Characterization of Biofilm Formation and Bacterial Resistance to Benzalkonium Chloride under Contrasting Cultivation Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation Conditions
2.2. Microbiological and Biochemical Testing of Bacterial Activity
2.2.1. Crystal Violet Assay for Biofilm Quantification
2.2.2. Fluorescein Diacetate Hydrolysis Activity (FDA)
2.2.3. Dehydrogenase Activity (DHA)
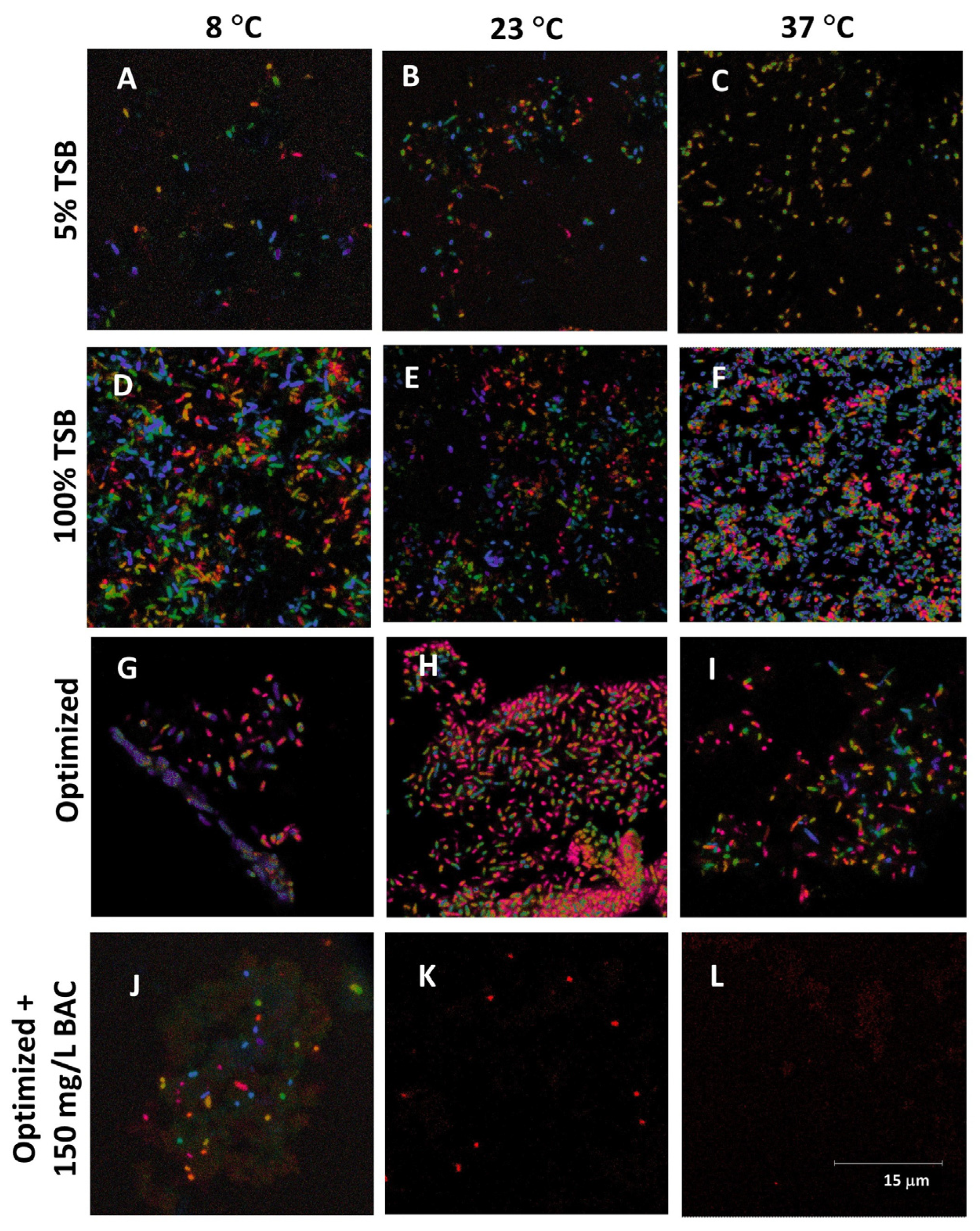
2.3. Microscopy Study
2.4. Statistical Analysis
3. Results
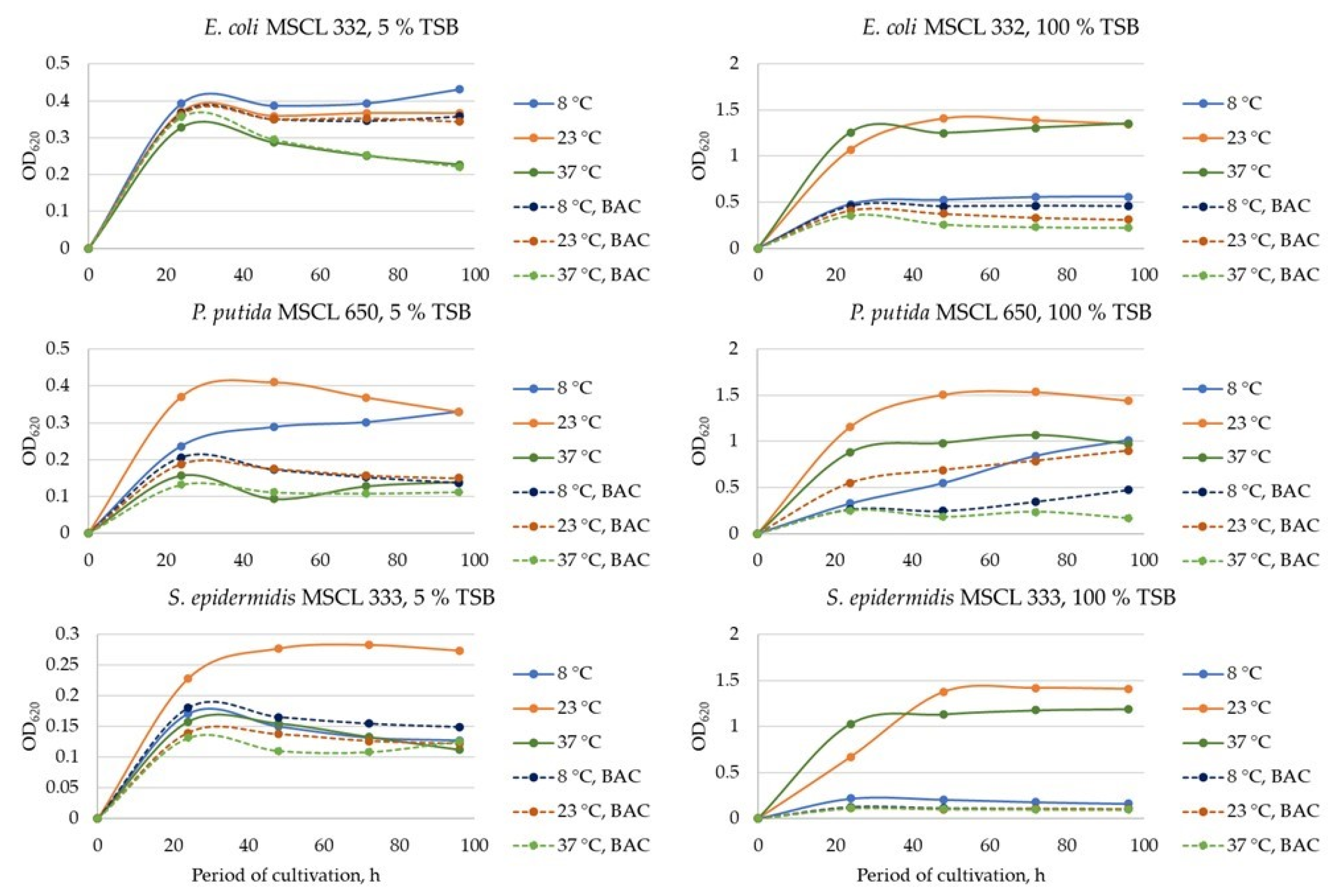
3.1. The Growth of Planktonic Cultures and Biofilm Formation under Various Cultivation Conditions
3.2. Enzyme Activity of Bacterial Cultures Grown in Different Cultivation Conditions
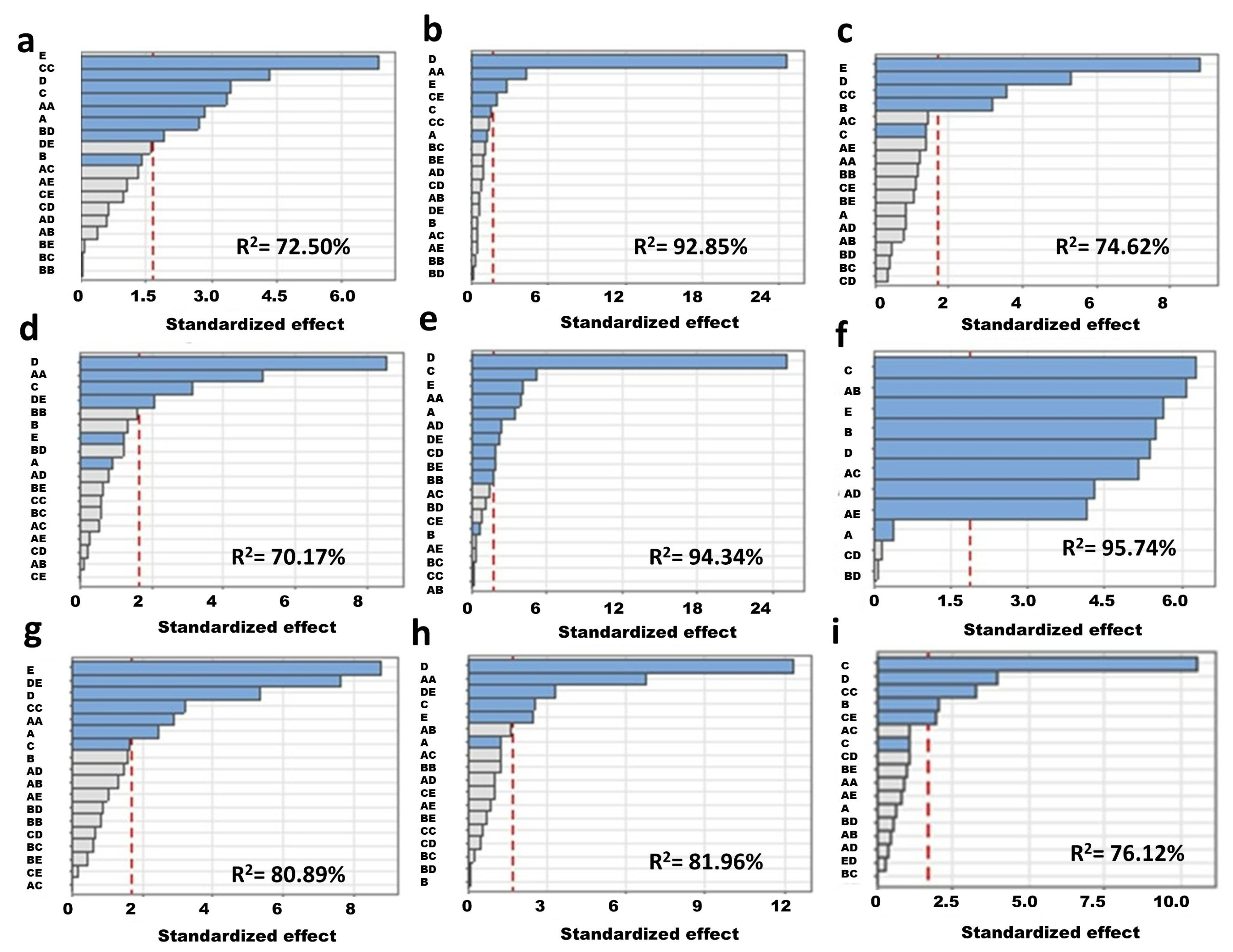
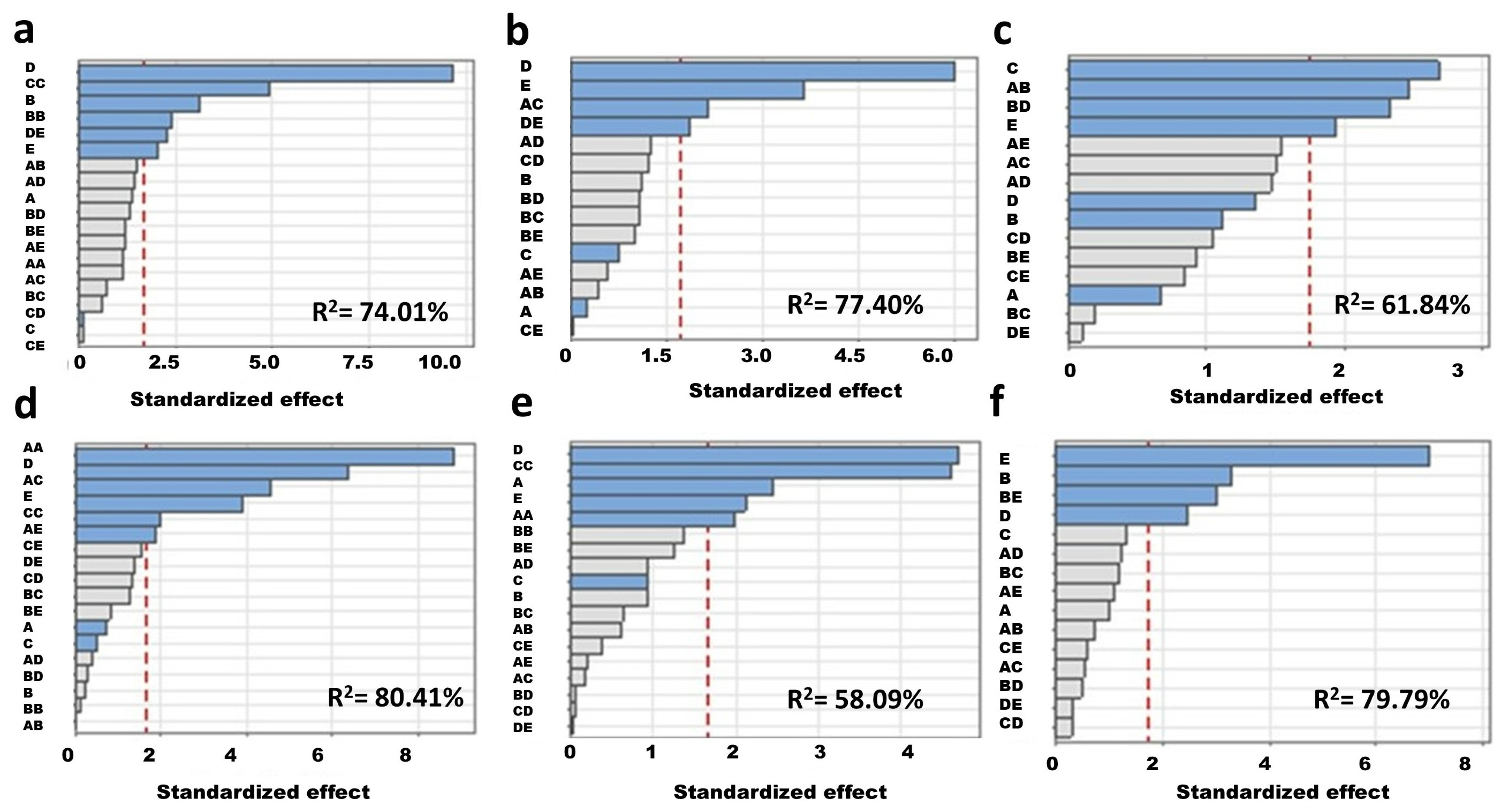
3.3. Statistical Optimization of the Media Variables for P. putida MSCL 650
3.4. Validation of the Optimized Broth Composition
4. Discussion
5. Conclusions
- -
- Planktonic cells of three tested bacterial cultures responded positively to increasing temperature nutrients, while biofilm formation was species-specific and was stimulated by increased temperatures (E. coli, S. epidermidis) and 100% TSB (P. putida).
- -
- Statistical optimization of broth composition for biofilm formation by P. putida showed the stimulatory effect of tryptone and glucose at the highest concentrations tested in this study, i.e., 17 g/L and 2.5 g/L, respectively. The optimized broth composition was temperature specific.
- -
- A stimulation effect of 150 mg/L BAC on the biofilm formation activity of P. putida in a 100% TSB and optimized broth need further investigation.
- -
- The optimized broth composition for biofilm formation by P. putida can be applied to different environmental biotechnological processes where immobilized cells are used. Special attention should be paid to the low-temperature processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Vickery, K. Special Issue: Microbial biofilms in healthcare: Formation, prevention and treatment. Materials 2019, 12, 2001. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gunawan, C.; Barraud, N.; Rice, S.A.; Harry, E.J.; Amal, R. Understanding, monitoring, and controlling biofilm growth in drinking water distribution systems. Environ. Sci. Technol. 2016, 50, 8954–8976. [Google Scholar] [CrossRef]
- Servais, P.; Laurent, P.; Randon, G. Comparison of the bacterial dynamics in various French distribution systems. Aqua London 1995, 44, 10–17. [Google Scholar]
- Giorgi, F.; Curran, J.M.; Patterson, E.A. Real-time monitoring of the dynamics and interactions of bacteria and the early-stage formation of biofilms. Sci. Rep. 2022, 12, 18146. [Google Scholar] [CrossRef]
- Suresh, M.K.; Biswas, R.; Biswas, L. An update on recent developments in the prevention and treatment of Staphylococcus aureus biofilms. Int. J. Med. Microbiol. 2019, 309, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Global Demand for Disinfectants and Antiseptics Predicted to Increase. 2021. Available online: http://www.issa.com/media/news/global-demand-for-disinfectants-and-antiseptics-predicted-to-increase (accessed on 22 June 2023).
- Ioannou, C.J.; Hanlon, G.W.; Denyer, S.P. Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 296–306. [Google Scholar] [CrossRef]
- Basiry, D.; Heravi, N.E.; Uluseker, C.; Kaster, K.M.; Kommedal, R.; Pala-Ozkok, I. The effect of disinfectants and antiseptics on co- and cross-selection of resistance to antibiotics in aquatic environments and wastewater treatment plants. Front. Microbiol. 2022, 13, 1050558. [Google Scholar] [CrossRef] [PubMed]
- Henly, E.L.; Dowling, J.A.R.; Maingay, J.B.; Lacey, M.M.; Smith, T.J.; Forbes, S. Biocide exposure induces changes in susceptibility, pathogenicity, and biofilm formation in uropathogenic Escherichia coli. Antimicrob. Agents Chemother. 2019, 63, e01892-18. [Google Scholar] [CrossRef]
- Møretrø, T.; Schirmer, B.C.T.; Heir, E.; Fagerlund, A.; Hjemli, P.; Langsrud, S. Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int. J. Food Microbiol. 2017, 241, 215–224. [Google Scholar] [CrossRef]
- Khelissa, S.; Gharsallaoui, A.; Fadel, A.; Barras, A.; Jama, C.; Jbilou, F.; Chihib, N.-E. Microencapsulation of benzalkonium chloride enhanced its antibacterial and antibiofilm activities against Listeria monocytogenes and Escherichia coli. J. Appl. Microbiol. 2021, 131, 1136–1146. [Google Scholar] [CrossRef]
- Yu, T.; Ma, M.; Sun, Y.; Xu, X.; Qiu, S.; Yin, J.; Chen, L. The effect of sublethal concentrations of benzalkonium chloride on the LuxS/AI-2 quorum sensing system, biofilm formation and motility of Escherichia coli. Int. J. Food Microbiol. 2021, 353, 109313. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, X.; Wang, Z.; Liu, M.; Wang, L.; Wu, Z. Impacts of quaternary ammonium compounds on membrane bioreactor performance: Acute and chronic responses of microorganisms. Water Res. 2018, 134, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Gupta, J.; Sharma, S.; Sharma, D.; Sharma, S. Focused review on dual inhibition of quorum sensing and efflux pumps: A potential way to combat multi drug resistant Staphylococcus aureus infections. Int. J. Biol. Macromol. 2021, 190, 33–43. [Google Scholar] [CrossRef]
- Turchi, B.; Bertellomi, F.; Marzoli, F.; Cerri, D.; Tola, S.; Azara, E.; Longheu, C.M.; Tassi, R.; Schiavo, M.; Ciliza, G.; et al. Coagulase negative staphylococci from ovine milk: Genotypic and phenotypic characterization of susceptibility to antibiotics, disinfectants and biofilm production. Small Rumin. Res. 2020, 183, 106030. [Google Scholar] [CrossRef]
- Merchel Piovesan Pereira, B.; Wang, X.; Tagkopoulos, I. Biocide-induced emergence of antibiotic resistance in Escherichia coli. Front. Microbiol. 2021, 12, 640923. [Google Scholar] [CrossRef]
- Doucet, A.N.; Slipski, C.J.; Golding, G.R.; Mulvey, M.R.; Bay, D.C. Generation of greater bacterial biofilm biomass using PCR-Plate deep well microplate devices. J. Vis. Exp. 2022, 182, e63069. [Google Scholar] [CrossRef]
- Nunez, C.; Kostoulias, X.; Peleg, A.; Short, F.; Qu, Y. A comprehensive comparison of biofilm formation and capsule production for bacterial survival on hospital surfaces. Biofilm 2023, 5, 100105. [Google Scholar] [CrossRef]
- Sapozhnikov, S.V.; Sabirova, A.E.; Shtyrlin, N.V.; Druk, A.Y.; Agafonova, M.N.; Chirkova, M.N.; Kazakova, R.R.; Grishaev, D.Y.; Nikishova, T.V.; Krylova, E.S.; et al. Design, synthesis, antibacterial activity and toxicity of novel quaternary ammonium compounds based on pyridoxine and fatty acids. Eur. J. Med. Chem. 2021, 211, 113100. [Google Scholar] [CrossRef]
- Barros, A.C.; Melo, L.F.; Pereira, A. Pseudomonas fluorescens cells’ recovery after exposure to BAC and DBNPA biocides. Antibiotics 2022, 11, 1042. [Google Scholar] [CrossRef]
- Rowson, C.; Townsend, R. Biofilms: Prevention and treatment. Br. J. Hosp. Med. 2016, 77, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Loya, M.; Tekin, E.; Kang, T.M.; Cardona, N.; Lozano-Huntelman, N.; Rodriguez-Verdugo, A.; Savage Van, M.; Yen, P.J. Antibiotics shift the temperature response curve of Escherichia coli growth. mSystems 2021, 6, 2–21. [Google Scholar] [CrossRef]
- Amir, M.; Bano, N.; Baker, A.; Zia, Q.; Banawas, S.; Zaheer, M.R.; Shariq, M.; Nawaz, M.S.; Khan, M.F.; Azad, Z.R.A.A.; et al. Isolation and optimization of extracellular PHB depolymerase producer Aeromonas caviae Kuk1-(34) for sustainable solid waste management of biodegradable polymers. PLoS ONE 2022, 17, e0264207. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.B.; Irie, Y.; Borlee, B.R.; Murakami, K.; Harrison, J.J.; Colvin, K.M.; Parsek, M.R. Different methods for culturing biofilms in vitro. In Biofilm Infections; Springer: New York, NY, USA, 2011; pp. 251–266. [Google Scholar] [CrossRef]
- Chen, W.; Hotink, H.; Schmithenner, A.F.; Tuovinen, O.H. The role of microbial activity in suppression of damping-off caused by Pythium ultimum. Phytopathology 1988, 78, 314–322. [Google Scholar] [CrossRef]
- Camiña, F.; Trasar-Cepeda, C.; Gil-Sotres, F.; Leirós, C. Measurement of dehydrogenase activity in acid soils rich in organic matter. Soil Biol. Biochem. 1998, 30, 1005–1011. [Google Scholar] [CrossRef]
- Gonthier, A.; Guérin-Faublée, V.; Tilly, B.; Delignette-Muller, M.L. Optimal growth temperature of O157 and non-O157 Escherichia coli strains. Lett. Appl. Microbiol. 2001, 33, 352–356. [Google Scholar] [CrossRef]
- Kikuchi, T.; Mizunoe, Y.; Takade, A.; Naito, S.; Yoshida, S.I. Curli fibers are required for development of biofilm architecture in Escherichia coli K-12 and enhance bacterial adherence to human uroepithelial cells. Microbiol. Immunol. 2005, 49, 875–884. [Google Scholar] [CrossRef]
- Kanegusuku, A.G.; Stankovic, I.N.; Cote-Hammarlof, P.A.; Yong, P.H.; White-Ziegler, C.A. A shift to human body temperature (37 °C) rapidly reprograms multiple adaptive responses in Escherichia coli that would facilitate niche survival and colonization. J. Bacteriol. 2021, 203, e0036321. [Google Scholar] [CrossRef]
- El-Sesy, M.E.; Ibrahim, S.S. Application of central composite design approach for optimization nitrate removal from aqueous solution by immobilized Pseudomonas putida. Water Sci. Technol. 2021, 83, 2931–2946. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Żur-Pińska, J.; Piński, A.; Pacek, G.; Mrozik, A. Adaptation of phenol-degrading Pseudomonas putida KB3 to suboptimal growth condition: A focus on degradative rate, membrane properties and expression of xylE and cfaB genes. Ecotoxicol. Environ. Saf. 2021, 221, 112431. [Google Scholar] [CrossRef]
- Patil, M.D.; Shinde, K.D.; Patel, G.; Chisti, Y.; Banerjee, U.C. Use of response surface method for maximizing the production of arginine deiminase by Pseudomonas putida. Biotechnol. Rep. 2016, 10, 29–37. [Google Scholar] [CrossRef]
- Holovan, V.; Andriichuk, O.; Budzanivska, I.; Zelena, P.; Kondratiuk, T.; Shevchenko, O. Bacteriophages and their microbial hosts in terrestrial biotopes of Antarctica. Antarct. Sci. 2022, 34, 120–136. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Koroleva, V.A.; Olsannikova, S.S.; Trizna, E.Y.; Bogachev, M.I.; Artyukhov, V.G.; Holyavka, M.G.; Kayumov, A.R. Biochemical properties and anti-biofilm activity of chitosan-immobilized papain. Mar. Drugs 2021, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Vibornijs, V.; Liepins, J.; Selga, T.; Bankovskis, V.; Cosemans, P.; Muter, O. Comparison of the antibacterial effect of a copper-coated surface on Staphylococcus epidermidis and Pseudomonas putida in different physiological states. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1186, 012004. [Google Scholar] [CrossRef]
- Onyango, L.A.; Dunstan, R.H.; Gottfries, J.; von Eiff, C.; Roberts, T.K. Effect of low temperature on growth and ultra-structure of Staphylococcus spp. PLoS ONE 2012, 7, e29031. [Google Scholar] [CrossRef]
- Dynes, J.J.; Lawrence, J.R.; Korber, D.R.; Swerhone, G.D.W.; Leppard, G.G.; Hitchcock, A.P. Morphological and biochemical changes in Pseudomonas fluorescens biofilms induced by sub-inhibitory exposure to antimicrobial agents. Can. J. Microbiol. 2009, 55, 163–178. [Google Scholar] [CrossRef]
- Forbes, S.; Morgan, N.; Humohreys, G.J.; Amezquita, A.; Mistry, H.; McBain, A.J. Loss of function in Escherichia coli exposed to environmentally relevant concentrations of benzalkonium chloride. Appl. Environ. Microbiol. 2019, 85, e02417-18. [Google Scholar] [CrossRef]
- Yu, M.; Jiang, C.; Meng, Y.; Wang, F.; Qian, J.; Fei, F.; Yin, Z.; Zhao, W.; Zhao, Y.; Liu, H. Effect of low temperature on the resistance of Listeria monocytogenes and Escherichia coli O157:H7 to acid electrolyzed water. Food Res. Int. 2023, 168, 112776. [Google Scholar] [CrossRef] [PubMed]
- Frolov, N.; Detusheva, E.; Fursova, N.; Ostashevskaya, I.; Vereshchagin, A. Microbiological Evaluation of Novel Bis-Quaternary Ammonium Compounds: Clinical Strains, Biofilms, and Resistance Study. Pharmaceuticals 2022, 15, 514. [Google Scholar] [CrossRef]
- Kocot, A.M.; Wróblewska, B.; Cabo, M.L. Operational culture conditions determinate benzalkonium chloride resistance in L. monocytogenes-E. coli dual species biofilms. Int. J. Food Microbiol. 2021, 360, 109441. [Google Scholar] [CrossRef]
- Fu, Y.; Peng, H.; Liu, J.; Nguyen, T.H.; Hashmi, M.Z.; Shen, C. Occurrence and quantification of culturable and viable but non-culturable (VBNC) pathogens in biofilm on different pipes from a metropolitan drinking water distribution system. Sci. Total Environ. 2021, 764, 142851. [Google Scholar] [CrossRef]
- Wanandy, S.; Brouwer, N.; Liu, Q.; Mahon, A.; Cork, S.; Karuso, P.; Vemulpad, S.; Jamie, J. Optimisation of the fluorescein diacetate antibacterial assay. J. Microbiol. Methods 2005, 60, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Jayapal, V.; Sharmila, K.M.; Selvibai, G.; Thyagarajan, S.P.; Shanmugasundaram Subramanian, N.S. Fluorescein diacetate and ethidium bromide staining to determine the viability of Mycobacterium smegmatis and Escherichia coli. Lepr. Rev. 1991, 62, 310–314. [Google Scholar] [PubMed]
- Chrzanowski, T.H.; Crotty, R.D.; Hubbard, J.G.; Welch, R.P. Applicability of the fluorescein diacetate method of detecting active bacteria in freshwater. Microb. Ecol. 1984, 10, 179–185. [Google Scholar] [CrossRef]
- Koreňová, J.; Lopašovská, J.; Kuchta, T. Comparison of three microtitre plate-based methods for quantification of biofilm formation ability of bacteria contaminating food technologies. J. Food Nutr. Res. 2008, 47, 100–104. [Google Scholar]
- Dmitrieva, M.V.; Zolotukhina, E.V.; Gerasimova, E.V.; Terent’ev, A.A.; Dobrovol’skii, Y.A. Dehydrogenase and electrochemical activity of Escherichia coli extracts. Appl. Biochem. Microbiol. 2017, 53, 458–463. [Google Scholar] [CrossRef]
- Uribe-Alvarez, C.; Chiquete-Felix, N.; Contreras-Zentella, M.; Guerro-Castilo, S.; Pena, A.; Uribe-Carvajal, S. Staphylococcus epidermidis: Metabolic adaptation and biofilm formation in response to different oxygen concentrations. Pathog. Dis. 2018, 74, ftv111. [Google Scholar] [CrossRef]
- Michalska, J.; Piński, A.; Zur, J.; Mrozik, A. Analysis of the bioaugmentation potential of Pseudomonas putida OR45a and Pseudomonas putida KB3 in the sequencing batch reactors fed with the phenolic landfill leachate. Water 2020, 12, 906. [Google Scholar] [CrossRef]
- Alisi, C.S.; Nwanyanwu, C.E.; Akujobi, C.O.; Ibegbulem, C.O. Inhibition of dehydrogenase activity in pathogenic bacteria isolates by aqueous extracts of Musa paradisiaca (var sapientum). Afr. J. Biotechnol. 2008, 7, 1821–1825. [Google Scholar] [CrossRef]
- Gül, Ş.; Öztürk, D. Determination of structure-toxicity relationship of amphiprotic compounds by means of the inhibition of the dehydrogenase activity of Pseudomonas putida. Turk. J. Chem. 1998, 22, 341–349. [Google Scholar]
- An, H.; Ren, J.; Ma, J.; Li, Z.; Liu, Y.; Liu, X. Effect of benzalkonium chloride on microbial activity of activated sludge. Chin. J. Environ. Eng. 2020, 14, 2701–2709. [Google Scholar] [CrossRef]
- Huang, Z.; Qi, Z.; Ding, X.; Liu, C. N-chlorosuccinimide enhancing the antimicrobial effect of benzalkonium chloride on biofilm Pseudomonas aeruginosa and its interaction mechanisms. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2022, 57, 593–600. [Google Scholar] [CrossRef]
- Hepburn, N.F.; MacRae, M.; Johnston, M.; Mooney, J.; Ogden, I.D. Optimizing enrichment conditions for the isolation of Escherichia coli O157 in soils by immunomagnetic separation. Lett. Appl. Microbiol. 2022, 34, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Al, M.S.; Saeed, A.M.; Hammad, A.A.; Swailam, H.M.; Abouzeid, M.A. Gamma-irradiation induced effects on histamine-forming bacteria isolated from the chilled mackerel fish. Egypt. J. Aquat. Biol. Fish. 2022, 26, 869–883. [Google Scholar] [CrossRef]
- Ma, D.; Jiang, Y.; Ahmed, S.; Qin, W.; Liu, Y. Physical and antimicrobial properties of edible films containing Lactococcus lactis. Int. J. Biol. Macromol. 2019, 141, 378–386. [Google Scholar] [CrossRef] [PubMed]




| Temperature | Ca2+, mM | Mg2+, mM | Yeast Extract, g/L | Tryptone, g/L | Glucose, g/L |
|---|---|---|---|---|---|
| 8 °C | 1.00 | 1.95 | 2.50 | 17.00 | 2.50 |
| 23 °C | 1.00 | 10.00 | 0.00 | 17.00 | 2.50 |
| 37 °C | 1.00 | 0.00 | 2.50 | 3.40 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žorža, L.; Dēliņa, A.; Selga, T.; Muter, O. Characterization of Biofilm Formation and Bacterial Resistance to Benzalkonium Chloride under Contrasting Cultivation Conditions. Fermentation 2023, 9, 699. https://doi.org/10.3390/fermentation9080699
Žorža L, Dēliņa A, Selga T, Muter O. Characterization of Biofilm Formation and Bacterial Resistance to Benzalkonium Chloride under Contrasting Cultivation Conditions. Fermentation. 2023; 9(8):699. https://doi.org/10.3390/fermentation9080699
Chicago/Turabian StyleŽorža, Laura, Aija Dēliņa, Tūrs Selga, and Olga Muter. 2023. "Characterization of Biofilm Formation and Bacterial Resistance to Benzalkonium Chloride under Contrasting Cultivation Conditions" Fermentation 9, no. 8: 699. https://doi.org/10.3390/fermentation9080699
APA StyleŽorža, L., Dēliņa, A., Selga, T., & Muter, O. (2023). Characterization of Biofilm Formation and Bacterial Resistance to Benzalkonium Chloride under Contrasting Cultivation Conditions. Fermentation, 9(8), 699. https://doi.org/10.3390/fermentation9080699







