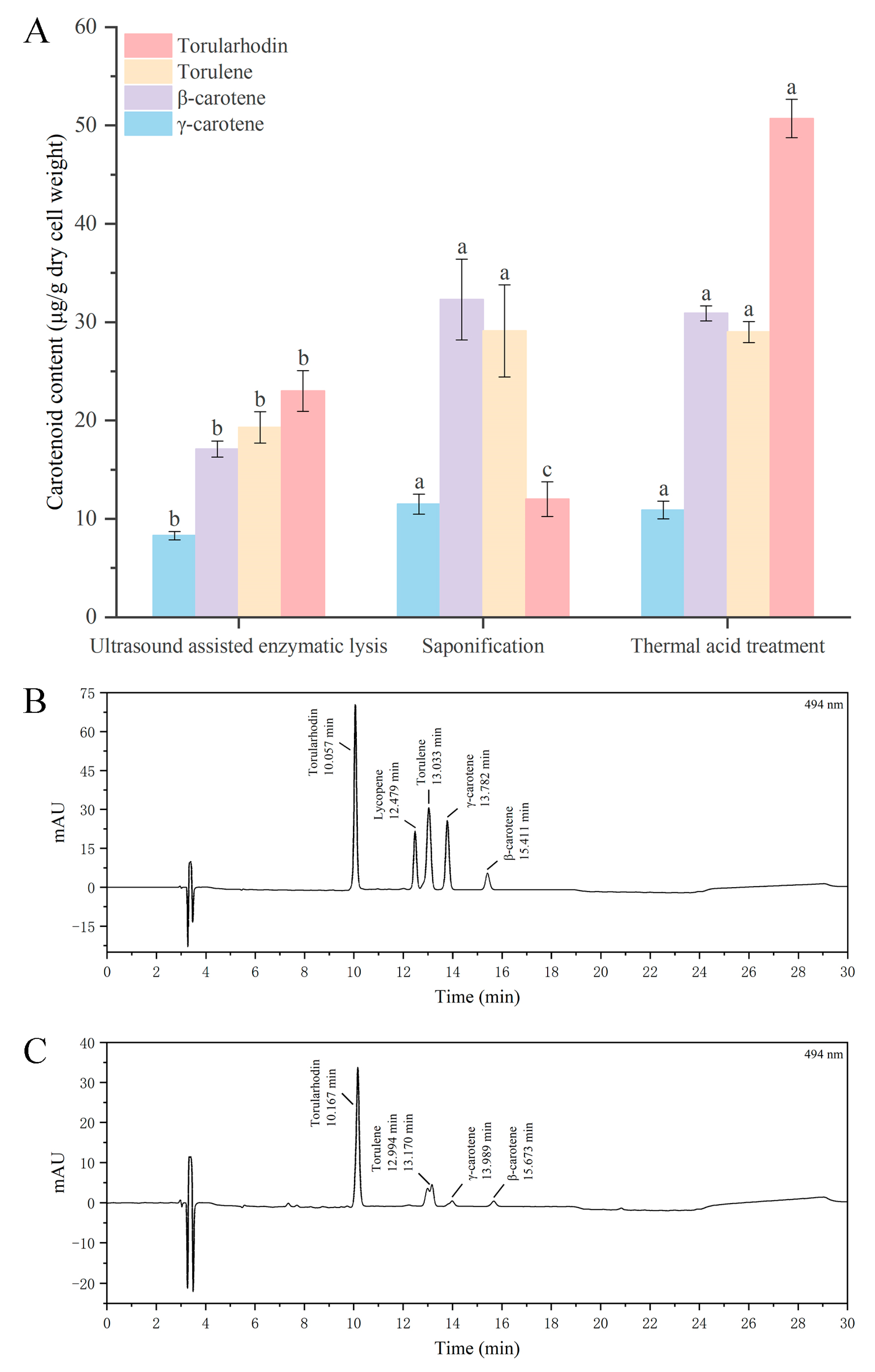
3.2. Effect of Different Cell Disruption Techniques on Release of Carotenoids
3.3. Effect of Different Cell Disruption Techniques on the Recovery of Torularhodin
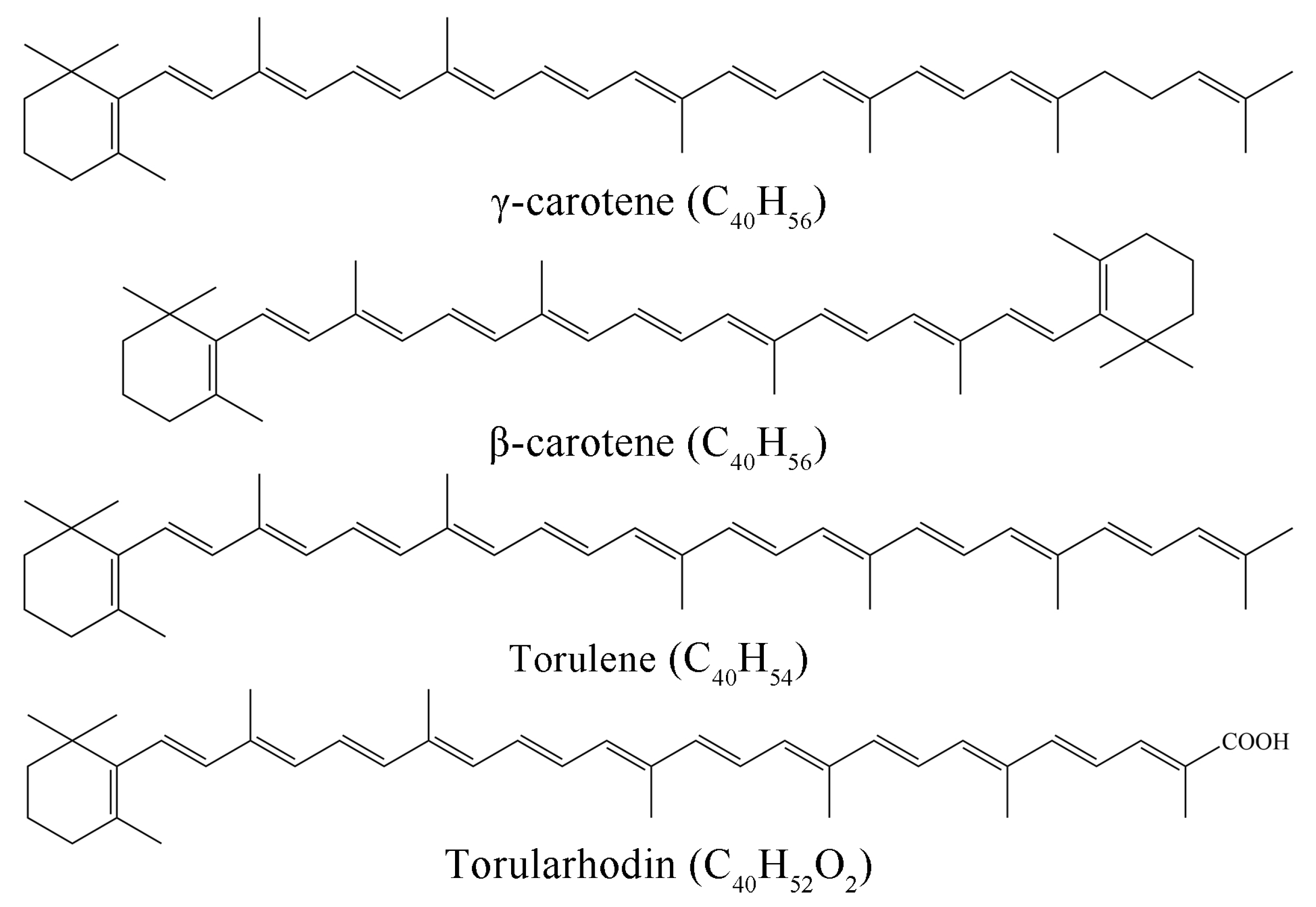
3.4. Absorption Coefficient () of Torularhodin in Different Solvents
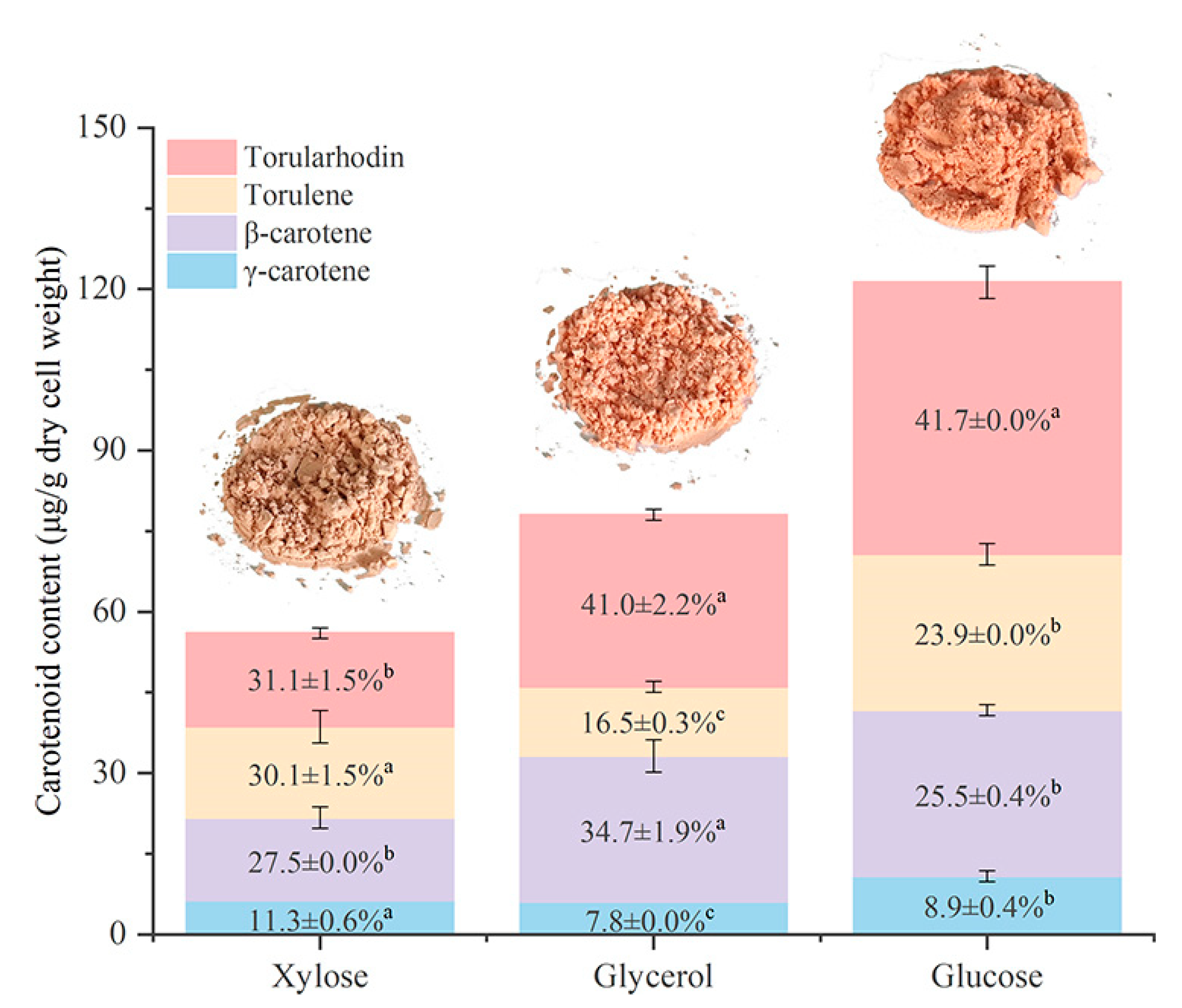
3.5. Effect of Different Carbon Sources on Carotenoid Production by R. mucilaginosa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; van den Berg, C.; Weusthuis, R.A.; Dragone, G.; Mussatto, S.I. Strategies for an Improved Extraction and Separation of Lipids and Carotenoids from Oleaginous Yeast. Sep. Purif. Technol. 2021, 257, 117946. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 Spectrophotometric Methods for Carotenoid Determination in Frequently Consumed Fruits and Vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, H.; Du, C.; Zhang, W.; Qian, H. Tentative Identification of Torulene Cis/Trans Geometrical Isomers Isolated from Sporidiobolus pararoseus by High-Performance Liquid Chromatography-Diode Array Detection-Mass Spectrometry and Preparation by Column Chromatography. Anal. Sci. 2013, 29, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.A.E.; Zaccarim, B.R.; de Lencastre Novaes, L.C.; Jozala, A.F.; dos Santos, C.A.; Teixeira, M.F.S.; Santos-Ebinuma, V.C. Natural Colorants from Filamentous Fungi. Appl. Microbiol. Biotechnol. 2016, 100, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Lopes, N.A.; Remedi, R.D.; dos Santos Sá, C.; Burkert, C.A.V.; de Medeiros Burkert, J.F. Different Cell Disruption Methods for Obtaining Carotenoids by Sporodiobolus pararoseus and Rhodothorula mucilaginosa. Food Sci. Biotechnol. 2017, 26, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, Y.N.; Burkina, V.; Okmane, L.; Blomqvist, J.; Rapoport, A.; Sandgren, M.; Pickova, J.; Sampels, S.; Passoth, V. Identification, Quantification and Kinetic Study of Carotenoids and Lipids in Rhodotorula toruloides CBS 14 Cultivated on Wheat Straw Hydrolysate. Fermentation 2022, 8, 300. [Google Scholar] [CrossRef]
- Li, J.; Qian, H.; Pi, F.; Wang, B.X. Bioavailability Evaluation of the Intestinal Absorption and Liver Accumulation of Torularhodin Using a Rat Postprandial Model. Food Funct. 2022, 13, 5946–5952. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Innocenti, M.; Turchetti, B.; Libkind, D.; Van Broock, M.; Mulinacci, N. Carotenoid Profiles of Yeasts Belonging to the Genera Rhodotorula, Rhodosporidium, Sporobolomyces, and Sporidiobolus. Can. J. Microbiol. 2007, 53, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Q.; Zhang, G. The Impact of the Physical Form of Torularhodin on Its Metabolic Fate in the Gastrointestinal Tract. Food Funct. 2021, 12, 9955–9964. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from Fungi and Microalgae: A Review on Their Recent Production, Extraction, and Developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.W.; Zeng, X.A.; Cheng, J.H.; Liu, D.B.; Aadil, R.M. The Efficiency and Comparison of Novel Techniques for Cell Wall Disruption in Astaxanthin Extraction from Haematococcus pluvialis. Int. J. Food Sci. Technol. 2018, 53, 2212–2219. [Google Scholar] [CrossRef]
- Michelon, M.; de Matos de Borba, T.; da Silva Rafael, R.; Burkert, C.A.V.; de Medeiros Burkert, J.F. Extraction of Carotenoids from Phaffia rhodozyma: A Comparison between Different Techniques of Cell Disruption. Food Sci. Biotechnol. 2012, 21, 1–8. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid Extraction Methods: A Review of Recent Developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Allahkarami, S.; Sepahi, A.A.; Hosseini, H.; Razavi, M.R. Isolation and Identification of Carotenoid-Producing Rhodotorula Sp. from Pinaceae Forest Ecosystems and Optimization of in Vitro Carotenoid Production. Biotechnol. Rep. 2021, 32, e00687. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of Physicochemical Properties and Analytical Characterization of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Cardoso, L.A.C.; Jäckel, S.; Karp, S.G.; Framboisier, X.; Chevalot, I.; Marc, I. Improvement of Sporobolomyces ruberrimus Carotenoids Production by the Use of Raw Glycerol. Bioresour. Technol. 2016, 200, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S.; French, W.T.; Hernandez, R.; Holmes, W.E.; Claupein, W. Effect of Different C/N Ratios on Carotenoid and Lipid Production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 6581–6588. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, P.; Qin, Z.; Li, Y.-Q.; Wang, G. Poly(3-Hydroxybutyrate) Anabolism in Cupriavidus necator Cultivated at Various Carbon-to-Nitrogen Ratios: Insights from Single-Cell Raman Spectroscopy. J. Biomed. Opt. 2016, 21, 097005. [Google Scholar] [CrossRef]
- Postma, P.R.; Pataro, G.; Capitoli, M.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M.; Olivieri, G.; Ferrari, G. Selective Extraction of Intracellular Components from the Microalga Chlorella vulgaris by Combined Pulsed Electric Field-Temperature Treatment. Bioresour. Technol. 2016, 203, 80–88. [Google Scholar] [CrossRef]
- Xiao, A.F.; Ni, H.; Cai, H.N.; Li, L.J.; Su, W.J.; Yang, Q.M. An Improved Process for Cell Disruption and Astaxanthin Extraction from Phaffia rhodozyma. World J. Microbiol. Biotechnol. 2009, 25, 2029–2034. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- FDA. Q3C—Tables and List Guidance for Industry. 2017. Available online: https://www.fda.gov/media/71737/download. (accessed on 10 September 2023).
- Machado, F.R.S.; Trevisol, T.C.; Boschetto, D.L.; Burkert, J.F.M.; Ferreira, S.R.S.; Oliveira, J.V.; Burkert, C.A.V. Technological Process for Cell Disruption, Extraction and Encapsulation of Astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2016, 218, 108–114. [Google Scholar] [CrossRef]
- Li, C.; Zhang, N.; Li, B.; Xu, Q.; Song, J.; Wei, N.; Wang, W.; Zou, H. Increased Torulene Accumulation in Red Yeast Sporidiobolus pararoseus NGR as Stress Response to High Salt Conditions. Food Chem. 2017, 237, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Lillie, S.H.; Pringle, J.R. Reserve Carbohydrate Metabolism in Saccharomyces cerevisiae: Responses to Nutrient Limitation. J. Bacteriol. 1980, 143, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. Ultrasonication Assisted Lipid Extraction from Oleaginous Microorganisms. Bioresour. Technol. 2014, 158, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Feist, A.M.; Dragone, G.; Mussatto, S.I. Lipid and Carotenoid Production from Wheat Straw Hydrolysates by Different Oleaginous Yeasts. J. Clean. Prod. 2020, 249, 119308. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil Production by Oleaginous Yeasts Using the Hydrolysate from Pretreatment of Wheat Straw with Dilute Sulfuric Acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef]
- Liu, Z.; Radi, M.; Mohamed, E.T.T.; Feist, A.M.; Dragone, G.; Mussatto, S.I. Adaptive Laboratory Evolution of Rhodosporidium toruloides to Inhibitors Derived from Lignocellulosic Biomass and Genetic Variations behind Evolution. Bioresour. Technol. 2021, 333, 125171. [Google Scholar] [CrossRef]
- Ungureanu, C.; Ferdes, M.; Chirvase, A.A. Torularhodin Biosynthesis and Extraction by Yeast Cells of Rhodotorula rubra. Rev. Chim. 2012, 63, 316–318. [Google Scholar]
- Maldonade, I.R.; Rodriguez-Amaya, D.B.; Scamparini, A.R.P. Carotenoids of Yeasts Isolated from the Brazilian Ecosystem. Food Chem. 2008, 107, 145–150. [Google Scholar] [CrossRef]
- Frengova, G.I.; Simova, E.D.; Beshkova, D.M. Effect of Temperature Changes on the Production of Yeast Pigments Co-Cultivated with Lacto-Acid Bacteria in Whey Ultrafiltrate. Biotechnol. Lett. 1995, 17, 1001–1006. [Google Scholar] [CrossRef]
- Liu, Z.; Natalizio, F.; Dragone, G.; Mussatto, S.I. Maximizing the Simultaneous Production of Lipids and Carotenoids by Rhodosporidium toruloides from Wheat Straw Hydrolysate and Perspectives for Large-Scale Implementation. Bioresour. Technol. 2021, 340, 125598. [Google Scholar] [CrossRef] [PubMed]
- Schwerdt, J.; Qiu, H.; Shirley, N.; Little, A.; Bulone, V. Phylogenomic Analyses of Nucleotide-Sugar Biosynthetic and Interconverting Enzymes Illuminate Cell Wall Composition in Fungi. MBio 2021, 12, e03540-20. [Google Scholar] [CrossRef]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast Cell Disruption Strategies for Recovery of Intracellular Bio-Active Compounds—A Review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Rech, R.; Marczak, L.D.F.; Mercali, G.D. Ultrasound as an Alternative Technology to Extract Carotenoids and Lipids from Heterochlorella luteoviridis. Bioresour. Technol. 2017, 224, 753–757. [Google Scholar] [CrossRef]
- Sun, M.; Hu, J.; Pan, S. Study on Stability of Carotenoids from Orange Peels. Food Sci. 2016, 28, 46–49. [Google Scholar]
- Squina, F.M.; Mercadante, A.Z. HPLC Analysis of Carotenoids from Five Rhodotorula Strains. Rev. Bras. Cienc. Farm. J. Pharm. Sci. 2003, 39, 309–318. [Google Scholar]
- Mussagy, C.U.; Santos-Ebinuma, V.C.; Gonzalez-Miquel, M.; Coutinho, J.A.P.; Pereira, J.F.B. Protic Ionic Liquids as Cell-Disrupting Agents for the Recovery of Intracellular Carotenoids from Yeast Rhodotorula glutinis CCT-2186. ACS Sustain. Chem. Eng. 2019, 7, 16765–16776. [Google Scholar] [CrossRef]
- Turcsi, E.; Nagy, V.; Deli, J. Study on the Elution Order of Carotenoids on Endcapped C18 and C30 Reverse Silica Stationary Phases. A Review of the Database. J. Food Compos. Anal. 2016, 47, 101–112. [Google Scholar] [CrossRef]
- Zoz, L.; Carvalho, J.C.; Soccol, V.T.; Casagrande, T.C.; Cardoso, L. Torularhodin and Torulene: Bioproduction, Properties and Prospective Applications in Food and Cosmetics—A Review. Braz. Arch. Biol. Technol. 2015, 58, 278–288. [Google Scholar] [CrossRef]
- Simpson, K.L.; Nakayama, T.O.; Chichester, C.O. Biosynthesis of Yeast Carotenoids. J. Bacteriol. 1964, 88, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Moliné, M.; Libkind, D.; Van Broock, M. Production of Torularhodin, Torulene, and β-Carotene by Rhodotorula Yeasts. Methods Mol. Biol. 2012, 898, 275–283. [Google Scholar] [PubMed]
- Sun, Z.; Lv, J.; Ji, C.; Liang, H.; Li, S.; Yang, Z.; Xu, W.; Zhang, S.; Lin, X. Analysis of Carotenoid Profile Changes and Carotenogenic Genes Transcript Levels in Rhodosporidium toruloides Mutants from an Optimized Agrobacterium tumefaciens-Mediated Transformation Method. Biotechnol. Appl. Biochem. 2021, 68, 71–81. [Google Scholar] [CrossRef]
- Gong, G.; Liu, L.; Zhang, X.; Tan, T. Comparative Evaluation of Different Carbon Sources Supply on Simultaneous Production of Lipid and Carotene of Rhodotorula glutinis with Irradiation and the Assessment of Key Gene Transcription. Bioresour. Technol. 2019, 288, 121559. [Google Scholar] [CrossRef]
- Tiukova, I.A.; Brandenburg, J.; Blomqvist, J.; Sampels, S.; Mikkelsen, N.; Skaugen, M.; Arntzen, M.O.; Nielsen, J.; Sandgren, M.; Kerkhoven, E.J. Proteome Analysis of Xylose Metabolism in Rhodotorula toruloides during Lipid Production. Biotechnol. Biofuels 2019, 12, 1–17. [Google Scholar] [CrossRef]
- Pinheiro, M.J.; Bonturi, N.; Belouah, I.; Miranda, E.A.; Lahtvee, P.J. Xylose Metabolism and the Effect of Oxidative Stress on Lipid and Carotenoid Production in Rhodotorula toruloides: Insights for Future Biorefinery. Front. Bioeng. Biotechnol. 2020, 8, 1008. [Google Scholar] [CrossRef]
- Bommareddy, R.R.; Sabra, W.; Maheshwari, G.; Zeng, A.P. Metabolic Network Analysis and Experimental Study of Lipid Production in Rhodosporidium toruloides Grown on Single and Mixed Substrates. Microb. Cell Fact. 2015, 14, 1–13. [Google Scholar] [CrossRef]
- Li, C.; Cheng, P.; Li, Z.; Xu, Y.; Sun, Y.; Qin, D.; Yu, G. Transcriptomic and Metabolomic Analyses Provide Insights into the Enhancement of Torulene and Torularhodin Production in Rhodotorula glutinis ZHK under Moderate Salt Conditions. J. Agric. Food Chem. 2021, 69, 11523–11533. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Li, Z.; Cheng, P.; Yu, G. Transcriptomic and Metabolomic Analysis Reveals the Potential Mechanisms Underlying the Improvement of β-Carotene and Torulene Production in Rhodosporidiobolus colostri under Low Temperature Treatment. Food Res. Int. 2022, 156, 111158. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Optimization of Carotenoids Production by Rhodotorula mucilaginosa (MTCC-1403) Using Agro-Industrial Waste in Bioreactor: A Statistical Approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Yang, C.F. Using Strain Rhodotorula Mucilaginosa to Produce carotenoids Using Food Wastes. J. Taiwan Inst. Chem. Eng. 2016, 61, 270–275. [Google Scholar] [CrossRef]
- Aksu, Z.; Tuǧba Eren, A. Carotenoids Production by the Yeast Rhodotorula mucilaginosa: Use of Agricultural Wastes as a Carbon Source. Process Biochem. 2005, 40, 2985–2991. [Google Scholar] [CrossRef]


| Sample Source | Solvents | The Maximum Absorption Wavelength (nm) | Reference | |
|---|---|---|---|---|
| Torularhodin standard | Acetone | 494 | 2303 ± 119 | - |
| Chloroform | 514 | 2829 ± 143 | - | |
| n-Hexane/ethanol (1/1, v/v) | 499 | 2174 ± 25 | - | |
| Extracted torularhodin | Acetone | 494 | 3257 ± 52 | - |
| Chloroform | 514 | 3342 ± 46 | - | |
| n-Hexane/ethanol (1/1, v/v) | 499 | 3578 ± 39 | - | |
| Previous reports | Petroleum ether | 474–500–533 | 2580 | [42] |
| Hexane | 468–502–539 | 2040 | [42] | |
| Chloroform | 515 | 1932 | [43] |
| Torularhodin Content (μg/g Dry Cell Weight) | Torularhodin Recovery (%) | ||
|---|---|---|---|
| Before Separation * | After Separation ** | ||
| Xylose | 17.4 ± 1.0 | 16.1 ± 2.0 | 92.3 ± 6.8 |
| Glycerol | 32.0 ± 1.0 | 28.1 ± 2.0 | 88.0 ± 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Wang, R.; Liang, J.; Zhang, H.; Yi, J.; Liu, Z. Strategies for Recovery, Purification and Quantification of Torularhodin Produced by Rhodotorula mucilaginosa Using Different Carbon Sources. Fermentation 2023, 9, 846. https://doi.org/10.3390/fermentation9090846
Zeng Y, Wang R, Liang J, Zhang H, Yi J, Liu Z. Strategies for Recovery, Purification and Quantification of Torularhodin Produced by Rhodotorula mucilaginosa Using Different Carbon Sources. Fermentation. 2023; 9(9):846. https://doi.org/10.3390/fermentation9090846
Chicago/Turabian StyleZeng, Yi, Rui Wang, Jiaqian Liang, Huixin Zhang, Junjie Yi, and Zhijia Liu. 2023. "Strategies for Recovery, Purification and Quantification of Torularhodin Produced by Rhodotorula mucilaginosa Using Different Carbon Sources" Fermentation 9, no. 9: 846. https://doi.org/10.3390/fermentation9090846
APA StyleZeng, Y., Wang, R., Liang, J., Zhang, H., Yi, J., & Liu, Z. (2023). Strategies for Recovery, Purification and Quantification of Torularhodin Produced by Rhodotorula mucilaginosa Using Different Carbon Sources. Fermentation, 9(9), 846. https://doi.org/10.3390/fermentation9090846