Sequential Inoculation of Metschnikowia pulcherrima and Saccharomyces cerevisiae as a Biotechnological Tool to Increase the Terpenes Content of Pecorino White Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of the Strains
2.2. Cellar Vinification
2.3. Determination of Oenological Parameters
2.4. Viable Yeast Count
2.5. Determination of Volatile Organic Compounds
2.6. Sensorial Analysis
2.7. Statistical Analysis
3. Results
3.1. Yeast Dynamics
3.2. Oenological Parameters
3.3. Effect of M. pulcherrima on Copper Reduction
3.4. Aroma Profile
3.5. Sensory Profile of Wines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, P.T.; Lu, L.; Duan, C.Q.; Yan, G.L. The contribution of indigenous non-Saccharomyces wine yeast to improved aromatic quality of Cabernet Sauvignon wines by spontaneous fermentation. LWT-Food Sci. Technol. 2016, 71, 356–363. [Google Scholar] [CrossRef]
- Wei, R.T.; Chen, N.; Ding, Y.T.; Wang, L.; Liu, Y.H.; Gao, F.F.; Zhang, L.; Li, H.; Wang, H. Correlations between microbiota with physicochemical properties and volatile compounds during the spontaneous fermentation of Cabernet Sauvignon (Vitis vinifera L.) wine. LWT-Food. Sci. Technol. 2022, 163, 113529. [Google Scholar] [CrossRef]
- Liu, D.; Howell, K. Community succession of the grapevine fungal microbiome in the annual growth cycle. Environ. Microbiol. 2020, 23, 1842–1857. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; Gonzalez-Arenzana, L.; Garijo, P.; Lopez, R.; Santamaría, P.; Gutierrez, A.R. Selection process of a mixed inoculum of non-Saccharomyces yeasts isolated in the D.O.Ca. Rioja. Fermentation 2021, 7, 148. [Google Scholar] [CrossRef]
- Suzzi, G.; Arfelli, G.; Schirone, M.; Corsetti, A.; Perpetuini, G.; Tofalo, R. Effect of grape indigenous Saccharomyces cerevisiae strains on Montepulciano d’Abruzzo red wine quality. Food Res. Int. 2012, 46, 22–29. [Google Scholar] [CrossRef]
- Suzzi, G.; Schirone, M.; Sergi, M.; Marianella, R.M.; Fasoli, G.; Aguzzi, I.; Tofalo, R. Multistarter from organic viticulture for red wine Montepulciano d’Abruzzo production. Front. Microbiol. 2012, 3, 135. [Google Scholar]
- Tofalo, R.; Patrignani, F.; Lanciotti, R.; Perpetuini, G.; Schirone, M.; Di Gianvito, P.; Pizzoni, D.; Arfelli, G.; Suzzi, G. Aroma profile of Montepulciano d’Abruzzo wine fermented by single and co-culture starters of autochthonous Saccharomyces and non-Saccharomyces yeasts. Front. Microbiol. 2016, 7, 610. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Zulian, L.; Ferreres, À.; Pastor, R.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Sequential inoculation of native non-Saccharomyces and Saccharomyces cerevisiae strains for wine making. Front. Microbiol. 2017, 8, 1293. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and phenotypic characterization of Metschnikowia pulcherrima strains from Douro wine region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-Q.; Shen, J.-Y.; Duan, C.-Q.; Yan, G.-L. Use of indigenous Hanseniaspora vineae and Metschnikowia pulcherrima co-fermentation with Saccharomyces cerevisiae to improve the aroma diversity of Vidal Blanc icewine. Front. Microbiol. 2018, 9, 2303. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascues, E.; Marquina, D.; Calderon, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef] [PubMed]
- Vicente, J.; Ruiz, J.; Belda, I.; Benito-Vázquez, I.; Marquina, D.; Calderón, F.; Santos, A.; Benito, S. The genus Metschnikowia in enology. Microorganisms 2020, 8, 1038. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Marechal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacon, J.J.; Ballester, J.; Vichi, S.; Guerin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Marangon, M.; Van Sluyter, S.C.; Robinson, E.M.; Muhlack, R.A.; Holt, H.E.; Haynes, P.A.; Godden, P.W.; Smith, P.A.; Waters, E.J. Degradation of white wine haze proteins by Aspergillopepsin I and II during juice flash pasteurization. Food Chem. 2012, 135, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Gambetta, J.M.; Jeffery, D.W.; Grbin, P.R.; Jiranek, V. Lower-alcohol wines produced by Metschnikowia pulcherrima and Saccharomyces cerevisiae co-fermentations: The effect of sequential inoculation timing. Int. J. Food Microbiol. 2020, 329, 108651. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef]
- Available online: https://biodiversita.umbria.parco3a.org/storymap/content/Scheda_Iscrizione_Registro_Regionale_Vitigno_Pecorino.pdf (accessed on 3 July 2023).
- Organisation International de la Vigne et du Vin (OIV). Compendium of International Methods of Wine and Must Analysis. Vol. 1 and Paris, France. Available online: http://188.165.107.123/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts-2-vol (accessed on 19 July 2023).
- Dournes, G.; Dufourcq, T.; Suc, L.; Roland, A.; Mouret, J.R. Unravelling copper effect on the production of varietal thiols during Colombard and Gros Manseng grape juices fermentation by Saccharomyces cerevisiae. Front. Microbiol. 2023, 14, 1101110. [Google Scholar] [CrossRef]
- Rossetti, A.P.; Perpetuini, G.; Battistelli, N.; Zulli, C.; Arfelli, G.; Suzzi, G.; Cichelli, A.; Tofalo, R. Capturing the fungal community associated with conventional and organic Trebbiano Abruzzese grapes and its influence on wine characteristics. Food Biosci. 2023, 52, 102382. [Google Scholar] [CrossRef]
- Perpetuini, G.; Rossetti, A.P.; Battistelli, N.; Zulli, C.; Piva, A.; Arfelli, G.; Corsetti, A.; Tofalo, R. Contribution of Starmerella bacillaris and oak chips to Trebbiano d’Abruzzo wine volatile and sensory diversity. Foods 2023, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- Kuchen, B.; Maturano, Y.P.; Mestre, M.V.; Combina, M.; Toro, M.E.; Vazquez, F. Selection of native non-Saccharomyces yeasts with biocontrol activity against spoilage yeasts in order to produce healthy regional wines. Fermentation 2019, 5, 60. [Google Scholar] [CrossRef]
- Checchia, I.; Binati, R.L.; Troiano, E.; Ugliano, M.; Felis, G.E.; Torriani, S. Unravelling the impact of grape washing, SO2, and multi-starter inoculation in lab-scale vinification trials of withered black grapes. Fermentation 2021, 7, 43. [Google Scholar] [CrossRef]
- Quiros, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Rojas, V.; Quiros, M.; Gonzalez, R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Droulia, F.; Charalampopoulos, I. A Review on the Observed Climate Change in Europe and Its Impacts on Viticulture. Atmosphere 2022, 13, 837. [Google Scholar] [CrossRef]
- Sadineni Naresh, V.; Kondapalli, N.; Obulam, V. Effect of co-fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii or Metschnikowia pulcherrima on the aroma and sensory properties of mango wine. Ann. Microbiol. 2012, 62, 1353–1360. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Jolly, N.; Augustyn, O.; Pretorius, I. The effect of non-Saccharomyces yeasts on fermentation and wine quality. J. South Afr. J. Enol. 2003, 24, 55–62. [Google Scholar] [CrossRef]
- Roca-Mesa, H.; Sendra, S.; Mas, A.; Beltran, G.; Torija, M.J. Nitrogen preferences during alcoholic fermentation of different non-Saccharomyces yeasts of oenological interest. Microorganisms 2020, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.-D.; Rauhut, D. Effect of sequential inoculation with non-Saccharomyces and Saccharomyces yeasts on Riesling wine chemical composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Ferreira, J.; Toit, M.; Toit, W. The effects of copper and high sugar concentrations on growth, fermentation efficiency and volatile acidity production of different commercial wine yeast strains. Aust. J. Grape Wine Res. 2006, 12, 50–56. [Google Scholar] [CrossRef]
- Robinson, N.; Winge, D. Copper Metallochaperones. Annu. Rev. Biochem. 2010, 79, 537–562. [Google Scholar] [CrossRef]
- Sun, X.Y.; Zhao, Y.; Liu, L.L.; Jia, B.; Zhao, F.; Huang, W.D.; Zhan, J.C. Copper Tolerance and biosorption of Saccharomyces cerevisiae during alcoholic fermentation. PLoS ONE 2015, 10, e0128611. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef]
- Milanovic, V.; Ciani, M.; Oro, L.; Comitini, F. Starmerella bombicola influences the metabolism of Saccharomyces cerevisiae at pyruvate decarboxylase and alcohol dehydrogenase level during mixed wine fermentation. Microb. Cell Fact. 2012, 11, 18. [Google Scholar] [CrossRef]
- Sadoudi, M.; Rousseaux, S.; David, V.; Alexandre, H.; Tourdot-Maréchal, R. Metschnikowia pulcherrima influences the expression of genes involved in PDH bypass and glyceropyruvic fermentation in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1137. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Galli, E.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima as biocontrol agent and wine aroma enhancer in combination with a native Saccharomyces cerevisiae. LWT-Food Sci. Technol. 2023, 181, 114758. [Google Scholar] [CrossRef]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, K.; Bauer, F.F.; Setati, M.E. Impact of oxygenation on the performance of three non-Saccharomyces yeasts in co-fermentation with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 2479–2491. [Google Scholar] [CrossRef]
- Tronchoni, J.; Curiel, J.A.; Saenz-Navajas, M.P.; Morales, P.; de-la-Fuente-Blanco, A.; Fernandez-Zurbano, P.; Ferreira, V.; Gonzalez, R. Aroma profiling of an aerated fermentation of natural grape must with selected yeast strains at pilot scale. Food Microbiol. 2018, 70, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Tourdot-Marechal, R.; Morge, C.; Sparrow, C.; Liu, Y.; Quintanilla-Casas, B.; Vichi, S.; Alexandre, H. Non-Saccharomyces yeasts nitrogen source preferences: Impact on sequential fermentation and wine volatile compounds profile. Front. Microbiol. 2017, 8, 2175. [Google Scholar] [CrossRef]
- Prior, K.J.; Bauer, F.F.; Divol, B. The utilisation of nitrogenous compounds by commercial non-Saccharomyces yeasts associated with wine. Food Microbiol. 2019, 79, 75–84. [Google Scholar] [CrossRef]
- Cordente, A.G.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-active wine yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, D.; Bauer, F.F. Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol. 2016, 55, 32–46. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Cabellos, J.M.; Arroyo, T. Sequential non-Saccharomyces and Saccharomyces cerevisiae fermentations to reduce the alcohol content in wine. Fermentation 2020, 6, 60. [Google Scholar] [CrossRef]
- Rodriguez, M.E.; Lopes, C.A.; Barbagelata, R.J.; Barda, N.B.; Caballero, A.C. Influence of Candida pulcherrima Patagonian strain on alcoholic fermentation behaviour and wine aroma. Int. J. Food Microbiol. 2010, 138, 19–25. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; Fresno, J.M.D.; González, C.; Suárez-Lepe, J.A. Contribution of non-Saccharomyces yeasts to wine freshness. A Review. Biomolecules 2019, 10, 34. [Google Scholar] [CrossRef]
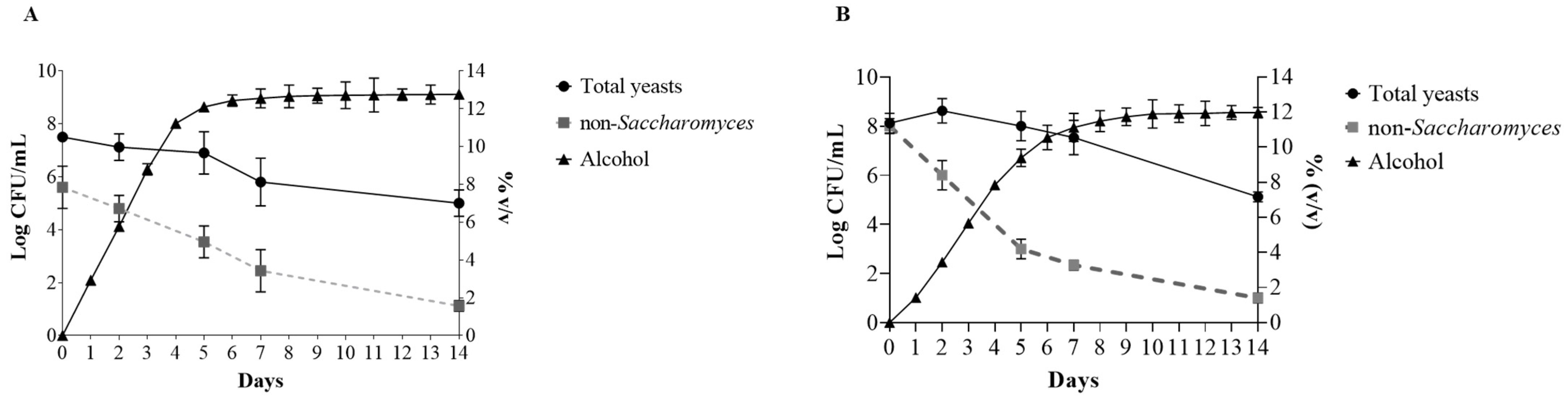
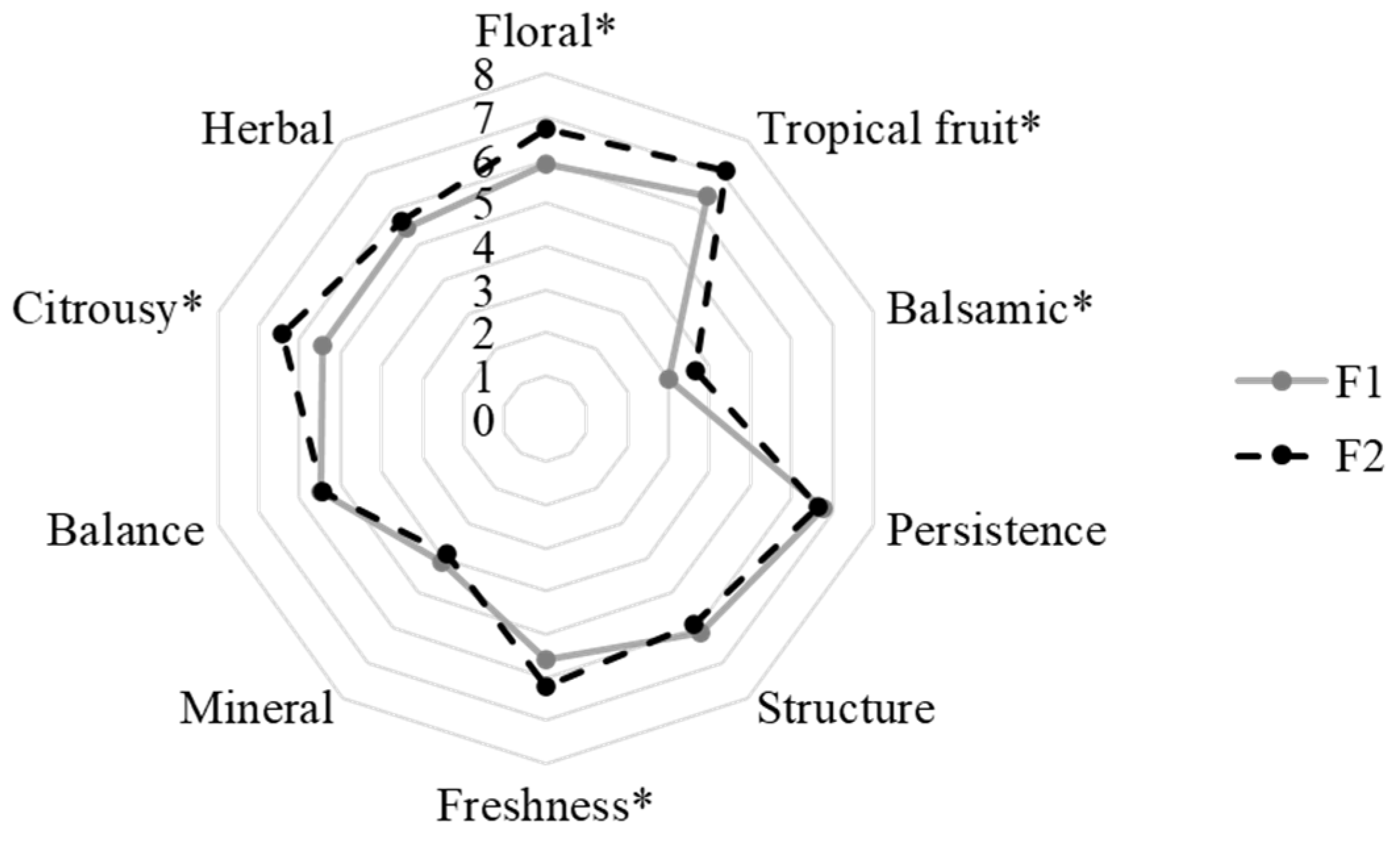
| Trial | Strain | Inoculation Type |
|---|---|---|
| F1 | S. cerevisiae (SRS1) | Pure culture |
| F2 | M. pulcherrima (GS80) + S. cerevisiae (SRS1) | Sequential inoculation (S. cerevisiae was inoculated after 48 h) |
| Parameters | F1 | F2 |
|---|---|---|
| Alcohol (% v/v) | 12.74 ± 0.12 A | 11.99 ± 0.11 B |
| Residual sugars (g/L) | 0.28 ± 0.02 A | 0.24 ± 0.03 A |
| Titratable acidity (g/L) * | 7.67 ± 0.14 A | 7.22 ± 0.08 B |
| Volatile acidity (g/L) ** | 0.43 ± 0.03 A | 0.34 ± 0.03 B |
| pH | 3.26 ± 0.78 A | 3.25 ± 0.36 A |
| Copper (mg/L) | 0.44 ± 0.08 A | 0.31 ± 0.06 B |
| Odor Description | F1 | F2 | |
|---|---|---|---|
| Organic acids | |||
| n-Decanoic acid | Rancid, sour, fatty, citrus | 1.33 ± 0.82 A | 1.55 ± 0.11 A |
| 2-Methylheptanoic acid | Fruity, cheesy, oily, fatty, lard | 0.11 ± 0.09 A | 0.11 ± 0.09 A |
| Acetic acid | Fatty, waxy, cheesy, sweet, creamy | 4.54 ± 0.55 A | 2.13 ± 0.23 B |
| Dodecanoic acid | Fatty, waxy, cheesy, sweet, creamy | 1.78 ± 0.22 A | 1.98 ± 0.22 A |
| Nonanoic acid | Fatty, waxy, rancid, oily, vegetable, cheesy | 1.77 ± 0.33 A | 1.33 ± 0.13 A |
| Octanoic acid | Acidic, sharp, cheesy, sour, milky, tobacco, fruity | 5.6 ± 0.22 A | 5.77 ± 1.23 A |
| Pentanoic acid | Sour, fatty, sweat, cheese | 1.33 ± 0.66 A | 1.44 ± 0.33 A |
| Hexanoic acid | Rancid, sour, fatty, citrus | 1.66 ± ±0.23 A | 1.77 ± 0.34 A |
| Tot | 18.12 | 16.08 | |
| Esters | |||
| Amyl acetate | Sweet, banana, fruity, ripe, estery | 1.32 ± 0.11 A | 1.44 ± 0.11 A |
| Benzyl benzoate | Sweet, balsamic, oily, herbal | 0.4 ± 0.12 A | 0.33 ± 0.08 A |
| Butyl 2-methylvalerate | N.f. | 0.05 ± 0.01 A | n.d.B |
| Butyl heptanoate | Fruity, green, oily, floral | n.d.B | 0.17 ± 0.12 A |
| Diethyl succinate | Fruity, apple, cooked, apple, ylang | 0.11 ± 0.02 B | 1.77 ± 0.33 A |
| Ethyl 2-methylbutanoate | Fruity, estery, berry, fresh, tropical | n.d.B | 3.22 ± 0.45 A |
| Ethyl 6-heptenoate | Fruity, pineapple, cognac, rummy winey | n.d.B | 0.33 ± 0.11 A |
| Ethyl 9-decenoate | Sweet, waxy, fruity, apple, grape, oily brandy | 1.33 ± 0.23 A | 2.22 ± 0.13 A |
| Ethyl butanoate | Waxy, sweet, musty, pineapple, fruity creamy, dairy, | 0.22 ± 0.03 A | 1.33 ± 0.13 B |
| Ethyl decanoate | Fruity, juicy, fruit, fruity, pineapple, cognac | 11.57 ± 0.22 B | 15.54 ± 0.23 A |
| Ethyl dodecanoate | Sweet, waxy, soapy, rummy, creamy, floral, | 1.26 ± 0.34 B | 4.44 ± 0.18 A |
| Ethyl hexanoate | Sweet, fruity, pineapple, waxy, green, banana | 5.3 ± 0.12 A | 7.33 ± 0.22 B |
| Ethyl lactate | Sharp, tart, fruity, buttery, butterscotch | n.d. | 3.22 ± 0.77 |
| Ethyl n-butyrate | Fruity, juicy, fruit, fruity, pineapple, cognac | n.d. | 0.97 ± 0.13 |
| Ethyl octanoate | Fruity, winey, waxy, sweet, apricot, banana, brandy, pear | 21.33 ± 0.45 B | 28.33 ± 0.33 A |
| Hexyl acetate | Fruity, green, apple, banana, sweet | 1.56 ± 0.33 A | 2.33 ± 0.77 A |
| Hexyl ethanoate | Green, fruity, estery, vegetable, waxy | n.d. | 3.44 ± 0.32 |
| Isoamyl acetate | Sweet, fruity, banana, solvent | 8.7 ± 1.22 A | 9.22 ± 2.22 A |
| Isobutyl acetate | Sweet, fruity, ethereal, apple, banana, | n.d. | 0.33 ± 0.03 |
| Isobutyl heptanoate | Fruity, green, oily, floral | n.d. | 0.22 ± 0.01 |
| Methyl 2.2-dimethylvalerate | N.f. | 4.2 ± 0.23 A | 4.33 ± 0.12 A |
| Methyl 2.4-dimethylpentanoate | N.f. | 0.3 ± 0.11 A | 0.22 ± 0.33 A |
| Methyl 2-methylhexanoate | N.f. | 0.07 ± 0.02 B | 2.33 ± 0.32 A |
| Methyl heptanoate | Sweet, fruity, green, orris, waxy, floral, berry | n.d. | 0.33 ± 0.01 |
| Phenethyl acetate | Floral, rose, sweet, honey, fruity, tropical | 1.71 ± 0.22 A | 2.22 ± 0.88 A |
| Sec-butyl 2-methylhexanoate | N.f. | n.d. | 0.11 ± 0.08 |
| Geranyl acetate | Floral, rose, lavender, green, waxy | 1.44 ± 0.17 A | 1.77 ± 0.22 A |
| Tot | 61.23 | 97.19 | |
| Higher alcohols | |||
| (S)-3.4-Dimethylpentanol | N.f. | 0.03 ± 0.02 A | 0.03 ± 0.01 A |
| 1-Butanol | Fusel, oily, sweet, balsamic, whiskey, | 1.33 ± 0.11 A | 1.26 ± 0.12 A |
| 1-Butanol 2-methyl- | N.f. | 1.18 ± 0.22 A | 1.44 ± 0.22 A |
| 1-Heptanol | Musty, leafy, violet, herbal, green, sweet, woody, peony | 0.33 ± 0.14 A | 0.44 ± 0.34 A |
| 1-Hepten-4-ol | Fresh, oily, fatty, creamy, vegetable, dairy, green, grassy | n.d. | 0.11 ± 0.02 |
| 1-Hexanol | Ethereal, fusel, oily, fruity, alcoholic, sweet | 0.15 ± 0.08 A | 0.33 ± 0.01 A |
| 1-Pentanol | Pungent, fermented bready, yeasty, fusel, winey, solvent | 0.22 ± 0.12 A | 0.23 ± 0.09 A |
| 2.3-Butanediol | Fruity, creamy, buttery | 1.22 ± 0.77 A | 0.99 ± 0.11 A |
| 2-Butanol | Sweet, apricot | 0.55 ± 0.34 A | 0.11 ± 0.09 A |
| Isoamyl alcohol | Fusel, alcoholic, whiskey, fruity, banana | 17.13 ± 1.45 A | 15.22 ± 1.66 A |
| Phenylethyl Alcohol | Sweet, floral fresh, bready, rose, honey | 4.49 ± 0.99 B | 7.38 ± 0.22 A |
| α-Methylphenethyl alcohol | N.f. | 0.02 ± 0.01 | n.d. |
| Tot | 26.65 | 27.54 | |
| Aldehydes | |||
| Hexanal | Green, fatty, leafy, vegetable, fruity, clean, woody | 0.01 ± 0.01 A | 0.01 ± 0.01 A |
| Acetaldehyde | Pungent, ethereal, aldehydic, fruity | 12.45 ± 1.45 A | 12.77 ± 2.77 A |
| Furfural | Almond, woody, sweet | 1.11 ± 0.22 A | 1.09 ± 0.01 A |
| Benzaldehyde | Almond | 0.44 ± 0.11 A | 0.77 ± 0.33 A |
| Tot | 14.01 | 14.64 | |
| Terpenes/C13-norisoprenoids/phenols | |||
| Geraniol | Floral, sweet, rose, fruity, citronella, citrus | 1.02 ± 0.22 A | 1.75 ± 0.11 A |
| β-citronellol | Floral, leather, waxy, rose, bud citrus | 0.34 ± 0.12 A | 0.74 ± 0.11 A |
| Linalool | Citrus, floral, sweet, bois de rose, woody, green, blueberry | 0.32 ± 0.11 B | 1.44 ± 0.23 A |
| Vanillin | Sweet, vanilla, creamy, chocolate | 0.12 ± 0.02 A | 0.33 ± 0.02 A |
| β-damascenone | Natural, sweet, fruity, rose plum, grape, raspberry, sugar | 0.01 ± 0.01 A | 0.02 ± 0.01 A |
| Tot | 1.81 | 4.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perpetuini, G.; Rossetti, A.P.; Quadrani, L.; Arfelli, G.; Piva, A.; Suzzi, G.; Tofalo, R. Sequential Inoculation of Metschnikowia pulcherrima and Saccharomyces cerevisiae as a Biotechnological Tool to Increase the Terpenes Content of Pecorino White Wines. Fermentation 2023, 9, 785. https://doi.org/10.3390/fermentation9090785
Perpetuini G, Rossetti AP, Quadrani L, Arfelli G, Piva A, Suzzi G, Tofalo R. Sequential Inoculation of Metschnikowia pulcherrima and Saccharomyces cerevisiae as a Biotechnological Tool to Increase the Terpenes Content of Pecorino White Wines. Fermentation. 2023; 9(9):785. https://doi.org/10.3390/fermentation9090785
Chicago/Turabian StylePerpetuini, Giorgia, Alessio Pio Rossetti, Luca Quadrani, Giuseppe Arfelli, Andrea Piva, Giovanna Suzzi, and Rosanna Tofalo. 2023. "Sequential Inoculation of Metschnikowia pulcherrima and Saccharomyces cerevisiae as a Biotechnological Tool to Increase the Terpenes Content of Pecorino White Wines" Fermentation 9, no. 9: 785. https://doi.org/10.3390/fermentation9090785
APA StylePerpetuini, G., Rossetti, A. P., Quadrani, L., Arfelli, G., Piva, A., Suzzi, G., & Tofalo, R. (2023). Sequential Inoculation of Metschnikowia pulcherrima and Saccharomyces cerevisiae as a Biotechnological Tool to Increase the Terpenes Content of Pecorino White Wines. Fermentation, 9(9), 785. https://doi.org/10.3390/fermentation9090785







