Efficient Biorefinery Based on Designed Lignocellulosic Substrate for Lactic Acid Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Alkaline Pretreatment
2.2. Enzymatic Hydrolysis
2.3. Microorganism and Inoculum Cultivation
2.4. Lactic Acid Fermentation
2.4.1. Separate Hydrolysis and Fermentation (SHF)
2.4.2. Simultaneous Saccharification and Fermentation (SSF)
2.5. Analytical Methods
3. Results and Discussion
3.1. Effect of Cellulase on Enzymatic Hydrolysis of Pretreated BSG
3.2. Comparison of SHF and SSF Processes for Lactic Acid Production
3.3. Optimal pH for Lactic Acid Production from Pretreated BSG
3.4. Improved SSF of Lactic Acid from Designed BSG Substrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mitri, S.; Salameh, S.-J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of Brewers’ Spent Grains: Pretreatments and Fermentation, a Review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Aliyu, S.; Bala, M. Brewer’s spent grain: A review of its potentials and applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar]
- Bachmann, S.A.L.; Calvete, T.; Féris, L.A. Potential applications of brewery spent grain: Critical an overview. J. Environ. Chem. Eng. 2022, 10, 106951. [Google Scholar] [CrossRef]
- Giacobbe, S.; Piscitelli, A.; Raganati, F.; Lettera, V.; Sannia, G.; Marzocchella, A.; Pezzella, C. Butanol production from laccase-pretreated brewer’s spent grain. Biotechnol. Biofuels. 2019, 12, 47. [Google Scholar] [CrossRef]
- Sibono, L.; Tronci, S.; Grosso, M.; Hajrizaj, R.; Errico, M. Brewer’s Spent Grain to Bioethanol Through a Hybrid Saccharification and Fermentation Process. Chem. Eng. Trans. 2023, 99, 7–12. [Google Scholar] [CrossRef]
- Camargo, F.P.; Rabelo, C.A.B.S.; Duarte, I.C.S.; Silva, E.L.; Varesche, M.B.A. Biogas from lignocellulosic feedstock: A review on the main pretreatments, inocula and operational variables involved in anaerobic reactor efficiency. Int. J. Hydrogen Energ. 2023, 48, 20613–20632. [Google Scholar] [CrossRef]
- Ogidi, C.O.; Emmanuel, O.P.; Daramola, O.O.; Bamigboye, O.; Malomo, O. Synthesis of Silver Nanoparticles using Cellulose and Starch Extracted from Brewer Spent Grain: Assessment of their Antimicrobial and Preservatives Activities. Turk. J. Agric. Food Sci. Technol. 2023, 11, 227–238. [Google Scholar] [CrossRef]
- Ciurko, D.; Neuvéglise, C.; Szwechłowicz, M.; Lazar, Z.; Janek, T. Comparative Analysis of the Alkaline Proteolytic Enzymes of Yarrowia Clade Species and Their Putative Applications. Int. J. Mol. Sci. 2023, 24, 6514. [Google Scholar] [CrossRef]
- Liu, C.; Ullah, A.; Gao, X.; Shi, J. Synergistic Ball Milling–Enzymatic Pretreatment of Brewer’s Spent Grains to Improve Volatile Fatty Acid Production through Thermophilic Anaerobic Fermentation. Processes 2023, 11, 1648. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Recent advances in biotechnological valorization of brewers’ spent grain. Food Sci. Biotechnol. 2021, 30, 341–353. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro)plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Latorre-Sánchez, M.; Coll Lozano, C.; Venus, J. Organic fraction of municipal solid waste for the production of l-lactic acid with high optical purity. J. Clean. Prod. 2020, 247, 119165. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; Gonzalez-Fernandez, C.; Ballesteros, M.; Tomas-Pejo, E. Biotechnological advances in lactic acid production by lactic acid bacteria: Lignocellulose as novel substrate. Biofuel Bioprod. Bior. 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Mancilha, I.M.; Roberto, I.C. Effects of medium supplementation and pH control on lactic acid production from brewer’s spent grain. Biochem. Eng. J. 2008, 40, 437–444. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Dragone, G.; Mancilha, I.M.; Roberto, I.C. Brewer’s spent grain as raw material for lactic acid production by Lactobacillus delbrueckii. Biotechnol. Lett. 2007, 29, 1973–1976. [Google Scholar] [CrossRef]
- Radosavljevic, M.; Pejin, J.; Kocic-Tanackov, S.; Mladenovic, D.; Djukic-Vukovic, A.; Mojovic, L. Brewers’ spent grain and thin stillage as raw materials in l-(+)-lactic acid fermentation. J. Inst. Brew. 2018, 124, 23–30. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljevic, M.; Pribic, M.; Kocic-Tanackov, S.; Mladenovic, D.; Djukic-Vukovic, A.; Mojovic, L. Possibility of l-(+)-lactic acid fermentation using malting, brewing, and oil production by-products. Waste Manag. 2018, 79, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Pejin, J.; Radosavljevic, M.; Kocic-Tanackov, S.; Djukic-Vukovic, A.; Mojovic, L. Lactic acid fermentation of brewer’s spent grain hydrolysate by Lactobacillus rhamnosus with yeast extract addition and pH control. J. Inst. Brew. 2017, 123, 98–104. [Google Scholar] [CrossRef]
- Kim, J.H.; Block, D.E.; Mills, D.A. Simultaneous consumption of pentose and hexose sugars: An optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl. Microbiol. Biot. 2010, 88, 1077–1085. [Google Scholar] [CrossRef]
- Wang, Y.; Abdel-Rahman, M.A.; Tashiro, Y.; Xiao, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. l-(+)-lactic acid production by co-fermentation of cellobiose and xylose without carbon catabolite repression using Enterococcus mundtii QU 25. RSC. Adv. 2014, 4, 22013–22021. [Google Scholar] [CrossRef]
- Biswas, R.; Persad, A.; Bisaria, V.S. Production of Cellulolytic Enzymes. In Bioprocessing of Renewable Resources to Commodity Bioproducts, 1st ed.; Sauer, M., Steiger, M., Mattanovich, D., Marx, H., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2014; Volume 1, pp. 105–132. [Google Scholar] [CrossRef]
- Tiwari, P.; Misra, B.N.; Sangwan, N.S.; Cihan, A.C. β-Glucosidases from the Fungus Trichoderma: An Efficient Cellulase Machinery in Biotechnological Applications. Biomed. Res. Int. 2013, 2013, 203735. [Google Scholar] [CrossRef]
- Sørensen, A.; Lübeck, M.; Lübeck, P.S.; Ahring, B.K. Fungal beta-glucosidases: A Bottleneck in industrial use of lignocellulosic materials. Biomolecules 2013, 3, 612–631. [Google Scholar] [CrossRef]
- Sørensen, A.; Lubeck, P.S.; Lubeck, M.; Teller, P.J.; Ahring, B.K. Beta-Glucosidases from a new Aspergillus species can substitute commercial beta-glucosidases for saccharification of lignocellulosic biomass. Can. J. Microbiol. 2011, 57, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Calderon, O.; Trajano, H.L.; Duff, S.J.B. Stability of commercial glucanase and beta-glucosidase preparations under hydrolysis conditions. PeerJ 2014, 2, e402. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2006. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. [Google Scholar]
- Zhao, T.; Tashiro, Y.; Abdel-Rahman, M.A.; Tan, J.; Hanamiya, M.; Sonomoto, K. Efficient open integrated lactic acid fermentation of undetoxified semi-hydrolysate of rice straw using thermophilic Enterococcus faecium QU 50. Ind. Crop. Prod. 2023, 196, 116494. [Google Scholar] [CrossRef]
- Tang, Y.; Bu, L.; He, J.; Jiang, J. l(+)-Lactic acid production from furfural residues and corn kernels with treated yeast as nutrients. Eur. Food. Res. Technol. 2013, 236, 365–371. [Google Scholar] [CrossRef]
- Arora, R.; Singh, P.; Sarangi, P.K.; Kumar, S.; Chandel, A.K. A critical assessment on scalable technologies using high solids loadings in lignocellulose biorefinery: Challenges and solutions. Crit. Rev. Biotechnol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, B.; Nie, K.; Ben, H.; Yang, X.; Zhang, Y.; Han, G.; Jiang, W. A novel cascade glycolic acid pretreatment-alkali degumming method for producing hemp fiber. Ind. Crop. Prod. 2023, 195, 116424. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, H.; Zhang, Y.; Wang, N.; Li, Y.; Liu, S.; Gao, M.; Wang, Y.; Wang, Q. Improving lactic acid yield of hemicellulose from garden garbage through pretreatment of a high solid loading coupled with semi-hydrolysis using low enzyme loading. Bioresour. Technol. 2023, 384, 129330. [Google Scholar] [CrossRef]
- Alrumman, S.A. Enzymatic saccharification and fermentation of cellulosic date palm wastes to glucose and lactic acid. Braz. J. Microbiol. 2016, 47, 110–119. [Google Scholar] [CrossRef]
- Cannella, D.; Hsieh, C.W.C.; Felby, C.; Jorgensen, H. Production and effect of aldonic acids during enzymatic hydrolysis of lignocellulose at high dry matter content. Biotechnol. Biofuels. 2012, 5, 26. [Google Scholar] [CrossRef]
- Kwan, T.H.; Pleissner, D.; Lau, K.Y.; Venus, J.; Pommeret, A.; Lin, C.S.K. Techno-economic analysis of a food waste valorization process via microalgae cultivation and co-production of plasticizer, lactic acid and animal feed from algal biomass and food waste. Bioresour. Technol. 2015, 198, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Andric, P.; Meyer, A.S.; Jensen, P.A.; Dam-Johansen, K. Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: I. Significance and mechanism of cellobiose and glucose inhibition on cellulolytic enzymes. Biotechnol. Adv. 2010, 28, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Sreenath, H.K.; Moldes, A.B.; Koegel, R.G.; Straub, R.J. Lactic acid production by simultaneous saccharification and fermentation of alfalfa fiber. J. Biosci. Bioeng. 2001, 92, 518–523. [Google Scholar] [CrossRef]
- Virunanon, C.; Ouephanit, C.; Burapatana, V.; Chulalaksananukul, W. Cassava pulp enzymatic hydrolysis process as a preliminary step in bio-alcohols production from waste starchy resources. J. Clean. Prod. 2013, 39, 273–279. [Google Scholar] [CrossRef]
- Lou, H.; Zhu, J.; Lan, T.Q.; Lai, H.; Qiu, X. pH-Induced lignin surface modification to reduce nonspecific cellulase binding and enhance enzymatic saccharification of lignocelluloses. Chemsuschem. 2013, 6, 919–927. [Google Scholar] [CrossRef]
- Hofvendahl, K.; Hahn-Hagerdal, B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- Zhou, J.; Ouyang, J.; Zhang, M.; Yu, H. Simultaneous saccharification and fermentation of bagasse sulfite pulp to lactic acid by Bacillus coagulans CC17. BioResoures 2014, 9, 2609–2620. [Google Scholar] [CrossRef][Green Version]
- Pleissner, D.; Demichelis, F.; Mariano, S.; Fiore, S.; Gutiérrez, I.M.N.; Schneider, R.; Venus, J. Direct production of lactic acid based on simultaneous saccharification and fermentation of mixed restaurant food waste. J. Clean. Prod. 2017, 143, 615–623. [Google Scholar] [CrossRef]
- He, J.; Zhang, W.; Liu, X.; Xu, N.; Xiong, P. Optimization of prehydrolysis time and substrate feeding to improve ethanol production by simultaneous saccharification and fermentation of furfural process residue. J. Biosci. Bioeng. 2016, 122, 563–569. [Google Scholar] [CrossRef]
- Chan, K.-L.; Dong, C.; Wong, M.S.; Kim, L.-H.; Leu, S.-Y. Plant chemistry associated dynamic modelling to enhance urban vegetation carbon sequestration potential via bioenergy harvesting. J. Clean Prod. 2018, 197, 1084–1094. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Kumar, A.; Hardwidge, P.R.; Tanaka, T.; Kondo, A.; Vadlani, P.V. d-lactic acid production from renewable lignocellulosic biomass via genetically modified Lactobacillus plantarum. Biotechnol. Progr. 2016, 32, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Németh, Á.; Sevella, B. Investigation and modeling of lactic acid fermentation on wheat starch via SSF, CHF and SHF technology. Period. Polytech. Chem. Eng. 2011, 55, 11–16. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljevic, M.; Kocic-Tanackov, S.; Mladenovic, D.; Djukic-Vukovic, A.; Mojovic, L. Fed-batch l-(+)-lactic acid fermentation of brewer’s spent grain hydrolysate. J. Inst. Brew. 2017, 123, 537–543. [Google Scholar] [CrossRef]
- Tanaka, T.; Hoshina, M.; Tanabe, S.; Sakai, K.; Ohtsubo, S.; Taniguchi, M. Production of d-lactic acid from defatted rice bran by simultaneous saccharification and fermentation. Bioresour. Technol. 2006, 97, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Givry, S.; Prevot, V.; Duchiron, F. Lactic acid production from hemicellulosic hydrolyzate by cells of Lactobacillus bifermentans immobilized in Ca-alginate using response surface methodology. World J. Microb. Biot. 2008, 24, 745–752. [Google Scholar] [CrossRef]
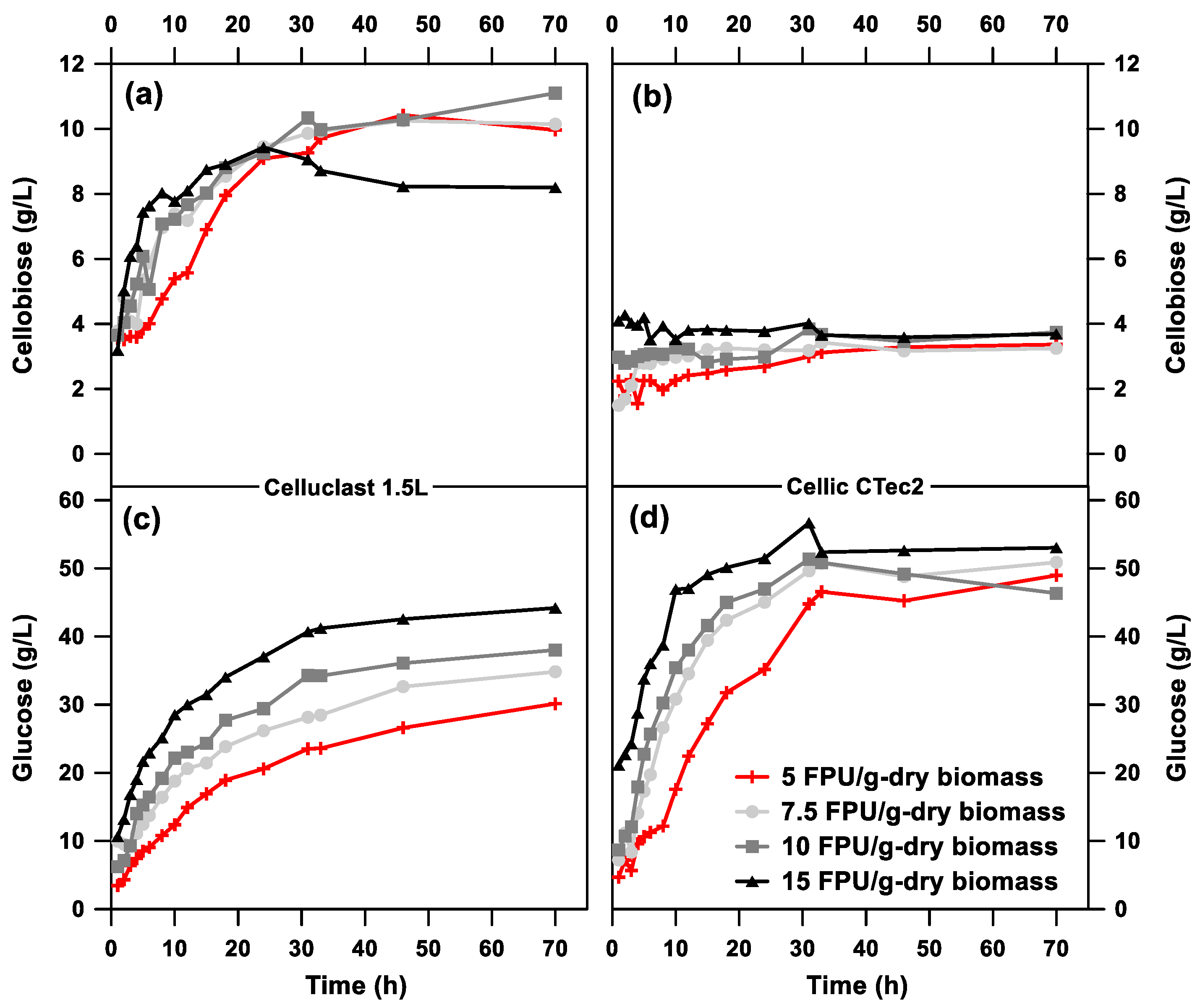
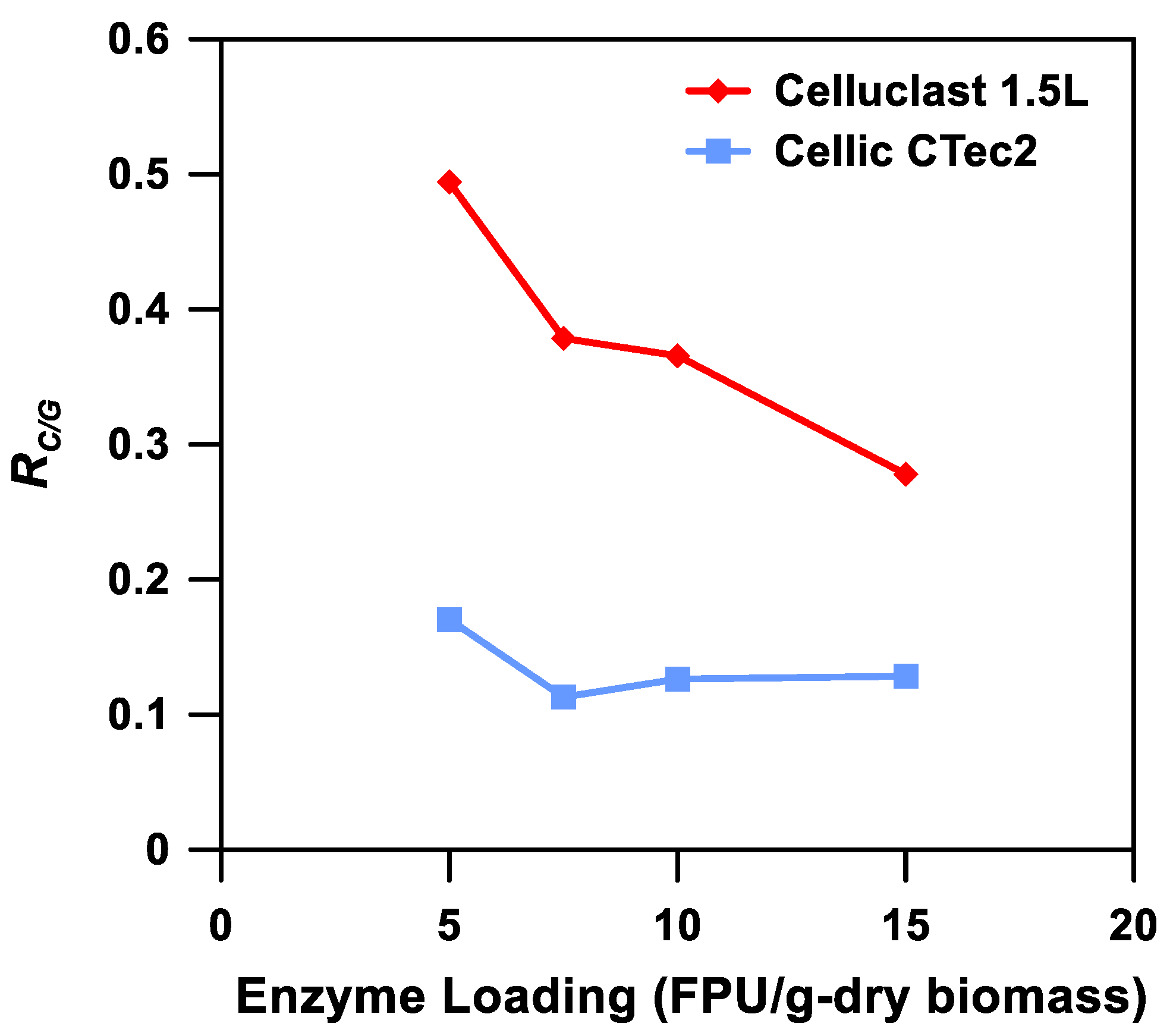
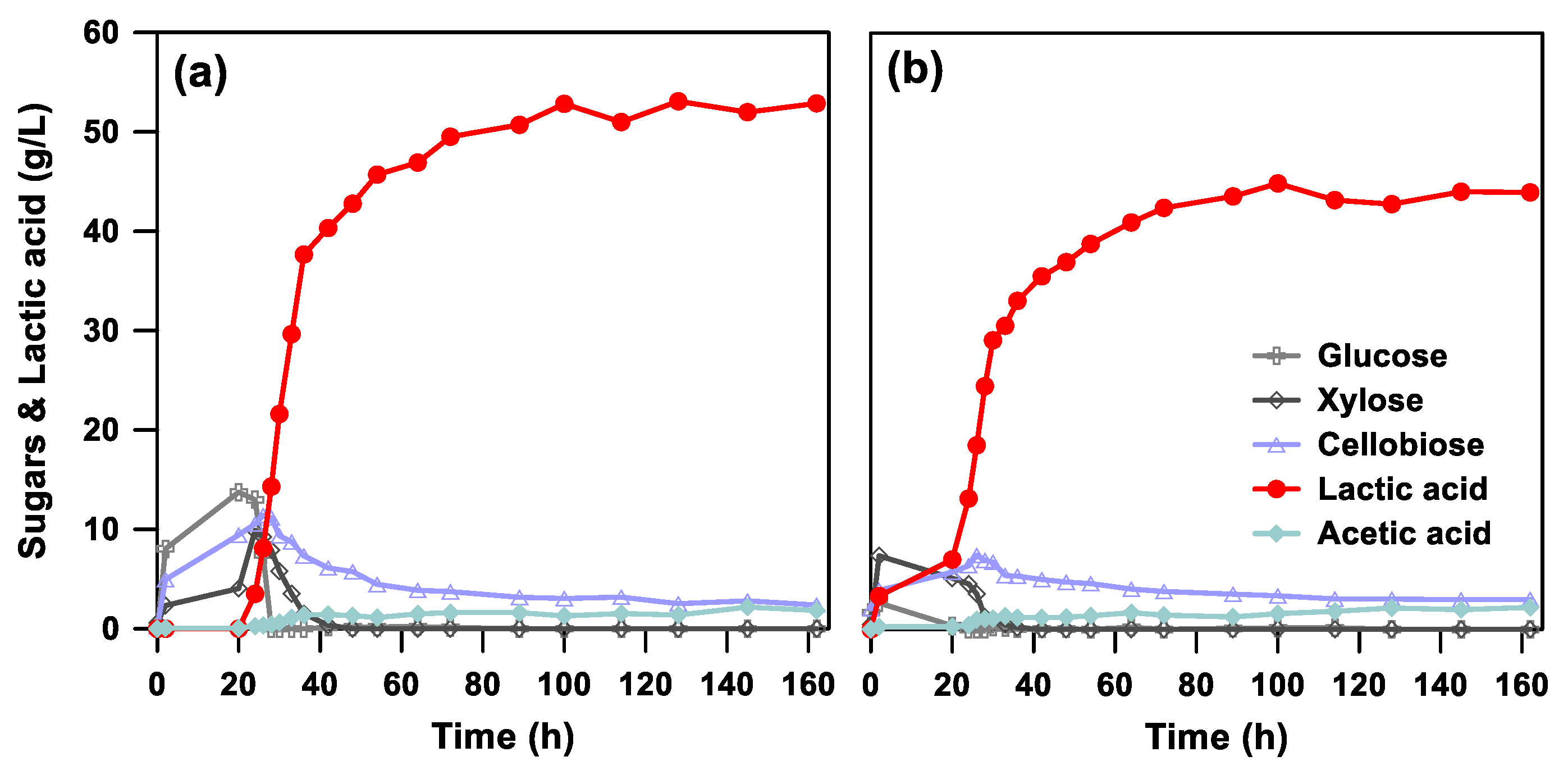

| Fermentation | Hydrolytic | CGlc 1 | Ccel 2 | CXyl 3 | CLA 4 | YLA 5 |
|---|---|---|---|---|---|---|
| Mode | Enzyme | (g/L) | (g/L) | (g/L) | (g/L) | (g/g) |
| SHF (121 h) | Celluclast 1.5L | 23.0 ± 3.43 | 5.99 ± 1.42 | 10.4 ± 0.88 | 21.9 ± 3.47 | 0.287 |
| SHF (121 h) | Cellic CTec2 | 35.9 ± 4.02 | 3.18 ± 0.33 | 13.0 ± 1.73 | 20.8 ± 1.62 | 0.273 |
| SSF (145 h) | Celluclast 1.5L | 18.2 ± 1.97 | 5.70 ± 1.02 | 7.38 ± 0.79 | 25.7 ± 2.27 | 0.337 |
| SSF (145 h) | Cellic CTec2 | 20.2 ± 0.73 | 4.15 ± 1.83 | 8.96 ± 1.65 | 23.1 ± 4.01 | 0.303 |
| pH | CGlc 1 (g/L) | CCel 2 (g/L) | CXyl 3 (g/L) | CLA 4 (g/L) | YLA 5 (g/g) |
|---|---|---|---|---|---|
| 5.5 | 38.7 ± 3.13 | 5.64 ± 1.50 | 16.8 ± 2.22 | 5.61 ± 0.53 | 0.074 |
| 6.0 | 29.6 ± 1.37 | 6.86 ± 0.16 | 14.9 ± 1.03 | 13.83 ± 2.18 | 0.181 |
| 6.5 | 22.0 ± 3.28 | 5.87 ± 1.76 | 12.5 ± 1.83 | 18.15 ± 1.79 | 0.238 |
| 7.0 | 16.2 ± 0.93 | 4.32 ± 0.69 | 9.36 ± 0.73 | 22.30 ± 3.02 | 0.292 |
| 7.5 | 14.2 ± 2.34 | 4.27 ± 1.02 | 7.94 ± 0.34 | 19.77 ± 1.28 | 0.259 |
| Microorganism | Substrate | Fermentation Mode | Nutrients Supplementation | CLA 1 (g/L) | YLA 2 (g/g) | PLA 3 (g/L/h) | Ref. |
|---|---|---|---|---|---|---|---|
| E. mundtii | BSG | SSF | No | 44.9 | 0.588 | 3.06 | This study |
| BSG | SSF with prehydrolysis | No | 53.1 | 0.696 | 3.65 | This study | |
| L. delbrueckii | BSG | SHF | MRS broth | 35.5 | 0.485 | 0.82 | [17] |
| L. rhamnosus | BSG | SHF | 50 g/L yeast extract | 39.4 | 0.913 | 1.69 | [21] |
| L. delbrueckii UFV H2b20 | BSG | SHF | No | 5.4 | 0.074 | 0.11 | [18] |
| L. rhamnosus ATCC 7469 | BSG supplemented with glucose | SHF | 50 g/L yeast extract | 116.1 | 0.933 | 2.0 | [50] |
| L. plantarum Δldh1 | Corn stover | SSF | mMRS broth | 21.1 | 0.505 | 0.5 | [48] |
| L. bifermentans DSM 20003T | Wheat bran | SHF | No | 62.8 | 0.647 | 1.2 | [52] |
| L. delbrueckii IFO 3202 | Rice bran | SSF | N2 gas | 28.0 | 0.483 | 0.78 | [51] |
| L. sp. MKT-878 | Wheat starch | SHF | 6 g/L yeast extract | 118 | 0.908 | 3.57 | [49] |
| Wheat starch | SSF with prehydrolysis | 6 g/L yeast extract | 121 | 0.931 | 4.32 | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Gao, M. Efficient Biorefinery Based on Designed Lignocellulosic Substrate for Lactic Acid Production. Fermentation 2023, 9, 744. https://doi.org/10.3390/fermentation9080744
Wang Y, Gao M. Efficient Biorefinery Based on Designed Lignocellulosic Substrate for Lactic Acid Production. Fermentation. 2023; 9(8):744. https://doi.org/10.3390/fermentation9080744
Chicago/Turabian StyleWang, Ying, and Ming Gao. 2023. "Efficient Biorefinery Based on Designed Lignocellulosic Substrate for Lactic Acid Production" Fermentation 9, no. 8: 744. https://doi.org/10.3390/fermentation9080744
APA StyleWang, Y., & Gao, M. (2023). Efficient Biorefinery Based on Designed Lignocellulosic Substrate for Lactic Acid Production. Fermentation, 9(8), 744. https://doi.org/10.3390/fermentation9080744






