Effects of Formic Acid and Lactic Acid Bacteria on the Fermentation Products, Bacterial Community Diversity and Predictive Functional Characteristics of Perennial Ryegrass Silage in Karst Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Analysis of Silage Microbial Populations, Chemical Composition and Fermentation Quality
2.3. Microbial Community Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Microbial Populations of Fresh Perennial Ryegrass
3.2. Effect of Additives on Chemical Composition
3.3. Additives’ Impact on Fermentation Properties
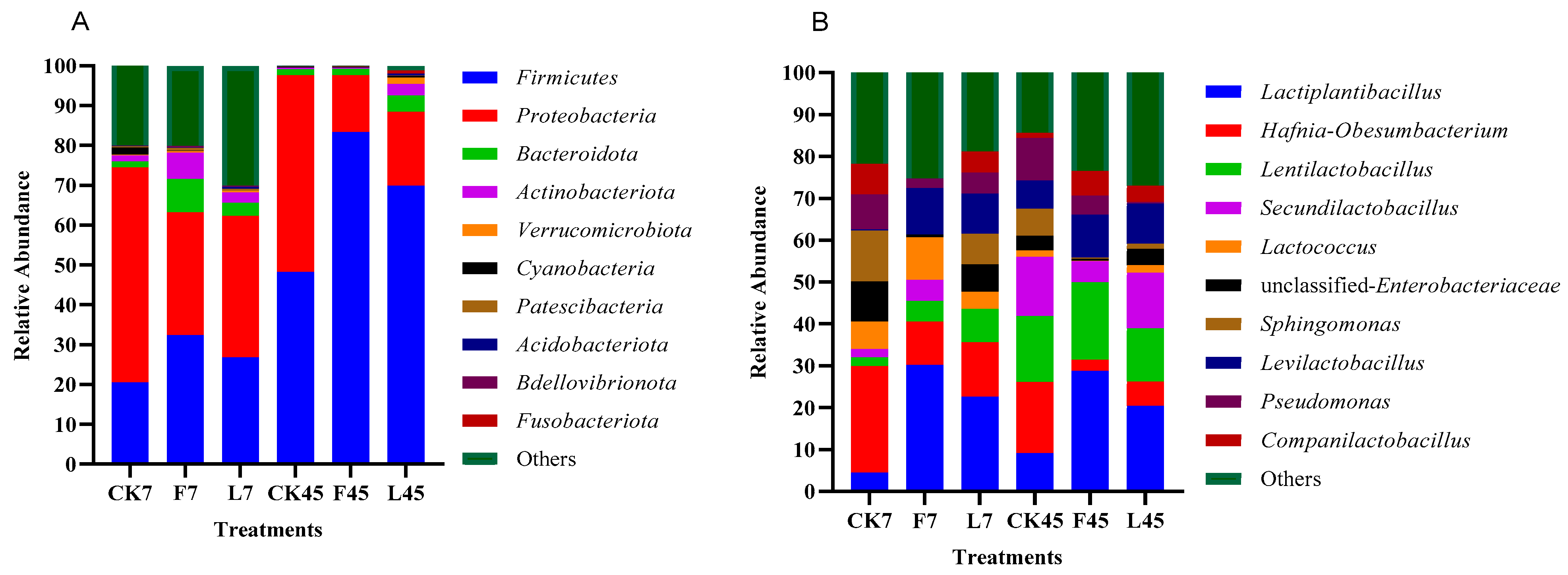
3.4. Effect of Additives on Microbial Communities
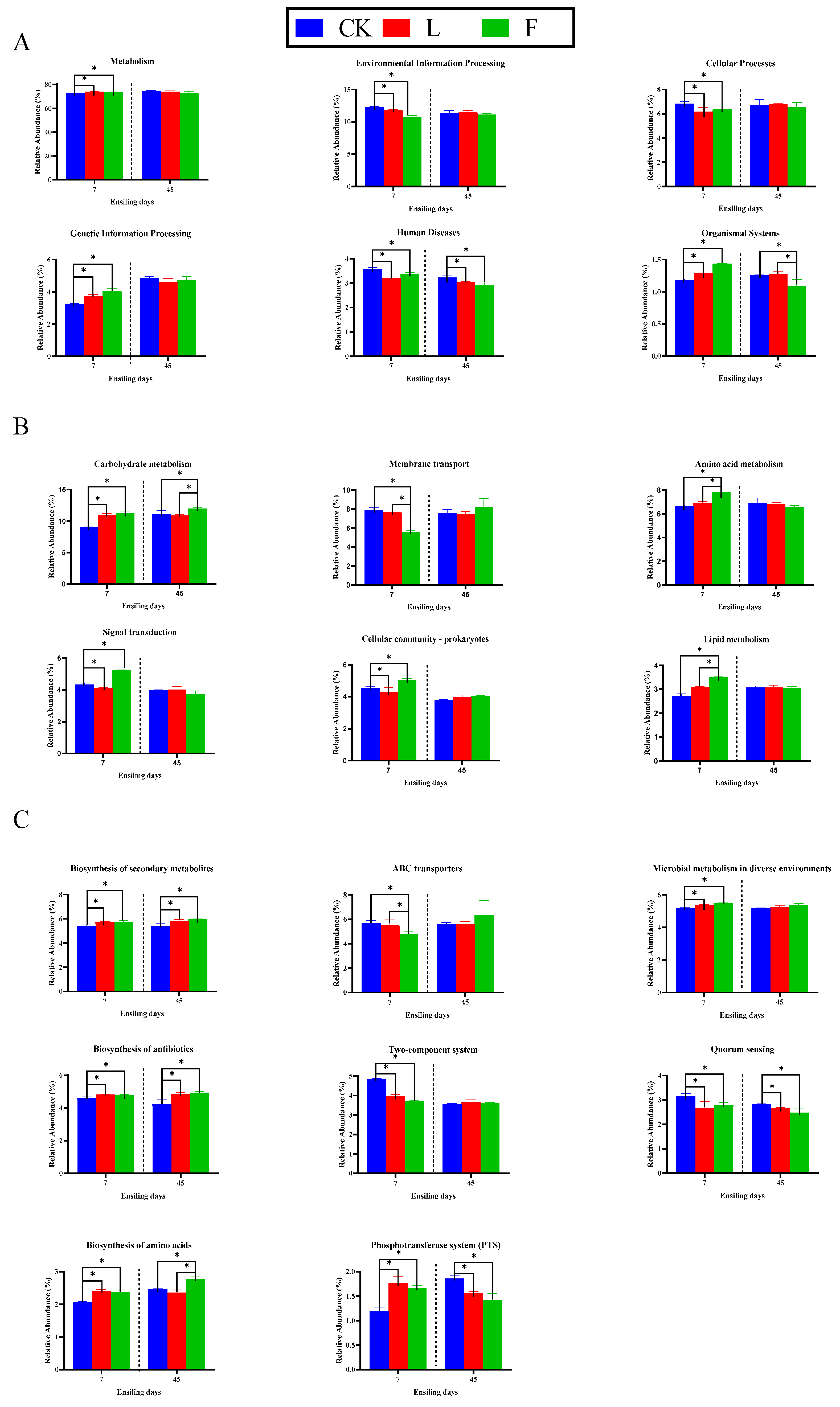
3.5. The Effect of Additives on the Pathways
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.J.; Zhu, J.X.; Xu, L.; Su, H.X.; Gao, Y.; Cai, X.L.; Peng, T.; Wen, X.F.; Zhang, J.J.; He, N.P. Rational land-use types in the karst regions of China: Insights from soil organic matter composition and stability. Catena 2018, 160, 345–353. [Google Scholar] [CrossRef]
- He, K.; Jia, Y.; Wang, F.; Lu, Y. Overview of karst geo-environments and karst water resources in north and south China. Environ. Earth Sci. 2011, 64, 1865–1873. [Google Scholar]
- Song, S.; Xiong, K.; Chi, Y.; Shen, X.; Guo, T.; Lu, N. Research Progress and Prospect of Grassland Establishment and Ecological Animal Husbandry in the Karst Rocky Desertification Area. Fresenius Environ. Bull. 2018, 27, 7017–7030. [Google Scholar]
- Wilkinson, J.M.; Lee, M.R.F.; Rivero, M.J.; Chamberlain, A.T. Some challenges and opportunities for grazing dairy cows on temperate pastures. Grass Forage Sci. 2020, 75, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela Saldinger, S. Bacterial dynamics of wheat silage. Front. Microbiol. 2019, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Sun, L.; Chen, C.; Lin, J.; Yang, F.; Cai, Y. Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed Sci. Technol. 2021, 275, 114766. [Google Scholar] [CrossRef]
- Yu, X.; Pijut, P.M.; Byrne, S.; Asp, T.; Bai, G.; Jiang, Y. Candidate gene association mapping for winter survival and spring regrowth in perennial ryegrass. Plant Sci. 2013, 235, 37–45. [Google Scholar] [CrossRef]
- Fu, Z.; Song, J.; Zhao, J.; Jameson, P.E. Identification and expression of genes associated with the abscission layer controlling seed shattering in Lolium perenne. AoB Plants 2019, 11, ply076. [Google Scholar] [CrossRef]
- Xie, L.; Teng, K.; Tan, P.; Chao, Y.; Li, Y.; Guo, W.; Han, L. PacBio single-molecule long-read sequencing shed new light on the transcripts and splice isoforms of the perennial ryegrass. Mol. Genet. Genomics. 2020, 295, 475–489. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.; Dong, Z.; Li, J.; Guo, G.; Bai, Y.; Zhang, J.; Shao, T. Characteristics of isolated lactic acid bacteria and their effects on the silage quality. Asian Australas. J. Anim. Sci. 2017, 30, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Zhao, S.; Wang, Y. Lactobacillus plantarum inoculants delay spoilage of high moisture alfalfa silages by regulating bacterial community composition. Front. Microbiol. 2020, 11, 1989. [Google Scholar] [CrossRef] [PubMed]
- Wambacq, E.; Latré, J.P.; Haesaert, G. The effect of Lactobacillus buchneri inoculation on the aerobic stability and fermentation characteristics of alfalfa-ryegrass, red clover and maize silage. Agr. Food Sci. 2013, 22, 127–136. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Tang, X.; Liao, C.; Li, P.; Xie, Y.X.; Cheng, Q.M.; Chen, C. Effect of co-inoculation of Lactobacillus plantarum and Lentilactobacillus buchneri on aerobic stability and microbial community composition of perennial sorghum silage. Biomass Bioenergy 2023, 173, 106801. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, A.; Desta, S.T.; Dong, Z.; Shao, T. Effects of four short-chain fatty acids or salts on the dynamics of nitrogen transformations and intrinsic protease activity of alfalfa silage. J. Sci. Food Agric. 2017, 97, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xie, F.; Guo, Y.; Liang, X.; Peng, L.; Li, M.; Tang, Z.H.; Peng, K.P.; Yang, C.J. Fermentation quality, nutritive value and in vitro ruminal digestion of Napier grass, sugarcane top and their mixed silages prepared using lactic acid bacteria and formic acid. Grassl. Sci. 2023, 69, 23–32. [Google Scholar] [CrossRef]
- Lv, J.; Fang, X.; Feng, G.; Zhang, G.; Zhao, C.; Zhang, Y.; Li, Y. Effects of Sodium Formate and Calcium Propionate Additives on the Fermentation Quality and Microbial Community of Wet Brewers Grains after Short-Term Storage. Animals 2020, 10, 1608. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.; Dong, Z.; Li, J.; Shao, T. Effect of ensiling corn stover with legume herbages in different proportions on fermentation characteristics, nutritive quality and in vitro digestibility on the Tibetan Plateau. Grassl. Sci. 2017, 63, 236–244. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Kaka, N.A.; Shao, T. Sequencing and microbiota transplantation to determine the role of microbiota on the fermentation type of oat silage. Bioresour. Technol. 2020, 309, 123371. [Google Scholar] [CrossRef]
- Krishnamoorthy, U.; Muscato, T.V.; Sniffen, C.J.; Van Soest, P.J. Nitrogen Fractions in Selected Feedstuffs. J. Dairy Sci. 1982, 65, 217–225. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Thomas, T. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Jia, T.; Sun, Z.; Gao, R.; Yu, Z. Lactic acid bacterial inoculant effects on the vitamin content of alfalfa and Chinese leymus silage. Asian Australas. J. Anim. Sci. 2019, 32, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Asshauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, H.; Gao, Y.; Diao, Q. Dynamic profiles of fermentation characteristics and bacterial community composition of Broussonetia papyrifera ensiled with perennial ryegrass. Bioresour. Technol. 2020, 310, 123396. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Y.; Gou, W.; Cheng, Q.; Bai, S.; Cai, Y. Silage fermentation and bacterial community of bur clover, annual ryegrass and their mixtures prepared with microbial inoculant and chemical additive. Anim. Feed Sci. Technol. 2019, 247, 285–293. [Google Scholar] [CrossRef]
- Conaghan, P.; O’Kiely, P.; O’Mara, F.P. Conservation characteristics of wilted perennial ryegrass silage made using biological or chemical additives. J. Dairy Sci. 2010, 93, 628–643. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Abdullah, N.; Magaji, U.; Hussin, G.; Ramli, A.; Miah, G. Fermentation Quality and Additives: A Case of Rice Straw Silage. Biomed. Res. Int. 2016, 2016, 7985167. [Google Scholar] [CrossRef]
- He, L.; Lv, H.; Xing, Y.; Wang, C.; You, X.; Chen, X.; Chen, X.; Zhang, Q. The nutrients in Moringa oleifera leaf contribute to the improvement of stylo and alfalfa silage: Fermentation, nutrition and bacterial community. Bioresour. Technol. 2020, 301, 122733. [Google Scholar] [CrossRef]
- Ran, Q.; Guan, H.; Li, H.; He, W.; Zhu, R.; Zhang, L.; Huang, Y.; Xu, Y.; Fan, Y. Effect of Formic Acid and Inoculants on Microbial Community and Fermentation Profile of Wilted or Un-Wilted Italian Ryegrass Silages during Ensiling and Aerobic Exposure. Fermentation 2022, 8, 755. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, X.; Li, J.; Dong, Z.; Shao, T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 2019, 275, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, L.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 2019, 284, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yin, X.J.; Wang, S.R.; Li, J.F.; Shao, T. Separating the effects of chemical and microbial factors on fermentation quality and bacterial community of Napier grass silage by using gamma-ray irradiation and epiphytic microbiota transplantation. Anim. Feed Sci. Technol. 2021, 280, 115082. [Google Scholar] [CrossRef]
- Guan, H.; Yan, Y.; Li, X.; Li, X.; Shuai, Y.; Feng, G.; Ran, Q.; Cai, Y.; Li, Y.; Zhang, X. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 2018, 265, 282–290. [Google Scholar] [CrossRef]
- He, L.; Wang, C.; Xing, Y.; Zhou, W.; Pian, R.; Chen, X.; Zhang, Q. Ensiling characteristics, proteolysis and bacterial community of high-moisture corn stalk and stylo silage prepared with Bauhinia variegate flower. Bioresour. Technol. 2020, 296. [Google Scholar] [CrossRef]
- Du, Z.; Lin, Y.; Sun, L.; Yang, F.; Cai, Y. Microbial community structure, co-occurrence network and fermentation characteristics of woody plant silage. J. Sci. Food Agric. 2022, 102, 1193–1204. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.Y.; Wang, C.; He, L.W.; Zhou, W.; Yang, F.Y.; Zhang, Q. The bacterial community and fermentation quality of mulberry (Morus alba) leaf silage with or without Lactobacillus casei and sucrose. Bioresour. Technol. 2019, 293, 122059. [Google Scholar] [CrossRef]
- Ma, S.S.; Fang, C.; Sun, X.X.; Han, L.J.; He, X.Q.; Huang, G.Q. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Bioresour. Technol. 2018, 259, 221–227. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Zhao, J.; Dong, Z.; Li, J.; Nazar, M.; Shao, T. Assessment of inoculating various epiphytic microbiota on fermentative profile and microbial community dynamics in sterile Italian ryegrass. J. Appl. Microbiol. 2020, 129, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, L.; Xing, Y.; Zhou, W.; Pian, R.; Yang, F.; Chen, X.; Zhang, Q. Bacterial diversity and fermentation quality of Moringa oleifera leaves silage prepared with lactic acid bacteria inoculants and stored at different temperatures. Bioresour. Technol. 2019, 284, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.N.; Li, Y.F.; Jeong, E.C.; Kim, H.J.; Kim, J.G. Effects of formic acid and lactic acid bacteria inoculant on main summer crop silages in Korea. J. Anim. Sci. Technol. 2021, 63, 91–103. [Google Scholar] [CrossRef] [PubMed]
| Item | Perennial Ryegrass |
|---|---|
| Dry matter (% FM) | 30.60 ± 1.21 |
| Crude protein (% DM) | 8.22 ± 0.79 |
| Neutral detergent fiber (% DM) | 47.43 ± 1.58 |
| Acid detergent fiber (% DM) | 24.22 ± 1.37 |
| Water-soluble carbohydrate (% DM) | 9.59 ± 0.96 |
| Lactic acid bacteria (Log10 cfu/g FM) | 4.97 ± 0.51 |
| Yeasts (Log10 cfu/g FM) | 3.31 ± 0.63 |
| Coliform bacteria (Log10 cfu/g FM) | <2.00 |
| Items | Treatment | Ensiling Period (D) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| (T) | Day 7 | Day 15 | Day 45 | T | D | T × D | ||
| DM (%FM) | CK | 19.89 A | 16.51 Ba | 16.31 B | 0.100 | 0.083 | <0.001 | 0.368 |
| L | 19.94 A | 16.60 Bb | 15.73 B | |||||
| F | 20.49 A | 16.83 Ba | 16.65 B | |||||
| CP (%DM) | CK | 5.99 b | 5.41 | 4.65 b | 0.208 | 0.026 | 0.069 | 0.369 |
| L | 6.50 Aab | 4.81 B | 6.16 Aab | |||||
| F | 7.30 a | 5.79 | 7.44 a | |||||
| NDF (%DM) | CK | 57.54 A | 51.35 B | 48.15 C | 0.714 | 0.961 | 0.132 | 0.095 |
| L | 50.00 | 52.52 | 53.06 | |||||
| F | 55.07 | 50.84 | 50.60 | |||||
| ADF (%DM) | CK | 42.64 | 43.52 a | 33.60 | 1.148 | 0.547 | 0.539 | 0.152 |
| L | 43.30 | 37.65 ab | 43.38 | |||||
| F | 39.02 | 34.55 b | 41.35 | |||||
| WSC (%DM) | CK | 4.52 Aa | 3.22 Bb | 1.85 Cb | 0.077 | <0.001 | <0.001 | <0.001 |
| L | 3.01 Ab | 2.09 Bb | 2.13 Bb | |||||
| F | 4.32 Aa | 4.34 Aa | 3.05 Ba | |||||
| Items | Treatment | Ensiling Period (D) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| (T) | Day 7 | Day 15 | Day 45 | T | D | T × D | ||
| pH | CK | 4.46 a | 4.47 a | 4.39 a | 0.019 | <0.001 | 0.035 | 0.955 |
| L | 4.48 a | 4.49 a | 4.34 a | |||||
| F | 4.13 b | 4.06 b | 3.98 b | |||||
| LA (%DM) | CK | 1.76 B | 4.12 Ab | 4.47 Ab | 0.179 | 0.002 | <0.001 | 0.351 |
| L | 2.64 B | 6.25 Aa | 6.94 Aa | |||||
| F | 2.16 C | 4.64 Bab | 6.56 Aa | |||||
| AA (%DM) | CK | 1.16 Ba | 1.29 B | 2.31 A | 0.178 | 0.071 | 0.070 | 0.141 |
| L | 2.08 a | 2.89 | 1.80 | |||||
| F | 0.05 Bb | 2.33 A | 1.18 AB | |||||
| PA (%DM) | CK | 0.28 a | 0.07 | 0.0 | 0.010 | <0.001 | <0.001 | <0.001 |
| L | 0.15 b | 0.0 | 0.18 | |||||
| F | 0.0 | 0.0 | 0.0 | |||||
| BA (%DM) | CK | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| L | 0.0 | 0.0 | 0.0 | |||||
| F | 0.0 | 0.0 | 0.0 | |||||
| NH3-N (%DM) | CK | 2.53 Ca | 3.07 Ba | 3.87 Aa | 0.224 | 0.390 | 0.104 | 0.979 |
| L | 2.13 Bb | 2.68 Bb | 3.32 Aa | |||||
| F | 2.03 b | 2.17 b | 2.30 b | |||||
| Items | Treatment | Ensiling Period (D) | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| (T) | Day 7 | Day 45 | T | D | T × D | ||
| Lactic acid bacteria (Log10 cfu/g FM) | CK | 5.08 Aa | 5.36 Ba | 0.013 | <0.001 | <0.001 | <0.001 |
| L | 6.16 Ab | 6.96 Bb | |||||
| F | 6.86 Ac | 7.42 Bc | |||||
| Yeasts (Log10 cfu/g FM) | CK | <2 | <2 | - | - | - | - |
| L | <2 | <2 | |||||
| F | <2 | <2 | |||||
| Coliform bacteria (Log10 cfu/g FM) | CK | <2 | <2 | - | - | - | - |
| L | <2 | <2 | |||||
| F | <2 | <2 | |||||
| Items | Treatment | Ensiling Period (D) | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|
| (T) | Day 7 | Day 45 | T | D | T × D | ||
| Observed species | CK | 458 | 439 | 2.790 | 0.249 | 0.246 | 0.976 |
| L | 473 | 452 | |||||
| F | 487 | 473 | |||||
| ACE | CK | 489.77 | 472.01 b | 2.059 | <0.001 | 0.735 | 0.004 |
| L | 513.07 | 477.48 b | |||||
| F | 509.95 | 554.74 a | |||||
| Chao1 | CK | 521.12 | 478.12 a | 2.184 | 0.005 | 0.843 | 0.016 |
| L | 509.42 | 463.42 b | |||||
| F | 490.91 | 452.48 b | |||||
| Simpson | CK | 0.85 b | 0.76 | 0.008 | 0.561 | 0.042 | 0.005 |
| L | 0.81 b | 0.920 | |||||
| F | 0.98 a | 0.69 | |||||
| Shannon | CK | 4.21 b | 3.46 ab | 0.0141 | 0.072 | 0.004 | <0.001 |
| L | 4.16 b | 5.30 a | |||||
| F | 6.74 a | 2.80 b | |||||
| Coverage | CK | 0.998 | 0.997 | ||||
| L | 0.998 | 0.999 | |||||
| F | 0.997 | 0.998 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Fan, X.; Li, M.; Chen, Y.; Li, P.; Xie, Y.; Zheng, Y.; Sun, H.; Wang, C.; Dong, R.; et al. Effects of Formic Acid and Lactic Acid Bacteria on the Fermentation Products, Bacterial Community Diversity and Predictive Functional Characteristics of Perennial Ryegrass Silage in Karst Regions. Fermentation 2023, 9, 675. https://doi.org/10.3390/fermentation9070675
Lei Y, Fan X, Li M, Chen Y, Li P, Xie Y, Zheng Y, Sun H, Wang C, Dong R, et al. Effects of Formic Acid and Lactic Acid Bacteria on the Fermentation Products, Bacterial Community Diversity and Predictive Functional Characteristics of Perennial Ryegrass Silage in Karst Regions. Fermentation. 2023; 9(7):675. https://doi.org/10.3390/fermentation9070675
Chicago/Turabian StyleLei, Yao, Xueying Fan, Maoya Li, Yulian Chen, Ping Li, Yixiao Xie, Yulong Zheng, Hong Sun, Chunmei Wang, Rui Dong, and et al. 2023. "Effects of Formic Acid and Lactic Acid Bacteria on the Fermentation Products, Bacterial Community Diversity and Predictive Functional Characteristics of Perennial Ryegrass Silage in Karst Regions" Fermentation 9, no. 7: 675. https://doi.org/10.3390/fermentation9070675
APA StyleLei, Y., Fan, X., Li, M., Chen, Y., Li, P., Xie, Y., Zheng, Y., Sun, H., Wang, C., Dong, R., Chen, C., & Cheng, Q. (2023). Effects of Formic Acid and Lactic Acid Bacteria on the Fermentation Products, Bacterial Community Diversity and Predictive Functional Characteristics of Perennial Ryegrass Silage in Karst Regions. Fermentation, 9(7), 675. https://doi.org/10.3390/fermentation9070675





