Enhanced CO2 Reduction by Electron Shuttle Molecules via Coupling Different Electron Transport Processes in Microbial Electrosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Startup and Operation Phase
2.2. Electrochemical Analyses
2.3. Analyses Methods
2.4. Calculations
2.5. High-Throughput Sequencing of 16S rRNA Gene
2.6. Tax4Fun Application for Predicting Functional Classification
2.7. Real-Time Quantitative PCR
3. Results and Discussion
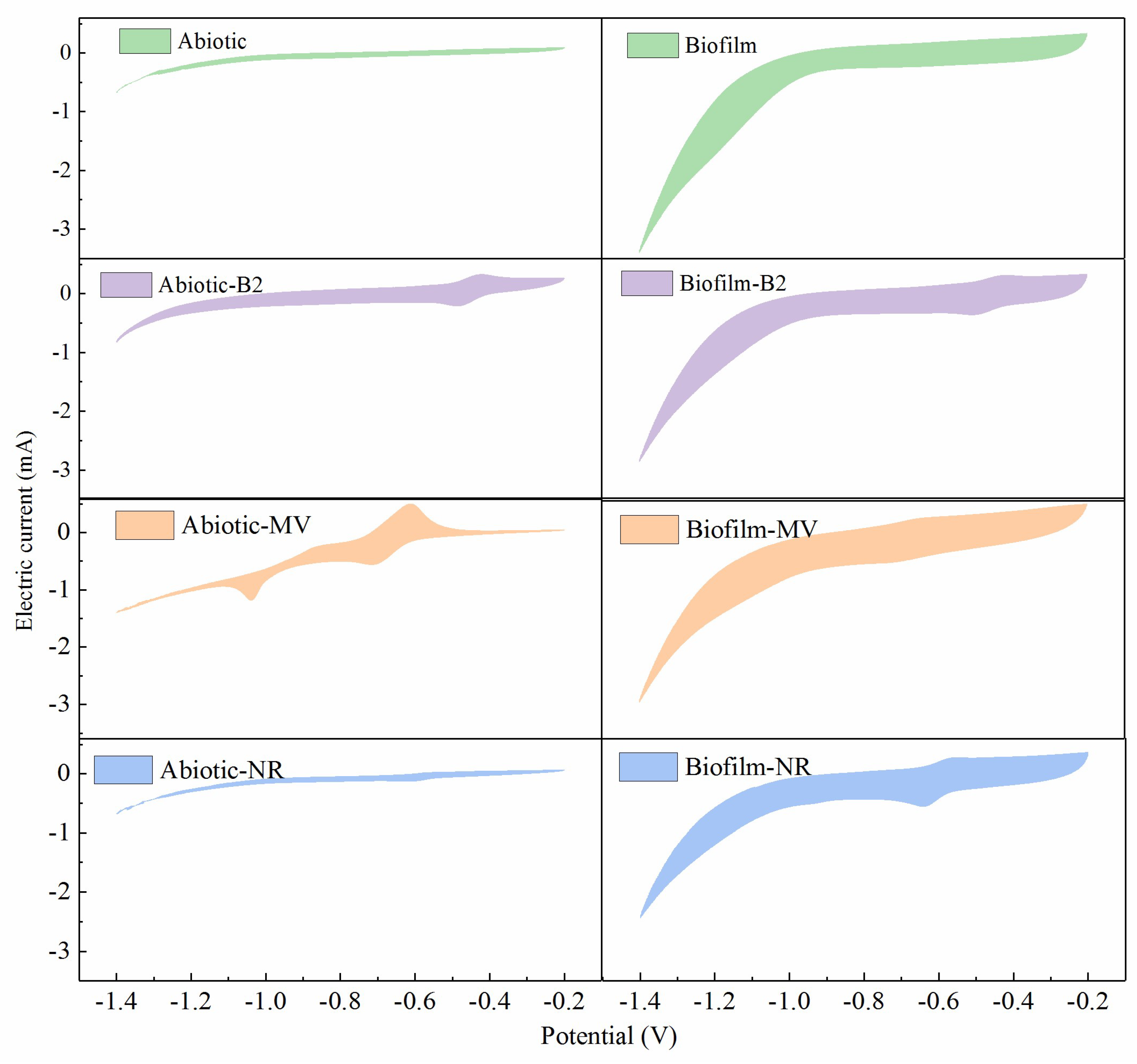
3.1. Electron Transfer Capacity and Redox Potential of the ESMs
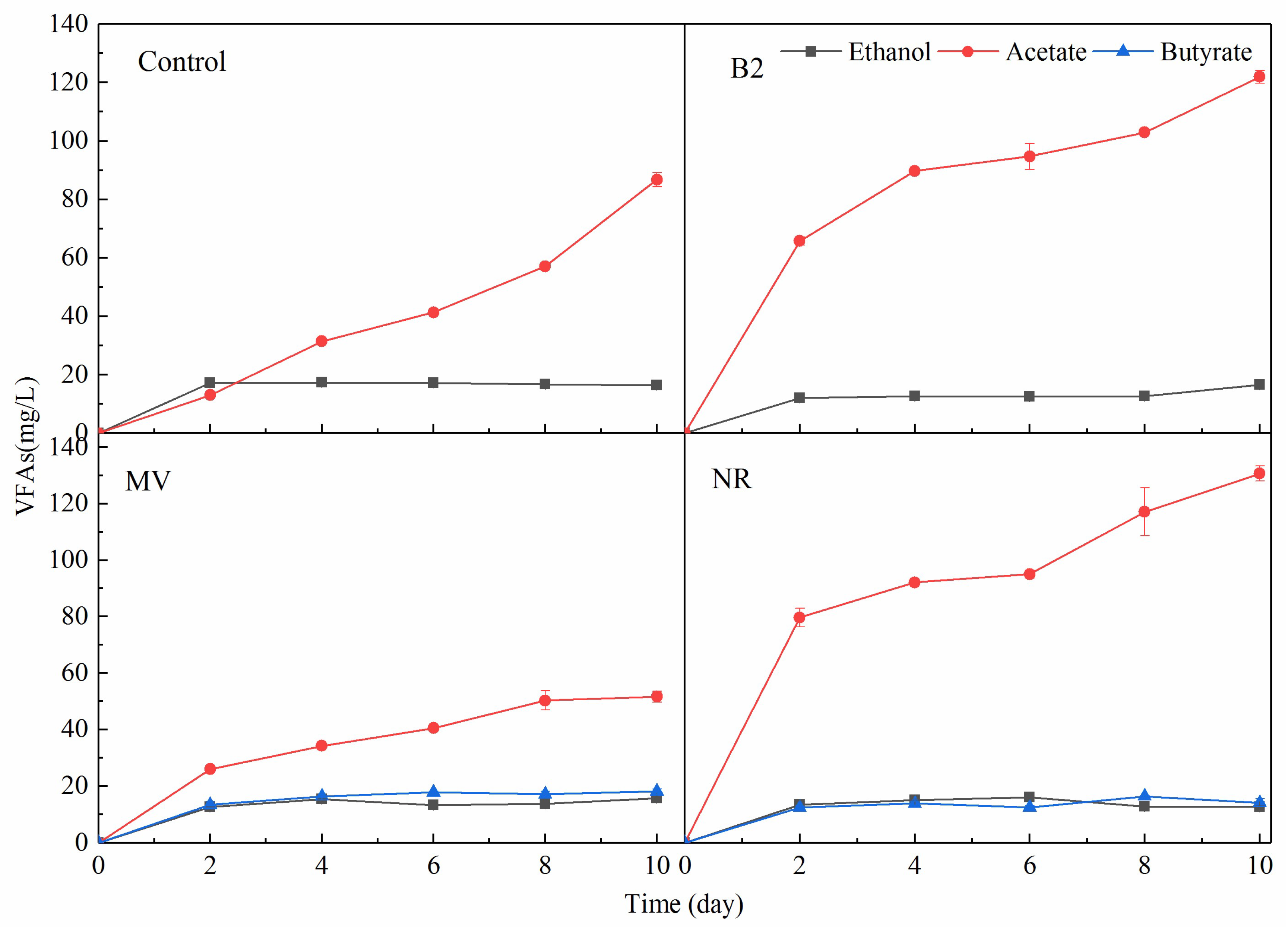
3.2. Chemicals Production with the Addition of ESMs in MES Reactors
3.3. Electron Conversion Efficiency of Different ESMs
3.4. Morphology of Biocathode Biofilm and Biomass Measurement
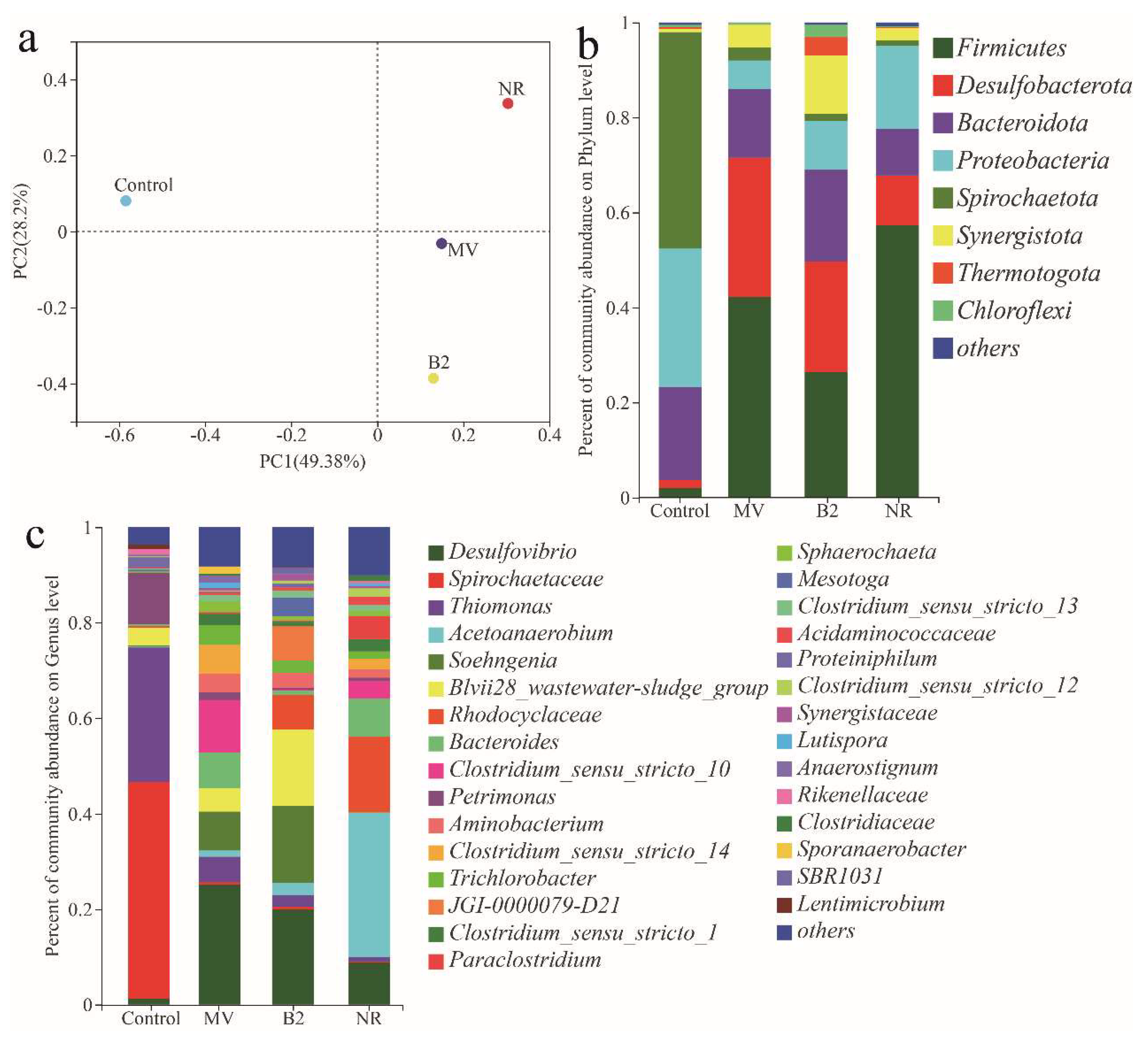
3.5. The 16S rRNA Gene Analysis of Microbial Community of Biocathode
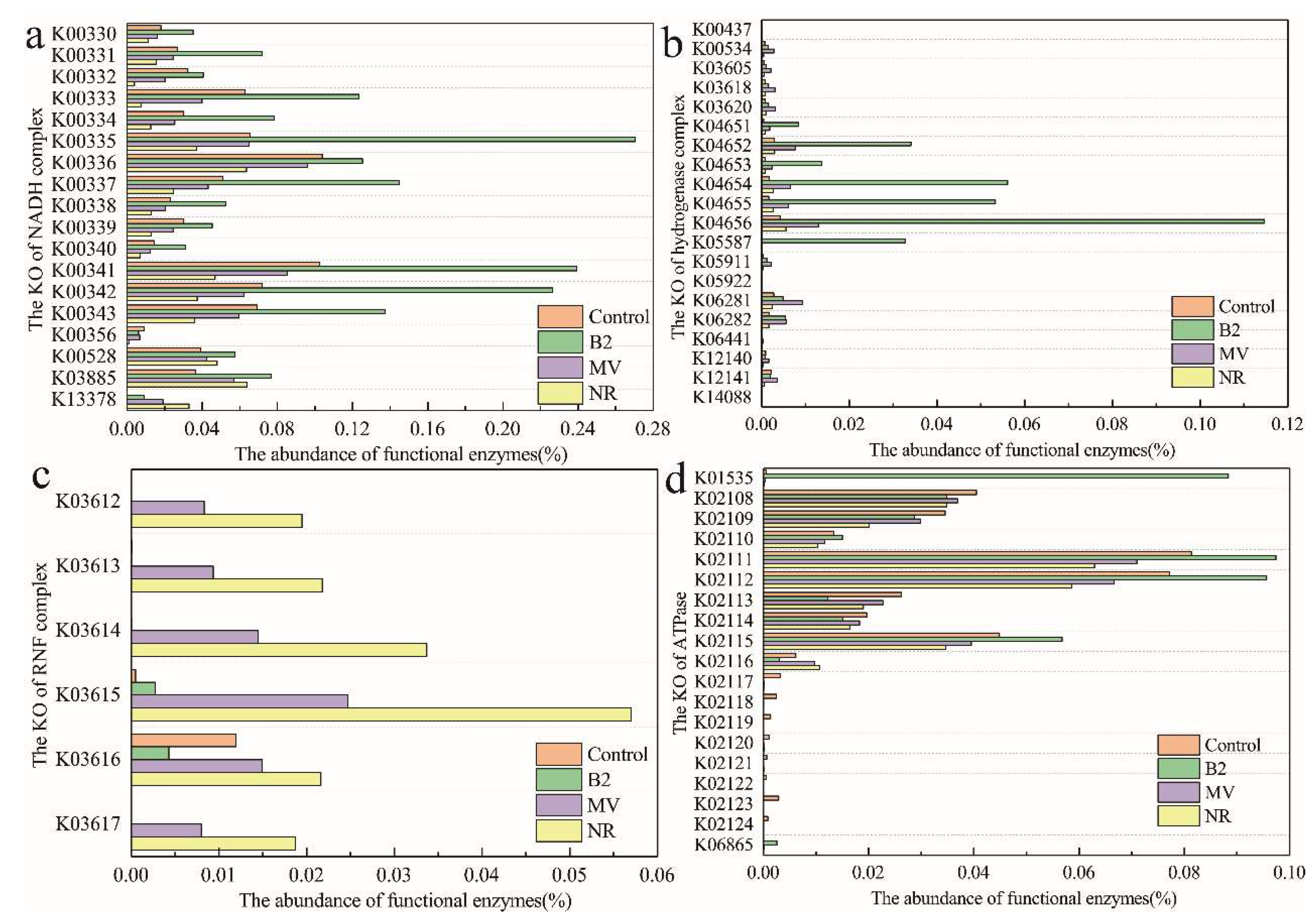
3.6. Predicted Functions of the Microbial Community on Biocathodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ElMekawy, A.; Hegab, H.M.; Mohanakrishna, G.; Elbaz, A.F.; Bulut, M.; Pant, D. Technological advances in CO2 conversion electro-biorefinery: A step toward commercialization. Bioresour. Technol. 2016, 215, 357–370. [Google Scholar] [CrossRef]
- Ammam, F.; Tremblay, P.-L.; Lizak, D.M.; Zhang, T. Effect of tungstate on acetate and ethanol production by the electrosynthetic bacterium Sporomusa ovata. Biotechnol. Biofuels 2016, 9, 163. [Google Scholar] [CrossRef]
- Batlle-Vilanova, P.; Ganigué, R.; Ramió-Pujol, S.; Bañeras, L.; Jiménez, G.; Hidalgo, M.; Balaguer, M.D.; Colprim, J.; Puig, S. Microbial electrosynthesis of butyrate from carbon dioxide: Production and extraction. Bioelectrochemistry 2017, 117, 57–64. [Google Scholar] [CrossRef]
- Martens, J.A.; Bogaerts, A.; De Kimpe, N.; Jacobs, P.A.; Marin, G.B.; Rabaey, K.; Saeys, M.; Verhelst, S. The Chemical Route to a Carbon Dioxide Neutral World. Chemsuschem 2016, 10, 1039–1055. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Vanbroekhoven, K.; Pant, D. Impact of dissolved carbon dioxide concentration on the process parameters during its conversion to acetate through microbial electrosynthesis. React. Chem. Eng. 2018, 3, 371–378. [Google Scholar] [CrossRef]
- Fontmorin, J.-M.; Izadi, P.; Li, D.; Lim, S.S.; Farooq, S.; Bilal, S.S.; Cheng, S.; Yu, E.H. Gas diffusion electrodes modified with binary doped polyaniline for enhanced CO2 conversion during microbial electrosynthesis. Electrochim. Acta 2021, 372, 137853. [Google Scholar] [CrossRef]
- Tahir, K.; Miran, W.; Jang, J.; Woo, S.H.; Lee, D.S. Enhanced product selectivity in the microbial electrosynthesis of butyrate using a nickel ferrite-coated biocathode. Environ. Res. 2021, 196, 110907. [Google Scholar] [CrossRef]
- Saini, R.; Kapoor, R.; Kumar, R.; Siddiqi, T.; Kumar, A. CO2 utilizing microbes—A comprehensive review. Biotechnol. Adv. 2011, 29, 949–960. [Google Scholar] [CrossRef]
- Luan, L.; Ji, X.; Guo, B.; Cai, J.; Dong, W.; Huang, Y.; Zhang, S. Bioelectrocatalysis for CO2 reduction: Recent advances and challenges to develop a sustainable system for CO2 utilization. Biotechnol. Adv. 2023, 63, 108098. [Google Scholar] [CrossRef]
- Steinbusch, K.J.J.; Hamelers, H.V.M.; Schaap, J.D.; Kampman, C.; Buisman, C.J.N. Bioelectrochemical Ethanol Production through Mediated Acetate Reduction by Mixed Cultures. Environ. Sci. Technol. 2009, 44, 513–517. [Google Scholar] [CrossRef]
- Choi, O.; Um, Y.; Sang, B.-I. Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor. Biotechnol. Bioeng. 2012, 109, 2494–2502. [Google Scholar] [CrossRef]
- Huang, B.; Gao, S.; Xu, Z.; He, H.; Pan, X. The Functional Mechanisms and Application of Electron Shuttles in Extracellular Electron Transfer. Curr. Microbiol. 2017, 75, 99–106. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, X.; Qin, B.; Li, F.; Häggblom, M.M.; Liu, T. Enhanced Current Production by Exogenous Electron Mediators via Synergy of Promoting Biofilm Formation and the Electron Shuttling Process. Environ. Sci. Technol. 2020, 54, 7217–7225. [Google Scholar] [CrossRef]
- Aryal, N.; Ammam, F.; Patil, S.A.; Pant, D. An overview of cathode materials for microbial electrosynthesis of chemicals from carbon dioxide. Green Chem. 2017, 19, 5748–5760. [Google Scholar] [CrossRef]
- Chatterjee, P.; Dessì, P.; Kokko, M.; Lakaniemi, A.-M.; Lens, P. Selective enrichment of biocatalysts for bioelectrochemical systems: A critical review. Renew. Sustain. Energy Rev. 2019, 109, 10–23. [Google Scholar] [CrossRef]
- Song, Y.E.; Mohamed, A.; Kim, C.; Kim, M.; Li, S.; Sundstrom, E.; Beyenal, H.; Kim, J.R. Biofilm matrix and artificial mediator for efficient electron transport in CO2 microbial electrosynthesis. Chem. Eng. J. 2021, 427, 131885. [Google Scholar] [CrossRef]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef]
- Kaden, J.; Galushko, A.S.; Schink, B. Cysteine-mediated electron transfer in syntrophic acetate oxidation by cocultures of Geobacter sulfurreducens and Wolinella succinogenes. Arch. Microbiol. 2002, 178, 53–58. [Google Scholar] [CrossRef]
- Wolf, M.; Kappler, A.; Jiang, J.; Meckenstock, R.U. Effects of Humic Substances and Quinones at Low Concentrations on Ferrihydrite Reduction by Geobacter metallireducens. Environ. Sci. Technol. 2009, 43, 5679–5685. [Google Scholar] [CrossRef]
- Huang, L.; Tang, J.; Chen, M.; Liu, X.; Zhou, S. Two Modes of Riboflavin-Mediated Extracellular Electron Transfer in Geobacter uraniireducens. Front. Microbiol. 2018, 9, 2886. [Google Scholar] [CrossRef]
- Wu, Y.D.; Liu, T.X.; Li, X.M.; Li, F.B. Exogenous Electron Shuttle-Mediated Extracellular Electron Transfer of Shewanella putrefaciens 200: Electrochemical Parameters and Thermodynamics. Environ. Sci. Technol. 2014, 48, 9306–9314. [Google Scholar] [CrossRef]
- Liang, T.; Zhou, L.; Irfan, M.; Bai, Y.; Liu, X.; Zhang, J.; Wu, Z.; Wang, W.; Liu, J.; Cheng, L.; et al. Assessment of Five Electron-Shuttling Molecules in the Extracellular Electron Transfer of Electromethanogenesis by using Methanosarcina barkeri. Chemelectrochem 2020, 7, 3783–3789. [Google Scholar] [CrossRef]
- Gemünde, A.; Lai, B.; Pause, L.; Krömer, J.; Holtmann, D. Redox Mediators in Microbial Electrochemical Systems. Chemelectrochem 2022, 9, e202200216. [Google Scholar] [CrossRef]
- Kracke, F.; Virdis, B.; Bernhardt, P.V.; Rabaey, K.; Krömer, J.O. Redox dependent metabolic shift in Clostridium autoethanogenum by extracellular electron supply. Biotechnol. Biofuels 2016, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, H.; Zhang, Y.; Wu, P.; Li, J.; Ding, P.; Jiang, Q.; Cui, M.-H. Heterotrophic precultivation is a better strategy than polarity reversal for the startup of acetate microbial electrosynthesis reactor. Biochem. Eng. J. 2022, 179, 108319. [Google Scholar] [CrossRef]
- He, S.; Gall, D.L.; McMahon, K.D. “Candidatus Accumulibacter” Population Structure in Enhanced Biological Phosphorus Removal Sludges as Revealed by Polyphosphate Kinase Genes. Appl. Environ. Microbiol. 2007, 73, 5865–5874. [Google Scholar] [CrossRef]
- Xu, K.W.; Liu, H.; Du, G.C.; Chen, J. Real-time PCR assays targeting formyltetrahydrofolate synthetase gene to enumerate acetogens in natural and engineered environments. Anaerobe 2009, 15, 204–213. [Google Scholar] [CrossRef]
- Peguin, S.; Delorme, P.; Goma, G.; Soucaille, P. Enhanced alcohol yields in batch cultures of Clostridium acetobutylicum using a three-electrode potentiometric system with methyl viologen as electron carrier. Biotechnol. Lett. 1994, 16, 269–274. [Google Scholar] [CrossRef]
- Paiano, P.; Menini, M.; Zeppilli, M.; Majone, M.; Villano, M. Electro-fermentation and redox mediators enhance glucose conversion into butyric acid with mixed microbial cultures. Bioelectrochemistry 2019, 130, 107333. [Google Scholar] [CrossRef]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.H.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of Organic Compounds from Carbon Dioxide Is Catalyzed by a Diversity of Acetogenic Microorganisms. Appl. Environ. Microbiol. 2011, 77, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Jourdin, L.; Freguia, S.; Donose, B.C.; Keller, J. Autotrophic hydrogen-producing biofilm growth sustained by a cathode as the sole electron and energy source. Bioelectrochemistry 2015, 102, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Aryal, N.; Tremblay, P.-L.; Lizak, D.M.; Zhang, T. Performance of different Sporomusa species for the microbial electrosynthesis of acetate from carbon dioxide. Bioresour. Technol. 2017, 233, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Aklujkar, M.; Leang, C.; Shrestha, P.M.; Shrestha, M.; Lovley, D.R. Transcriptomic profiles of Clostridium ljungdahlii during lithotrophic growth with syngas or H2 and CO2 compared to organotrophic growth with fructose. Sci. Rep. 2017, 7, 13135. [Google Scholar] [CrossRef] [PubMed]
- Bes, M.; Merrouch, M.; Joseph, M.; Quemeneur, M.; Payri, C.; Pelletier, B.; Ollivier, B.; Fardeau, M.L.; Erausol, G.; Postec, A. Acetoanaerobium pronyense sp nov., an anaerobic alkaliphilic bacterium isolated from a carbonate chimney of the Prony Hydrothermal Field (New Caledonia). Int. J. Syst. Evol. Microbiol. 2015, 65, 2574–2580. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Mattiasson, B. Anaerobes in Industrial- and Environmental Biotechnology. In Anaerobes in Biotechnology; Hatti-Kaul, R., Mamo, G., Mattiasson, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–33. [Google Scholar]
- Rodrigues, T.D.C.; Rosenbaum, M.A. Microbial Electroreduction: Screening for New Cathodic Biocatalysts. Chemelectrochem 2014, 1, 1916–1922. [Google Scholar] [CrossRef]
- Peguin, S.; Soucaille, P. Modulation of metabolism of Clostridium acetobutylicum grown in chemostat culture in a three-electrode potentiostatic system with methyl viologen as electron carrier. Biotechnol. Bioeng. 1996, 51, 342–348. [Google Scholar] [CrossRef]
- Barton, L.L. Microbial Ecology; John Wiley & Sons, Incorporated: Newark, NJ, USA, 2011. [Google Scholar]
- Perona-Vico, E.; Feliu-Paradeda, L.; Puig, S.; Bañeras, L. Bacteria coated cathodes as an in-situ hydrogen evolving platform for microbial electrosynthesis. Sci. Rep. 2020, 10, 19852. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, G.; Zhang, R.; Lu, Y.; Luo, H. Acetate production and electron utilization facilitated by sulfate-reducing bacteria in a microbial electrosynthesis system. Bioresour. Technol. 2017, 241, 821–829. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Genet. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Hirose, A.; Kasai, T.; Aoki, M.; Umemura, T.; Watanabe, K.; Kouzuma, A. Electrochemically active bacteria sense electrode potentials for regulating catabolic pathways. Nat. Commun. 2018, 9, 1083. [Google Scholar] [CrossRef]
- Schut, G.J.; Adams, M.W.W. The Iron-Hydrogenase of Thermotoga maritima Utilizes Ferredoxin and NADH Synergistically: A New Perspective on Anaerobic Hydrogen Production. J. Bacteriol. 2009, 191, 4451–4457. [Google Scholar] [CrossRef] [PubMed]
- Schuchmann, K.; Müller, V. A Bacterial Electron-bifurcating Hydrogenase. J. Biol. Chem. 2012, 287, 31165–31171. [Google Scholar] [CrossRef] [PubMed]
- Biegel, E.; Schmidt, S.; González, J.M.; Müller, V. Biochemistry, evolution and physiological function of the Rnf complex, a novel ion-motive electron transport complex in prokaryotes. Cell. Mol. Life Sci. 2010, 68, 613–634. [Google Scholar] [CrossRef]
- Müller, V. Energy Conservation in Acetogenic Bacteria. Appl. Environ. Microbiol. 2003, 69, 6345–6353. [Google Scholar] [CrossRef]
- Edel, M.; Sturm, G.; Sturm-Richter, K.; Wagner, M.; Ducassou, J.N.; Couté, Y.; Horn, H.; Gescher, J. Extracellular riboflavin induces anaerobic biofilm formation in Shewanella oneidensis. Biotechnol. Biofuels 2021, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W.; Thauer, R.K. Flavin-Based Electron Bifurcation, Ferredoxin, Flavodoxin, and Anaerobic Respiration With Protons (Ech) or NAD+ (Rnf) as Electron Acceptors: A Historical Review. Front. Microbiol. 2018, 9, 401. [Google Scholar] [CrossRef]
- Velazquez, I.; Nakamaru-Ogiso, E.; Yano, T.; Ohnishi, T.; Yagi, T. Amino acid residues associated with cluster N3 in the NuoF subunit of the proton-translocating NADH-quinone oxidoreductase from Escherichia coli. FEBS Lett. 2005, 579, 3164–3168. [Google Scholar] [CrossRef]
- Beckmann, S.; Welte, C.; Li, X.; Oo, Y.M.; Kroeninger, L.; Heo, Y.; Zhang, M.; Ribeiro, D.; Lee, M.; Bhadbhade, M.; et al. Novel phenazine crystals enable direct electron transfer to methanogens in anaerobic digestion by redox potential modulation. Energy Environ. Sci. 2015, 9, 644–655. [Google Scholar] [CrossRef]





| Reactors | Inorganic Carbon Consumption (mg/L) | Carbon Concentration of VFAs (mg/L) | Carbon Conversion Efficiency (%) | ||
|---|---|---|---|---|---|
| Ethanol | Acetate | Butyrate | |||
| control | 81.5 ± 1.27 | 8.6 ± 0.08 | 34.7 ± 0.98 | - | 53.2 |
| B2 | 60.9 ± 0.42 | 8.7 ± 0.04 | 48.8 ± 0.86 | - | 94.3 |
| MV | 70.8 ± 1.70 | 8.2 ± 0.22 | 20.7 ± 0.78 | 9.9 ± 0.47 | 54.7 |
| NR | 112.8 ± 2.55 | 6.6 ± 0.17 | 52.3 ± 1.08 | 7.7 ± 0.76 | 59.1 |
| ESMs | 16S rRNA Gene (Copy) | fhs (Copy) | fhs/16S rRNA Gene |
|---|---|---|---|
| control | 1.80 × 109 | 4.13 × 105 | 2.29 × 10−4 |
| B2 | 7.81 × 109 | 5.82 × 105 | 7.45 × 10−5 |
| MV | 7.56 × 109 | 5.17 × 106 | 6.84 × 10−4 |
| NR | 1.00 × 1010 | 5.86 × 106 | 5.86 × 10−4 |
| Shannon | Simpson | Ace | Chao | Coverage | |
|---|---|---|---|---|---|
| control | 2.00 | 0.25 | 137.78 | 138.09 | 0.99 |
| B2 | 3.09 | 0.08 | 178.43 | 187.00 | 0.99 |
| MV | 3.07 | 0.09 | 179.58 | 179.40 | 0.99 |
| NR | 2.88 | 0.13 | 179.36 | 181.91 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, H.; Zhang, Y.; Fu, B.; Zhang, C.; Cui, M.; Wu, P.; Chen, C. Enhanced CO2 Reduction by Electron Shuttle Molecules via Coupling Different Electron Transport Processes in Microbial Electrosynthesis. Fermentation 2023, 9, 679. https://doi.org/10.3390/fermentation9070679
Zhang J, Liu H, Zhang Y, Fu B, Zhang C, Cui M, Wu P, Chen C. Enhanced CO2 Reduction by Electron Shuttle Molecules via Coupling Different Electron Transport Processes in Microbial Electrosynthesis. Fermentation. 2023; 9(7):679. https://doi.org/10.3390/fermentation9070679
Chicago/Turabian StyleZhang, Jie, He Liu, Yan Zhang, Bo Fu, Chao Zhang, Minhua Cui, Ping Wu, and Chongjun Chen. 2023. "Enhanced CO2 Reduction by Electron Shuttle Molecules via Coupling Different Electron Transport Processes in Microbial Electrosynthesis" Fermentation 9, no. 7: 679. https://doi.org/10.3390/fermentation9070679
APA StyleZhang, J., Liu, H., Zhang, Y., Fu, B., Zhang, C., Cui, M., Wu, P., & Chen, C. (2023). Enhanced CO2 Reduction by Electron Shuttle Molecules via Coupling Different Electron Transport Processes in Microbial Electrosynthesis. Fermentation, 9(7), 679. https://doi.org/10.3390/fermentation9070679






