Solid-State Fermentation of Green Tea Residues as Substrates for Tannase Production by Aspergillus niger TBG 28A: Optimization of the Culture Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Substrate and Pretreatment
2.3. Operational Conditions for SSF System
2.4. Determination of Fungal Biomass
2.5. Enzyme Assay
2.6. Determination of Hydrolysable and Condensed Tannins, Sugar Totals, and Soluble Protein
2.7. Optimization of Tannase Production by Statistical Design
2.8. Statistical Analysis
2.9. Purification of the Enzyme
2.10. Determination of Molecular Weight via Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.11. Zymogram Analysis
3. Results and Discussion
3.1. Proximal Characterization of the Spent Green Tea Leaves
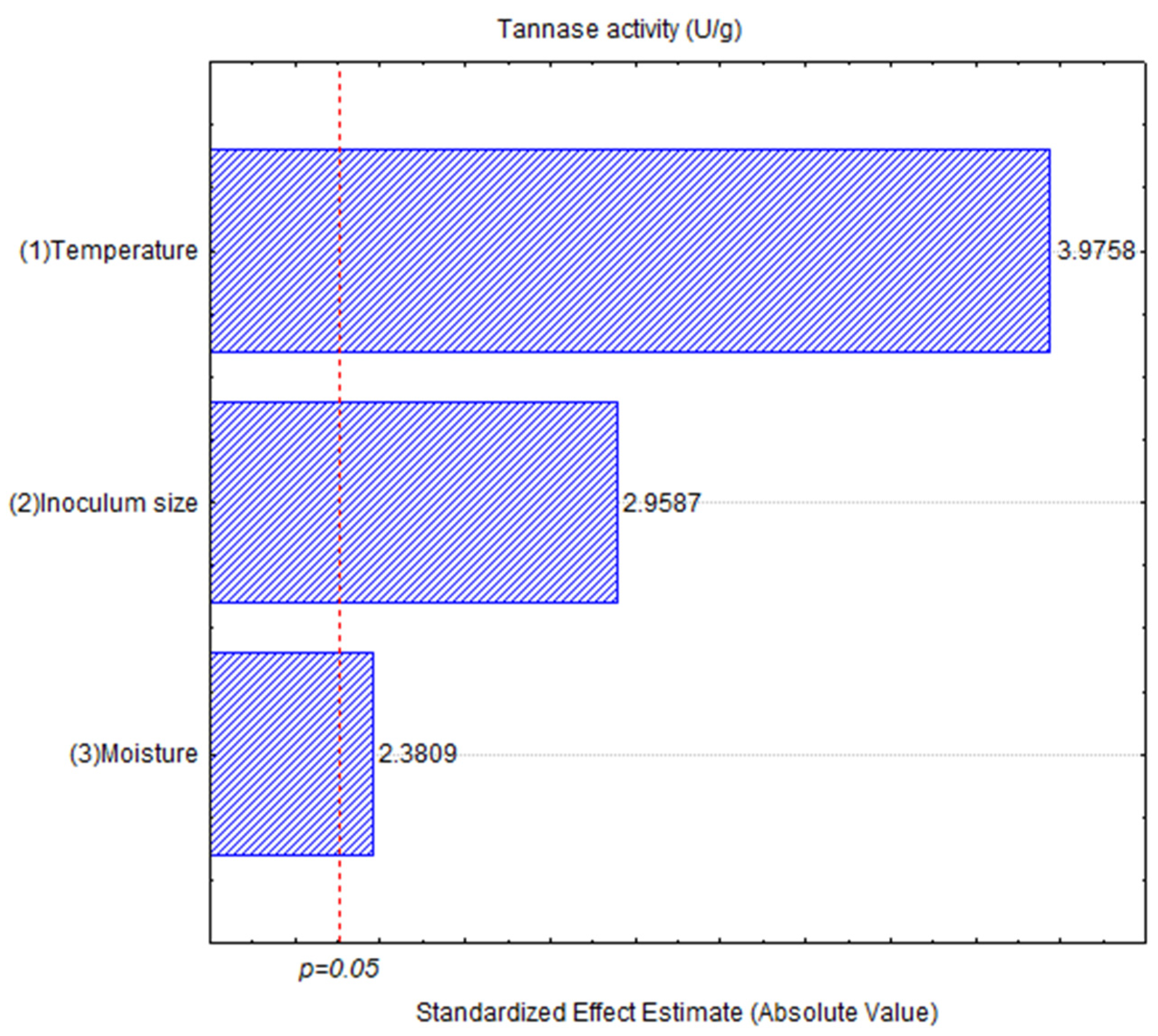
3.2. Exploratory Hunter and Hunter Design
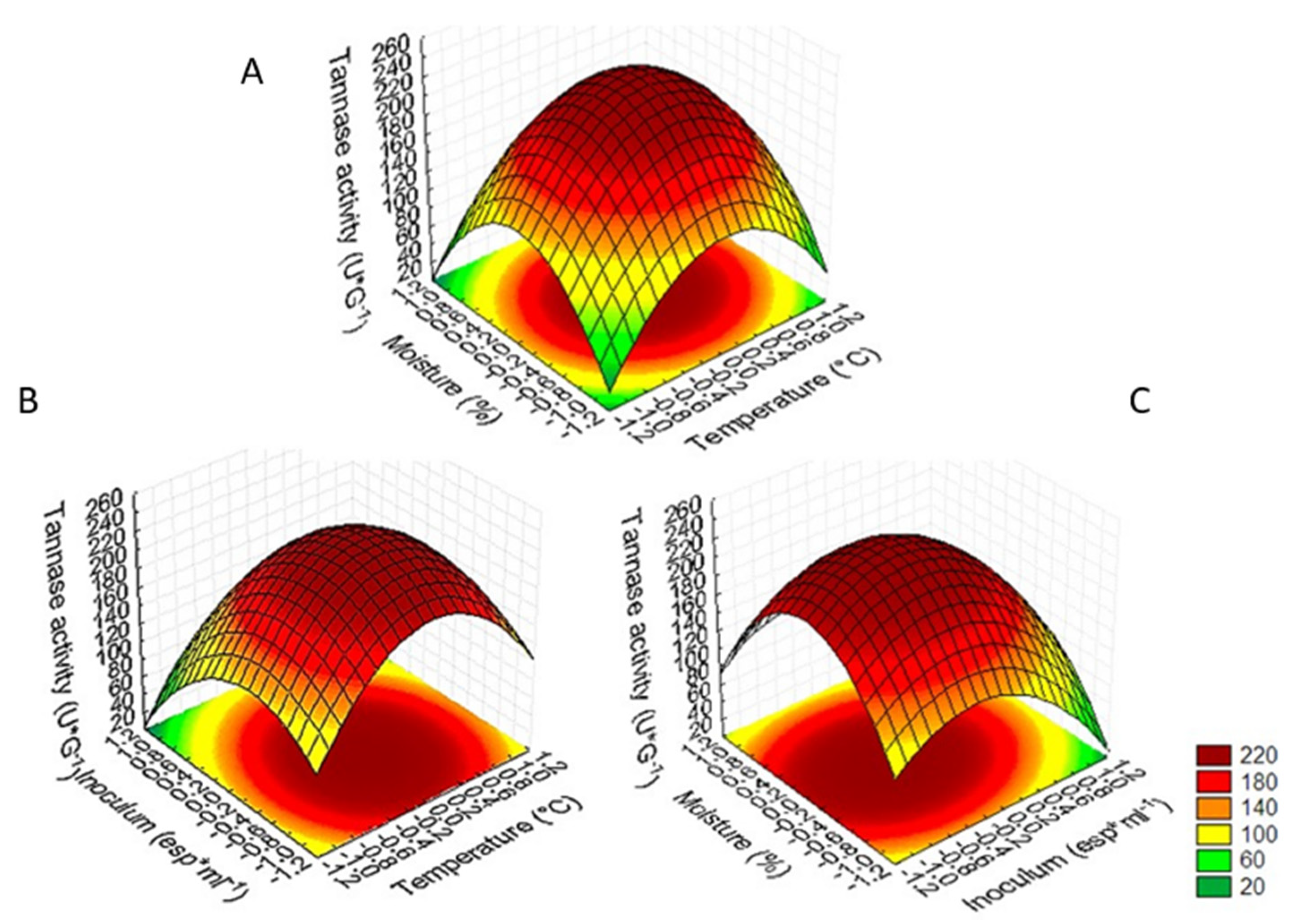
3.3. Optimization of Tannase Production
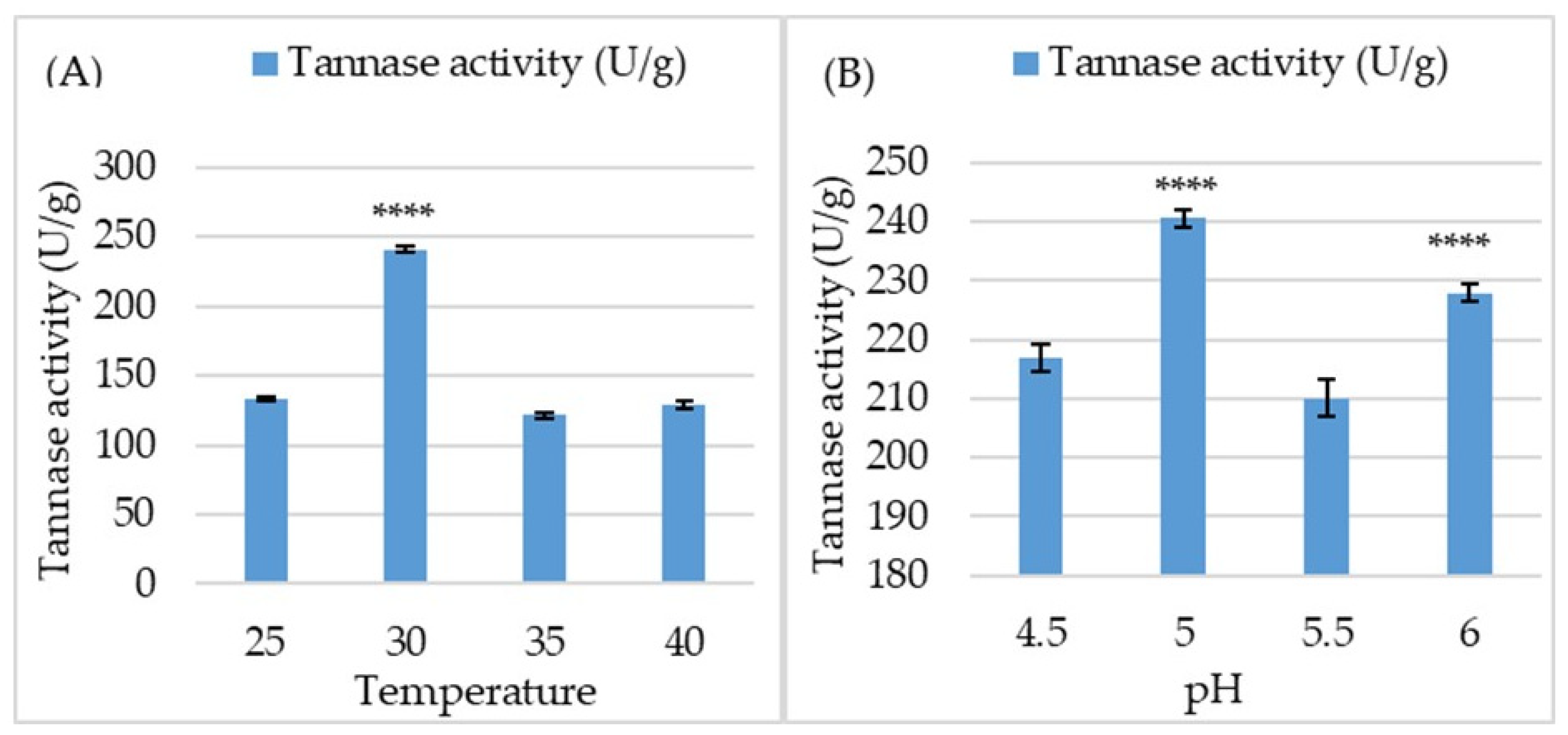
3.4. Production of Tannase, Biomass, and Soluble Protein in SSF
3.5. Kinetical Behavior of Tannase Enzyme in SSF
3.6. Purification of Tannase and Molecular Mass Determination
3.7. Biochemical Characterization of the Purified Tannase
3.8. Applications of Tannase in Food Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govindarajan, R.K.; Krishnamurthy, M.; Neelamegam, R.; Shyu, D.J.H.; Muthukalingan, K.; Nagarajan, K. Purification, structural characterization, and biotechnological potential of tannase enzyme produced by Enterobacter cloacae strain 41. Process Biochem. 2018, 77, 37–47. [Google Scholar] [CrossRef]
- Yao, J.; Guo, G.S.; Ren, G.H.; Liu, Y.H. Production, characterization and applications of tannase. J. Mol. Catal. B Enzym. 2014, 101, 137–147. [Google Scholar] [CrossRef]
- Madeira, J.V., Jr.; Ferreira, L.R.; Macedo, J.A.; Macedo, G.A. Efficient tannase production using Brazilian citrus residues and potential application for orange juice valorization. Biocat. Agricult. Biotechnol. 2015, 4, 91–97. [Google Scholar] [CrossRef]
- Govindarajan, R.K.; Revathi, S.; Rameshkumar, N.; Krishnan, M.; Kayalvizhi, N. Microbial tannase: Current perspectives and biotechnological advances. In Biocatalysis and Agricultural Biotechnology; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 6, pp. 168–175. [Google Scholar] [CrossRef]
- Coello, C.L. Tannins in commercial poultry feed. Braz. Anim. Sci. 2006, 1, 5–22. Available online: https://revistas.ufg.br/vet/article/view/242 (accessed on 1 July 2023).
- Chávez-González, M.; Rodríguez-Durán, L.V.; Balagurusamy, N.; Prado-Barragán, A.; Rodríguez, R.; Contreras, J.C.; Aguilar, C.N. Biotechnological Advances and Challenges of Tannase: An Overview. Food Bioprocess Technol. 2012, 5, 445–459. Available online: http://link.springer.com/10.1007/s11947-011-0608-5 (accessed on 1 July 2023). [CrossRef]
- Wang, F.; Ni, H.; Cai, H.; Xiao, A. Tea stalks—A novel agro-residue for the production of tannase under solid-state fermentation by Aspergillus niger. Ann. Microbiol. 2013, 63, 897–904. [Google Scholar] [CrossRef]
- Sharma, N.K.; Beniwal, V.; Kumar, N.; Kumar, S.; Pathera, A.K.; Ray, A. Production of tannase under solid-state fermentation and its application in detannification of guava juice. Prep. Biochem. Biotechnol. 2014, 44, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Graminha, E.B.N.; Goncalves, A.Z.L.; Pirota, R.D.P.B.; Balsalobre, M.A.A.; da Silva, R.; Gomes, E. Enzyme production by solid-state fermentation: Application to animal nutrition. Anim. Feed Sci. Technol. 2008, 144, 1–22. [Google Scholar] [CrossRef]
- Ibrahim, S.; Yunus Shukor, M.; Arif Syed, M.; Arina Ab Rahman, N.; Abdul Khalil, K.; Khalid, A.; Aqlima Ahmad, S.; Author, C. Bacterial Degradation of Caffeine: A Review. Asian J. Plant Biol. 2014, 2, 19–28. [Google Scholar] [CrossRef]
- Belmares, R.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Coronel, A.R.; Aguilar, C.N. Microbial production of tannase: An enzyme with potential use in food industry. LWT-Food Sci. Technol. 2004, 37, 857–864. [Google Scholar] [CrossRef]
- Lekshmi, R.; Arif Nisha, S.; Kaleeswaran, B.; Alfarhan, A.H. Pomegranate peel is a low-cost substrate to produce tannase by Bacillus velezensis TA3 under solid state fermentation. J. King Saud Univ.-Sci. 2020, 32, 1831–1837. [Google Scholar] [CrossRef]
- Liu, T.P.S.L.; Brandão Costa, R.M.P.; de Vasconcelos Freitas, D.J.; Oliveira Nacimento, C.; de Souza Motta, C.M.; Bezerra, R.P.; Nunes Herculano, P.; Porto, A.L.F. Tannase from Aspergillus melleus improves the antioxidant activity of green tea: Purification and biochemical characterisation. Int. J. Food Sci. Technol. 2017, 52, 652–661. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- de Las Rivas, B.; Rodríguez, H.; Anguita, J.; Muñoz, R. Bacterial tannases: Classification and biochemical properties. Appl. Microbiol. Biotechnol. 2019, 103, 603–623. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Nomoto, R.; Yoshida, K.I.; Osawa, R. Comparison of three tannases cloned from closely related Lactobacillus species: L. Plantarum, L. Paraplantarum, and L. Pentosus. BMC Microbiol. 2014, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Chaitanyakumar, A.; Anbalagan, M. Expression, purification, and immobilization of 768 tannase from Staphylococcus lugdunensis MTCC 3614. Appl. Microb. Biotech. Express 2016, 6, 89. [Google Scholar] [CrossRef]
- Jiménez, N.; Barcenilla, J.M.; López de Felipe, F.; de las Rivas, B.; Muñoz, R. Characterization of a bacterial tannase from Streptococcus gallolyticus UCN34 810 suitable for tannin biodegradation. Appl. Microbiol. Biotechnol. 2014, 98, 6329–6337. [Google Scholar] [CrossRef]
- Beena, P.S.; Basheer, S.M.; Bhat, S.G. Propyl Gallate Synthesis Using Acidophilic Tannase and Simultaneous Production of Tannase and Gallic Acid by Marine Aspergillus awamori BTMFW032. Appl. Biochem. Biotechnol. 2011, 164, 612–628. [Google Scholar] [CrossRef]
- Mannan, S.; Fakhrul-Razi, A.; Alam, M.Z. Optimization of process parameters for the bioconversion of activated sludge by Penicillium corylophilum, using response surface methodology. J. Environ. Sci. 2007, 19, 23–28. [Google Scholar] [CrossRef]
- Bajpai, B.; Patil, S. Tannin acyl hydrolase activity (EC 3.1.1.20) of Aspergillus, Penicillium, Fusarium and Trichoderma. World J. Microbiol. Biotechnol. 1996, 12, 217–220. [Google Scholar] [CrossRef]
- Viswanath, V.; Leo, V.V.; Prabha, S.S.; Potty, V.P.; Jisha, M.S. Optimized Production of Tannase from Cashew Testa using Aspergillus niger MTCC 5898. Food Biotechnol. 2016, 30, 249–262. [Google Scholar] [CrossRef]
- Daryoush Davoudi, O.; Neslihan, B.; Ellidokuz, H.; Özlem, Y. Evaluation of different cryoprotective agents in maintenance of viability of helicobacter pylori in stock culture media. Braz. J. Microbiol. 2010, 90, 1038–1046. [Google Scholar] [CrossRef][Green Version]
- Nava Villagómez, J.I. Fermentation of Coffee Pulp with Penicillium commune at the Laboratory Level and Semi-Pilot. Master´s Thesis, Autonomous Metropolitan University, Mexico City, Mexico, 2003; pp. 33–35. Available online: http://148.206.53.233/tesiuami/UAMI11058.pdf (accessed on 1 July 2023).
- Medina-Morales, M.; Rojas-Molina, R.; Rodríguez-Herrera, R.; Aguilar-Gonzalez, C.N. Manual of Laboratory Methods of the Department of Food Research of the Autonomous, 1st ed.; University of Coahuila: Saltillo, Mexico, 2012. [Google Scholar]
- Sharma, S.; Bhat, T.K.; Dawra, R.K. A Spectrophotometric Method for Assay of Tannase Using Rhodanine. Anal. Biochem. 2000, 89, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Galvão, M.A.M.; Arruda, A.O.; de Bezerra, I.C.F.; Ferreira, M.R.A.; Soares, L.A.L. Evaluation of the Folin-Ciocalteu Method and Quantification of Total Tannins in Stem Barks and Pods from Libidibia ferrea (Mart. ex Tul) L. P. Queiroz. Braz. Arch. Biol. Technol. 2018, 61, 1–20. [Google Scholar] [CrossRef]
- Kruger, N. The Bradford Method for Protein Quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Raaman, N.; Mahendran, B.; Jaganathan, C.; Sukumar, S.; Chandrasekaran, V. Optimization of extracellular tannase production from Paecilomyces variotii. World J. Microbiol. Biotechnol. 2010, 26, 1033–1039. [Google Scholar] [CrossRef]
- Aguirre, N.; Cancelado Vergara, D. Evaluation of the green tea extract Camellia sinensis as an antioxidant agent in the preparation of Frankfourt sausage. Bachelor’s Thesis, La Salle University, Faculty of Engineering, Bogota, Colombia, 2016. Available online: https://ciencia.lasalle.edu.co/ing_alimentos/13 (accessed on 1 July 2023).
- Ni, H.; Chen, F.; Jiang, Z.D.; Cai, M.Y.; Yang, Y.F.; Xiao, A.F.; Cai, H.N. Biotransformation of tea catechins using Aspergillus niger tannase prepared by solid state fermentation on tea byproduct. LWT-Food Sci. Technol. 2015, 60, 1206–1213. [Google Scholar] [CrossRef]
- Aguilar-gonzález, C.; Viniegra-gonzález, G. Culture Conditions Dictate Protease and Tannase Production in Submerged and Solid-State Cultures of Aspergillus niger Aa-20. Int. J. Biotechnol. Appl. Biochem. 2002, 102, 407–414. [Google Scholar] [CrossRef]
- Dalsenter, F.D.H.; Viccini, G.; Barga, M.C.; Mitchell, D.A.; Krieger, N. A mathematical model describing the effect of temperature variations on the kinetics of microbial growth in solid-state culture. Process Biochem. 2005, 40, 801–807. [Google Scholar] [CrossRef]
- Bhoite, R.N.; Murthy, P.S. Biodegradation of coffee pulp tannin by Penicillium verrucosum for production of tannase, statistical optimization and its application. Food Bioprod. Process. 2015, 94, 727–735. [Google Scholar] [CrossRef]
- Xiao, A.; Huang, Y.; Ni, H.; Cai, H.; Yang, Q. Statistical optimization for tannase production by Aspergillus tubingensis in solid-state fermentation using tea stalks. Electron. J. Biotechnol. 2015, 18, 143–147. [Google Scholar] [CrossRef]
- Kumar, M.; Rana, S.; Beniwal, V.; Salar, R.K. Optimization of tannase production by a novel Klebsiella pneumoniae KP715242 using central composite design. Biotechnol. Rep. 2015, 7, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sabu, A.; Kiran, G.S.; Pandey, A. Purification and characterization of tannin acyl hydrolase from Aspergillus niger ATCC 16620. Food Technol. Biotechnol. 2005, 43, 133–138. [Google Scholar]
- Curiel, J.A.; Rodriguez, H.; Acebron, I.; Mancheno, J.M.; Rivas de las, B.; Munoz, R. Production and physicochemical properties of recombinant Lactobacillus plantarum tannase. J. Agric. Food Chem. 2009, 57, 6224–6230. [Google Scholar] [CrossRef]
- Rodríguez-Durán, L.V.; Contreras-Esquivel, J.C.; Rodríguez, R.; Prado-Barragán, L.A.; Aguilar, C.N. Optimization of tannase production by Aspergillus niger in solid-state packed-bed bioreactor. J. Microbiol. Biotechnol. 2011, 21, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.J.S.; Sato, H.H. Synergistic effects of agroindustrial wastes on simultaneous production of protease and α-amylase under solid state fermentation using a simplex centroid mixture design. Ind. Crops Prod. 2013, 49, 813–821. [Google Scholar] [CrossRef]
- Renovato, J.; Gutiérrez-Sánchez, G.; Rodríguez-Durán, L.V.; Bergman, C.; Rodríguez, R.; Aguilar, C.N. Differential Properties of Aspergillus niger Tannase Produced Under Solid-State and Submerged Fermentations. Appl. Biochem. Biotechnol. 2011, 165, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.G.; Rodríguez, L.v.; Ramos, E.L.; Renovato, J.; Cruz-Hernández, M.A.; Rodríguez, R.; Contreras, J.; Aguilar, C.N. A novel tannase from the xerophilic fungus Aspergillus niger GH1. J. Microbiol. Biotechnol. 2009, 19, 987–996. [Google Scholar] [CrossRef]
- Khanahmadi, M.; Arezi, I.; Amiri, M.s.; Miranzadeh, M. Bioprocessing of agro-industrial residues for optimization of xylanase production by solid-state fermentation in flask and tray bioreactor. Biocatal. Agric. Biotechnol. 2017, 13, 272–282. [Google Scholar] [CrossRef]
- Amorim, G.M.; Oliveira, A.C.; Gutarra, M.L.E.; Godoy, M.G.; Freire, D.M.G. Solid-state fermentation as a tool for methylxanthine reduction and simultaneous xylanase production in cocoa meal. Biocatal. Agric. Biotechnol. 2017, 11, 34–41. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, F.; Li, L.; Jiang, Z.; Ni, H.; Xiao, A. Novel optimization strategy for tannase production through a modified solid-state fermentation system. Biotechnol. Biofuels 2018, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Rozes, N.; Peres, C. Effects of phenolic compounds on the growth and the fatty acid composition of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 1998, 49, 108–111. [Google Scholar] [CrossRef]
- Mahmoud, A.E.; Fathy, S.A.; Rashad, M.M.; Ezz, M.K.; Mohammed, A.T. Purification and characterization of a novel tannase produced by Kluyveromyces marxianus using olive pomace as solid support, and its promising role in gallic acid production. Int. J. Biol. Macromol. 2018, 107 Pt B, 2342–2350. [Google Scholar] [CrossRef]
- Selwal, K.K. High-level tannase production by Penicillium atramentosum KM using agro residues under submerged fermentation. Ann. Microbiol. 2012, 62, 139–148. [Google Scholar] [CrossRef]
- Bradoo, S.; Gupta, R.; Saxena, R.K. Screening of extracellular tannase- producing fungi: Development of a rapid and simple plate assay. J. Gen. Appl. Microbiol. 1996, 42, 325–329. [Google Scholar] [CrossRef]
- Walker, G.M.; White, N.A. Introduction to fungal physiology. Fungi Biol. Appl. 2017, 3, 1–35. [Google Scholar] [CrossRef]
- Rojas, J.U.; Verreth, J.A.J.; Amato, S.; Huisman, E.A. Biological treatments affect the chemical composition of coffee pulp. Bioresour. Technol. 2003, 89, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Beltran, J.G.; Leask, R.L.; Brown, W.A. Activity and stability of caffeine demethylases found in Pseudomonas putida IF-3. Biochem. Eng. J. 2006, 31, 8–13. [Google Scholar] [CrossRef]
- Saxena, R.K.; Sharmila, P.; Singh, V.P. Microbial degradation of tannins. In Biotransformation: Microbial Degradation of Health risk Compounds Progress in Industrial Microbiology (Singh VP); Elsevier Science Publishers: Amsterdam, The Netherlands, 1995; Volume 32, pp. 259–270. [Google Scholar]
- Aguilar, C.N.; Gutierrez-Sanchez, G. Review: Sources, Properties, Applications and Potential uses of Tannin Acyl Hydrolase. Food Sci. Technol. Int. 2001, 7, 373–382. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Guyot, S.; Rodríguez-Herrera, R.; Prado-Barragán, A.; Aguilar, C.N. Production profiles of phenolics from fungal tannic acid biodegradation in submerged and solid-state fermentation. Process Biochem. 2014, 49, 541–546. [Google Scholar] [CrossRef]
- Lekha, P.K.; Lonsane, B.K. Production and application of tannin acyl hydrolase: State of the art. Adv. Appl. Microbiol. 1997, 44, 215–260. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Beniwal, V.; Kumar, R. Purification and characterization of a thermophilic tannase from Klebsiella pneumoniae KP715242. Biocatal. Agric. Biotechnol. 2015, 4, 745–751. [Google Scholar] [CrossRef]
- Jana, A.; Maity, C.; Halder, S.K.; Das, A.; Pati, B.R.; Mondal, K.C.; Mohapatra, P.K.D. 369 Structural characterization of thermostable, solvent tolerant, cytosafe tannase from 370 Bacillus subtilis PAB2. Biochem. Eng. J. 2013, 77, 161–170. [Google Scholar] [CrossRef]
- Mingshu, L.; Kai, Y.; Qiang, H.; Dongying, J. Biodegradation of gallotannins and ellagitannins. J. Basic Microbiol. 2006, 46, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.K.; Viruthagiri, T.; Arunkumar, C. Application of response surface methodology for the maximum production of tannase using Aspergillus foetidus (MTCC 3557). Int. Pharm. Dev. Technol. 2015, 4, 151–156. [Google Scholar]
- Kumar, R.A.; Gunasekaran, P.; Lakshman, M. Biodegradation of tannic acid by Citrobacter freundii isolated from a tannery effluent. J. Basic Microbiol. 1999, 39, 161–168. [Google Scholar] [CrossRef]
- Hiura, T.; Hashidoko, Y.; Kobayashi, Y.; Tahara, S. Effective degradation of tannic acid by immobilized rumen microbes of a sika deer (Cervus nippon yesoensis) in winter. Anim. Feed Sci. Technol. 2010, 155, 1–8. [Google Scholar] [CrossRef]
- Sivashanmugam, K.; Jayaraman, G. Production and partial purification of extracellular tannase by Klebsiella pneumoniae MTCC 7162 isolated from tannery effluent. Afr. J. Biotechnol. 2011, 10, 1364–1374. [Google Scholar]
- Beniwal, V.; Kumar, A.; Goel, G.; Chhokar, V. A novel low molecular weight acido-thermophilic tannase from Enterobacter cloacae MTCC 9125. Biocat. Agric. Biotechnol. 2013, 2, 132–137. [Google Scholar] [CrossRef]
- Riul, A.J.; Goncalves, H.B.; Jorge, J.A.; Guimarães, L.H.S. Characterization of a glucose-and solvent-tolerant extracellular tannase from Aspergillus phoenicis. J. Mol. Catal. B Enzym. 2013, 85–86, 126–133. [Google Scholar] [CrossRef]
- Sharma, K.P.; John, P.J. Purification, and characterization of tannase and tannase gene 367 from Enterobacter sp. Process Biochem. 2011, 46, 240–244. [Google Scholar] [CrossRef]
- Rodríguez, H.; de las Rivas, B.; Gómez-Cordovés, C.; Muñoz, R. Characterization of tannase activity in cell-free extracts of Lactobacillus plantarum CECT 748T. Int. J. Food Microbiol. 2008, 121, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.B.; Pashova, S.B.; Slokoska, L.S. Comparison of antioxidant enzyme biosynthesis by free and immobilized Aspergillus niger cells. Enzyme. Microb. Tech. 2000, 26, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Shiono, Y.; Koseki, T. Biochemical characterization of Aspergillus oryzae native tannase and the recombinant enzyme expressed in Pichia pastoris. J. Biosci. Bioeng. 2014, 118, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Battestin, V.; Macedo, G.A. Tannase production by Paecilomyces variotii. Bioresour. Technol. 2007, 98, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Skene, I.K.; Brooker, J.D. Characterization of tannin acyl hydrolase activity in the ruminal bacterium Selenomonas ruminantium. Anaerobe 1995, 1, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Gayen, S.; Ghosh, U. Purification and characterization of tannin acyl hydrolase produced by mixed solid-state fermentation of wheat bran and marigold flower by Penicillium notatum NCIM 923. Biomed. Res. Int. 2013, 2013, 596380. [Google Scholar] [CrossRef]
- Lekshmi, R.; Arif Nisha, S.; Thirumalai Vasan, P.; Kaleeswaran, B. A comprehensive review on tannase: Microbes associated production of tannase exploiting tannin rich agro-industrial wastes with special reference to its potential environmental and industrial applications. Environ. Res. 2021, 201, 111625. [Google Scholar] [CrossRef]
- Kasieczka-Burnecka, M.; Kuc, K.; Kalinowska, H.; Knap, M.; Turkiewicz, M. Purification and characterization of two cold-adapted extracellular tannin acylhydrolases from an Antarctic strain Verticillium sp. P9. Appl. Microbiol. Biotechnol. 2007, 77, 77–89. [Google Scholar] [CrossRef]
- Al-Mraai, S.T.Y.; Al-Fekaiki, D.F.; Al-Manhel, A.J.A. Purification and characterization of tannase from the local isolate of Aspergillus niger. J. Appl. Biol. Biotechnol. 2019, 7, 29–34. [Google Scholar] [CrossRef]
- Chhokar, V.; Sangwan, M.; Beniwal, V.; Nehra, K.; Nehra, K.S. Effect of additives on the activity of tannase from Aspergillus awamori MTCC9299. Appl. Biochem. Biotechnol. 2010, 160, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Aithal, M.; Belur, P.D. Enhancement of propyl gallate yield in nonaquous medium using novel cell-associated tannase of Bacillus massiliensis. Prep. Biochem. Biotechnol. 2013, 43, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Belur, P.D.; Mugeraya, G. Microbial production of Tannase: State of art. Res. J. Microbiol. 2011, 6, 25–40. [Google Scholar] [CrossRef]
- Nuero, O.M.; Reyes, F. Enzymes for animal feeding from Penicillium chrysogenum mycelial wastes from penicillin manufacture. Lett. Appl. Microbiol. 2002, 34, 413–416. [Google Scholar] [CrossRef]
- Das Mohapatra, P.K.; Maity, C.; Rao, R.S.; Pati, B.R.; Mondal, K.C. Tannase production by Bacillus licheniformis KBR6: Optimization of submerged culture conditions by Taguchi DOE methodology. Food Res. Int. 2009, 42, 430–435. [Google Scholar] [CrossRef]
- Madeira, J.V., Jr.; Macedo, J.A.; Macedo, G.A. Detoxification of castor bean residues and the simultaneous production of tannase and phytase by solid-state fermentation using Paecilomyces variotii. Bioresour. Technol. 2011, 102, 7343–7348. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Jani, S. Tannins: Antinutrient with positive effect to Manage diabetes. J. Recent Sci. 2012, 1, 70–73. [Google Scholar]



| Treatments | Temperature | Inoculum Size | Moisture |
|---|---|---|---|
| 1 | −1.00000 | −1.00000 | 1.00000 |
| 2 | 1.00000 | −1.00000 | −1.00000 |
| 3 | −1.00000 | 1.00000 | −1.00000 |
| 4 | 1.00000 | 1.00000 | 1.00000 |
| 5 | −1.00000 | −1.00000 | 1.00000 |
| 6 | 1.00000 | −1.00000 | −1.00000 |
| 7 | −1.00000 | 1.00000 | −1.00000 |
| 8 | 1.00000 | 1.00000 | 1.00000 |
| 9 | −1.00000 | −1.00000 | 1.00000 |
| 10 | 1.00000 | −1.00000 | −1.00000 |
| 11 | −1.00000 | 1.00000 | −1.00000 |
| 12 | 1.00000 | 1.00000 | 1.00000 |
| Analysis | Concentration (mg/g) | Standard Deviation |
|---|---|---|
| Sugar totals | 120 * | ±0.05 |
| Condensed tannins | 209.05 * | ±0.012 |
| Hydrolysable tannins | 242.05 * | ±0.014 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value < 0.05 |
|---|---|---|---|---|---|
| Temperature | 294.82 | 1 | 294.820 | 1.12711 | 0.0399640 |
| Inoculum | 3292.46 | 1 | 3292.457 | 12.58723 | 0.071079 |
| Moisture | 8415.91 | 1 | 8415.908 | 32.17443 | 0.029703 |
| Error | 523.14 | 2 | 261.571 | ||
| Total SS | 16,409.03 | 8 |
| Time (h) | Tannase Activity (U/g) | Biomass (mg/g) | Soluble Protein (mg/g) |
|---|---|---|---|
| 0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 44.21 ± 4.22 |
| 24 | 30.51 ± 1.77 | 0.41 ± 0.05 | 45.11 ± 1.17 ** |
| 48 | 246.83 ± 0.67 ** | 0.51 ± 0.01 ** | 60.04 ± 3.43 ** |
| 72 | 133.38 ± 1.06 ** | 0.20 ± 0.08 ** | 42.19 ± 1.76 |
| 96 | 69.01 ± 2.83 | 0.31 ± 0.03 | 43.65 ± 1.96 |
| Stage | Volume (mL) | Total Activity (U) 1 | Total Protein (mg) 1 | Specific Activity (U/mg Protein) 1 | Yield (%) 1 |
|---|---|---|---|---|---|
| Crude Extract | 250 | 247.12 ± 0.53 | 487 ± 0.35 | 0.5 ± 0.21 | 100 |
| Dialysis (Membrane 10 kDa) | 170 | 168.04 ± 0.10 | 6.64 ± 0.15 | 25.30 ± 0.19 | 67.99 |
| Ultracentrifugation (Microcon) | 40 | 40.40 ± 0.12 | 4.72 ± 0.03 | 8.55 ± 0.50 | 16.35 |
| Metal Ions | Concentration (5 mM) | |
|---|---|---|
| % Relative Activity | % Inhibition | |
| Control | 100 | - |
| ZnCl2 | 98.67 | 1.33 |
| MgSO4 | 119.93 | - |
| CuSO4 | 112.52 | - |
| FeCl3 | 102.63 | - |
| CaCl2 | 74.15 | 26.85 |
| NaCl | 94.11 | 5.89 |
| MnCl2 | 56.65 | 43.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Lucio, E.M.; Chávez-González, M.L.; Londoño-Hernandez, L.; Ruiz, H.A.; Martínez-Hernandez, J.L.; Govea-Salas, M.; Nediyaparambil Sukumaran, P.; Abdulhameed, S.; Aguilar, C.N. Solid-State Fermentation of Green Tea Residues as Substrates for Tannase Production by Aspergillus niger TBG 28A: Optimization of the Culture Conditions. Fermentation 2023, 9, 781. https://doi.org/10.3390/fermentation9090781
Peña-Lucio EM, Chávez-González ML, Londoño-Hernandez L, Ruiz HA, Martínez-Hernandez JL, Govea-Salas M, Nediyaparambil Sukumaran P, Abdulhameed S, Aguilar CN. Solid-State Fermentation of Green Tea Residues as Substrates for Tannase Production by Aspergillus niger TBG 28A: Optimization of the Culture Conditions. Fermentation. 2023; 9(9):781. https://doi.org/10.3390/fermentation9090781
Chicago/Turabian StylePeña-Lucio, Erick M., Mónica L. Chávez-González, Liliana Londoño-Hernandez, Héctor A. Ruiz, José L. Martínez-Hernandez, Mayela Govea-Salas, Pradeep Nediyaparambil Sukumaran, Sabu Abdulhameed, and Cristóbal N. Aguilar. 2023. "Solid-State Fermentation of Green Tea Residues as Substrates for Tannase Production by Aspergillus niger TBG 28A: Optimization of the Culture Conditions" Fermentation 9, no. 9: 781. https://doi.org/10.3390/fermentation9090781
APA StylePeña-Lucio, E. M., Chávez-González, M. L., Londoño-Hernandez, L., Ruiz, H. A., Martínez-Hernandez, J. L., Govea-Salas, M., Nediyaparambil Sukumaran, P., Abdulhameed, S., & Aguilar, C. N. (2023). Solid-State Fermentation of Green Tea Residues as Substrates for Tannase Production by Aspergillus niger TBG 28A: Optimization of the Culture Conditions. Fermentation, 9(9), 781. https://doi.org/10.3390/fermentation9090781









