Abstract
As a response to the environmental and societal issues that emanate from the high reliance on fossil fuels, the world is now transitioning toward a circular bioeconomy. Acidogenic biohydrogen production is envisaged as a clean fuel of the future due to its non-polluting features and affordability. The major encumbrance for the industrialization of this process is due to the accumulation of metabolic inhibitors (volatile fatty acids (VFAs)), which lower the H2 yields. This review discusses novel methods that can be adopted to valorize the acidogenic VFAs via a “cascade microbial biorefinery-based” approach that enables this process to be economically feasible as it leads to the concomitant production of diverse high-value-added products. The work also elucidates the key setpoint parameters governing the recovery of VFAs during the acidogenic H2 process. It further explores the recent advances in the use of VFAs in microbial biorefineries. Finally, the paper provides some recommendations that might help develop acidogenic microbial biorefineries in the future. Studies focusing on microbial biorefineries tailored towards the valorization/beneficiation of acidogenic VFAs are very scarce in the literature. This work aims to provide new insights into microbial biorefinery-based processes involving the use of acidogenic VFAs as substrates.
1. Introduction
With the heightening risk of climate change, dwindling fossil reserves, and ecological imbalances, the global community is now devoted to making a transition to a sustainable circular bioeconomy to mitigate these issues [1]. Acidogenic biohydrogen fermentation is a sustainable biochemical process that uses anaerobic microorganisms for the conversion of organic substrates into biohydrogen and volatile fatty acids (VFAs) [2]. This bioprocess has gained the interest of many researchers due to its role in renewable H2 process development. Nevertheless, the scalability of this process is hindered by the accumulation of inhibitory metabolites (VFAs), which lowers its H2 yield [3]. This has triggered the development of robust biotechnological approaches that can valorize/beneficiate these chemical precursors—which are considered inhibitors in the bio-H2 production process [4].
The use of these metabolites could provide many breakthroughs in biorefineries as these technologies have reached pilot-scale, implying that they have a potential for industrialization. The term biorefinery encompasses the production of biobased products, such as fuels and chemicals, from biomass feedstocks through integrated processes [5]. This technology was developed several years ago as an economical and sustainable approach to synthesizing industrially-relevant products. It is envisaged that such biorefineries will replace petroleum-based refineries, whose processes are energy-intensive and contribute to CO2 emissions [6]. It is worth noting that this field is broad as it encapsulates various research domains such as chemistry, chemical engineering, and industrial microbiology, and each research domain defines the term based on its biomass valorization/beneficiation strategies. Several market-based products such as xylitol, furfural, sorbitol, and aspartic acid are already being produced at large-scale using chemical-based biorefinery technologies [7]. From an economic standpoint, microbial biorefineries are desirable as these technologies rely solely on the use of microbial cell factories that serve as biocatalytic agents, resulting in low operational expenditure as opposed to chemical biorefineries that use expensive chemicals to fractionate and/or produce the targeted compounds [7]. In addition, microbial biorefineries offer many opportunities in the field of sustainable engineering as they contribute and/or address several thematic issues that fall within the United Nations’ Sustainable Development Goals, and these include (i) waste valorization, (ii) environmental remediation, (iii) clean fuels, and (iv) circular bioeconomy.
However, most pilot-scale microbial biorefineries documented in the literature apply edible crops such as sugarcane, wheat, rice, sorghum, and corn as raw materials. These are typically used to produce first-generation biofuels. Microbial biorefineries focusing on non-edible crops are now being explored in the production of clean fuels and chemicals [2]. These bioprocesses will be key in the development of a circular bioeconomy as they use feedstocks that are considered wastes; thus, they do not pose a threat to food security.
Against this background, this review explores the utilization of acidogenic VFAs as building blocks for the synthesis of market-based products (e.g., polymers, clean fuels, and chemicals). This paper also discusses the key setpoint parameters that govern the extraction of VFAs during acidogenesis. A short section is included to explain the scientific milestones that have been accomplished in this research field. Finally, the work provides suggestions that could help fast-track the development of acidogenic biorefineries.
Contribution of this Work to the Scientific Knowledge
The pursuit of scalable acidogenic fermentation has led to the development of novel biorefinery concepts that enable the coupling of this technology with other biotechnological processes. Research is now headed towards the valorization of acidogenic effluents into different market-based products (e.g., fuels, polymeric compounds, and chemicals) to make this technology scalable and industrially appealing. At the end of the acidogenic fermentation, the effluents which primarily consist of VFAs, can be used as valuable precursors (substrates) in other microbial biorefinery-based processes. The VFAs are important chemical precursors because they consist of functional groups which allow them to be used in diverse scientific fields, including chemicals, polymers, clean fuels, wastewater treatment, biofertilizer/composting, etc.
While there are sufficient amounts of scientific articles focusing on the valorization of VFAs obtained from different environments [3,4,5], none of these scholarly articles focused on coupling the acidogenic biohydrogen fermentation with other microbial biorefinery-based technologies in order to enhance the economic value of this biocatalytic process. Such an approach could be industrially appealing as it will concomitantly produce renewable H2 and cheap precursors in the form of acidogenic VFAs. The approach may also pave the way for the scalability of acidogenic biohydrogen fermentation.
Therefore, in a microbial biorefinery-based framework, we believe that acidogenic biohydrogen fermentation could serve as a potent VFA-producing process followed by the valorization of these chemical precursors in secondary bioprocesses leading to the production of various products (discussed in Section 5). It is hoped that this review article will reinvigorate research interest in bio-H2 process development, serve as a foundation upon which further studies can be undertaken in this scientific field, and ultimately, achieve the goal of showcasing the attainability of a large-scale acidogenic biohydrogen fermentation when this technology is integrated within a “cascade microbial biorefinery” framework/approach.
2. What Are Acidogenic-Derived VFAs?
Acidogenic VFAs are organic compounds consisting of C2–C6 atoms (e.g., acetic acid, lactic acid, butyric acid, and propionic acid). They are primarily produced during acidogenic fermentation—the main biohydrogen production process. These compounds are also readily accessible; they are found in soils, water, and digestive tracts of mammals and other habitats where microbes are the “key process drivers” [8].
From an economic standpoint, VFAs are important because they are applicable in many areas, such as medicine, chemicals, polymers, biofuels, and wastewater treatment. The industrial production of VFAs is expected to increase in the next few years due to their diverse uses. Currently, more than 15 million tons of acetic acid are generated worldwide, and this value is expected to reach 20 million tons in 2025 [9]. Similarly, the demand for butyric acid is growing due to its use in pharmaceuticals, animal feed, and the food industry. Its global market is currently projected at USD 450 million, and it has an annual growth rate of 6.7% [10]. Lactic acid and propionic acid are other VFA types that are also important in bioprocesses. The global demand for lactic acid and propionic acid currently sits at 150,000 and 400,000 tons, respectively [11]. The global market for these compounds is projected to grow from USD 13.7 billion in 2018 to USD 18.0 billion by 2023 [11]. The issue is that these building blocks are mainly produced using fossil-based technologies like the oxidation or carboxylation of compounds such as aldehyde and alkenes [12].
However, the high reliance on carbon-intensive VFA production processes contributes to environmental pollution. Researchers are now exploring the valorization/beneficiation of acidogenic-based VFAs to make this biocatalytic process economical, sustainable, and environmentally-friendly.
3. Types of Feedstocks Used for VFA Production during Acidogenesis
A wide variety of organic wastes, including solid and liquid wastes, have been used for acidogenic VFA production, as shown in Table 1. These include sludge, industrial waste, agricultural residues, food waste, and organic fraction of municipal solid waste (OFMSW). Amongst these, food waste, activated sludge, and OFMSW are the most utilized solid wastes, whereas wastewaters from dairy industries [13], pulp and paper industries [14], and brewing industries [15] are the most exploited effluents/liquids in VFA studies. Co-digested feedstocks are used in VFA production studies to enhance the production of these compounds, VFAs in this context [16].
4. The Key Parameters Governing the Recovery of VFAs during Acidogenesis
As mentioned earlier, VFAs are primarily produced through acidogenic fermentation, and this involves the regulation of the key setpoint variables such as carbon-to-nitrogen (C/N) ratio, pH, temperature, hydraulic retention time, and organic loading rate (see Figure 1). It is imperative to acquire deeper insights into these variables as they impact the dominant microbiota and VFA recovery [17]. They are usually evaluated one-at-the-time in the literature in order to ascertain their individualist roles [18,19]. Hence, the individualist impact of these variables on VFA recovery will be explained in this section. Temperature is one of the most studied parameters as it impacts the overall process dynamics, i.e., the proliferation of dominant VFA-producing microbial assemblages, the hydrolysis of feedstocks, accumulation of inhibitors, etc. [20]. Acidogenesis is mostly carried out under mesophilic and thermophilic conditions [21], though some studies have reported the production of these precursors under psychrophilic conditions [22]. From an economic standpoint, mesophilic conditions are well-suited for VFA augmentation studies because they require less energy inputs in comparison to thermophilic conditions [23]. Thermophilic conditions are applied as they enhance the hydrolysis of substrates and inhibit unwanted metabolites such as ammonia and hydrogen sulfide. It was also reported that thermophiles increased the VFAs due to enhanced acidogenic activity, leading to high VFA recovery [21]. In one study, a VFA yield that was three times higher under thermophilic conditions compared to under mesophilic conditions was recorded [24]. Thermophilic conditions also promoted the degradability of feedstock waste and microbial activity [24]. Contradicting results have also been reported regarding the impact of thermophilic conditions on VFA production. He et al. [25] observed that varying the temperature from 35–55 °C decreased the VFA yield from 17–11 g/L. A plausible explanation for this phenomenon could be due to the high solubilization of organics, but this reduced the rate of acidogenesis, resulting in low VFA recovery [20].
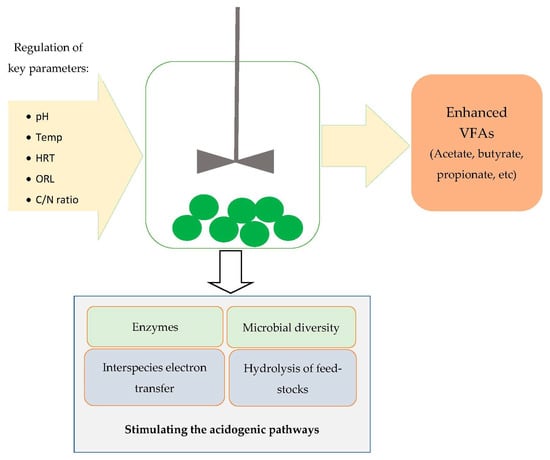
Figure 1.
Showing the effects of the key parameters on the recovery of VFAs.
Elsewhere, it was shown that the type of feedstocks alongside the microbial cultures also contributes to the process performance despite the temperature regimes that are chosen during acidogenesis [25], as further corroborated by the studies outlined in Table 1. In addition, temperature can be used to target specific types of VFAs [26]. It is, therefore, evident from the aforementioned results that an in-depth assessment needs to be conducted when choosing a particular temperature profile during acidogenic fermentation.
pH is another crucial setpoint parameter in biocatalytic VFA production, as it mainly affects the acidogenic microbes and their enzymatic activities. Acidogens usually thrive at a pH range of 5.5–6.5, while methanogens proliferate at pH 7.0–9.0 [27]. It is, therefore, important to regulate the fermentation pH so that VFA-consuming pathways such as acetogenesis and methanogenesis can be inhibited during the process [28]. It has also been shown that pH can be used to obtain specific types of VFAs [29,30]. A neutral pH range (pH 6.5 to 7.5) was reported to favor the production of acetic acid, butyric acid, and propionic acid [31], whereas an acidic pH (pH 4.5 to 5.0) favored the production of mainly ethanol and lactate [32]. In addition, it was revealed that the pH range of 5.5–7.0 favored the growth of the VFA-producing phyla such as Bacteroidetes, Proteobacteria, and Firmicutes during the digestion of cow manure, and this was helpful in the inhibition of VFA-consumers such as methanogens [33].
The accumulation and composition of VFAs are significantly affected by the C/N ratio of the chosen feedstocks during acidogenic fermentation [34]. Usually, a C/N ratio of 20–30 is deemed favorable for acidogenic bioprocesses [35]; however, some researchers have reported C/N ratios that fall outside this range, and this could be attributed to the bioconvertability of the feedstocks that were used during VFA production [36]. Liu et al. [37] reported that the C/N ratios of 20, 25, and 30 increased the VFA yields by 70%, 140%, and 170%, respectively, during the microbial production of VFAs using sewage sludge. A similar C/N ratio (20–30) was also observed by Paranjpe et al. [38] and Franceschi et al. [39]. On the contrary, a low C/N ratio has been shown to release excess ammonia, which inhibits the formation of VFAs [40], while a high C/N ratio prolongs microbial activities leading to ineffective substrate utilization [38]. It is noteworthy to highlight that the C/N ratio of the chosen substrate is determined by its chemical composition. The substrate should be thoroughly assessed before being used in acidogenic fermentations.
Hydraulic retention time (HRT) is a measure of the period it takes for the compound/substrate to remain in the reactor during acidogenic fermentation [8]. HRT should be strategically chosen so that the solubilization of substrates will be promoted while preventing the growth of methanogens that consume the acidogenic metabolites, including the VFAs [41]. Generally, short HRTs (<5 days) are used to promote acidogenic consortia as these are fast-growing microorganisms compared to methanogens which are slowing-growing microorganisms [17]. However, some researchers observed that prolonging the HRTs was effective in the enrichment of VFAs [42]—this is highly attributed to the biodegradability of the feedstock used [43]. Farouk et al. [44] reported an optimal HRT of 2 days for high production of VFAs 4019 mg/L, with acetic acid, butyric acid, and isobutyric acid being the major VFA components. Tsegaye et al. [45] also reported the HRT of 3 days as suitable for maximizing the production of VFAs during acidogenic fermentation.
The organic loading rate (OLR) determines the amount of feedstock that needs to be fed daily into the reactor (per unit reactor volume) for acidogenic fermentation to occur [43]. There are inconsistent results regarding the optimal ORL for VFA production. Wijekoon et al. [46] reported a remarkable increase in VFA production when varying the OLR from 5 to 12 kg chemical oxygen demand (COD) m−3 d−1 during the two-stage thermophilic digestion of molasses-based wastewater. Whereas Magdalena et al. [47] demonstrated that increasing the OLR beyond 12 g COD L−1 d−1 did not increase the VFA production as this led to the proliferation of Euryarchaeota species which are not the dominant VFA-producers compared to Firmicutes and Bacteroidetes. These contradicting results occur because other operational variables, such as HRT, pH, and temperature, synergistically contribute to the process’s overall performance.
The selection of an appropriate acidogenic reactor Is also critical—this is linked to other factors such as the mode of fermentation that is going to be chosen (e.g., batch, fed-batch, or continuous fermentation), the inoculum source alongside its growth requirements, and the efficacy of that particular design. Hitherto, batch reactors are mostly used in VFA studies due to their cost-effectiveness and their ease of operation [48]; however, they produce low yields compared to other reactor designs [49]. Some researchers have used fluidized-bed reactors as they have been shown to eliminate the clogging issues caused by the accumulation of biomass within the reactor [50]. Continuously stirred tank reactors are widely used in bioprocesses, including VFA studies, because they maximize the hydrolysis of substrates, offer better mixing, and produce high process yields [51]. In addition, other reactor configurations have recently been tested In acidogenic fermentations, and these include the up-flow anaerobic sludge blanket reactor [52], anaerobic biofilm reactor [53], anaerobic membrane reactors [54], packed-bed reactor [55], and anaerobic plug-flow reactor [56]. Additives are essential in acidogenic fermentations because they enhance the activity of microbial cultures, stimulate the hydrolysis of substrates, and maximize the VFA yields [57]. Trace metals such as sodium (Na), iron (Fe), gold (Au), nickel (Ni), copper (Cu), silver (Ag), zinc (Zn), palladium (Pd), and cobalt (Co) are added in acidogenic reactors as nutritional supplements [58,59]. Lin and Shei [59] observed that low concentrations of trace metals (Zn, Cu, and Cr) were instrumental in the enhancement of acidogenic reactions when using bacteria from sewage sludge. However, a further increase in the concentration of these metals resulted in the inhibition of acidogenic pathways. These chemical additives not only stimulated the acidogenic reactions but maximized the key enzymatic activities, reduced lag-phase, and accumulated acidogenic metabolites [59]. Likewise, Liu et al. [60] reported that the use of trace metals promoted the abundance of Firmicutes, which play an important role in the acidification process.
In recent years, nano-additives (nanoparticles) have been gaining increasing prominence in acidogenic fermentation owing to their remarkable features, such as large surface area, chemical and mechanical stability, high catalytic behavior, and high VFA recovery [61]. The biochemistry (mechanism) of nanoparticles in acidogenic consortia is still not clearly understood in the literature, but it has been postulated that these nano-based additives act as catalytic agents which stimulate acidogenic pathways during the fermentation reaction [62]. Kim and Lee [63] evaluated the effect of dual-nanoparticles on acidogenic fermentation using Clostridium ljungdahlii. The methyl-functionalized silica and methyl-functionalized cobalt ferrite–silica (CoFe2O4@SiO2-CH3) nanoparticles led to the enhancement in syngas fermentation, bioethanol production, biomass formation, and acetic acid production. Moreover, the supplementation of CoFe2O4@SiO2-CH3 increased the acetic acid, ethanol, and biomass formation by 59.6%, 213.5%, and 227.6%, respectively, in comparison to the control experiments [63]. Although these are promising results, it should be noted that some nanoparticles have inhibitory effects on acidogenic fermentations. For example, a recent study that examined the effect of copper nanoparticles (Cu NPs) on acidogenic fermentation demonstrated that these nano-based additives inhibited the hydrolysis and acidification processes [64]. Further investigation revealed that the Cu NPs enriched the growth of VFA-consuming consortia. These findings show that these stimulatory additives need to be cautiously used in acidogenic fermentations.
5. Microbial Biorefinery-Based Processes Involving the Use of Acidogenic VFAs
Acidogenic VFAs are seen as valuable building blocks for a wide spectrum of biobased technologies such as polymers, alternative fuels, chemicals, and environmental remediation [23]. These compounds are desirable in the circular bioeconomy due to their chemical structure, high abundance, recyclability, and non-hazardous nature. The use of these metabolites in these microbial biorefinery-based processes is elucidated below.
5.1. Biobased Polymers
Biopolymers, such as polyhydroxyalkanoates (PHAs), are polymeric compounds that are produced via the use of microbial cell factories, i.e., they accumulate inside the cells of microorganisms under nutrient-limiting conditions of nitrogen, phosphorous, sulfur, or oxygen during fermentation [65]. These biobased polymers are proposed as suitable substitutes for petroleum-based plastics due to their biodegradability, environmental friendliness, and affordability [66,67]. The application of acidogenic-derived VFAs is advantageous in PHAs as this can reduce operational costs by more than 40%, thereby significantly improving the competitiveness of this process [68,69].
However, preliminary methods need to be implemented before the acidogenic-derived VFAs can be used in PHA studies. Firstly, it is important to regulate the concentration of nutrients (e.g., carbon, nitrogen, phosphorous, and sulfur) during the microbial conversion of VFAs into PHAs; this phenomenon is known as the feast/famine strategy [70]. Secondly, it has been shown that the fermented effluents need some pretreatments (filtration) to ensure that the inhibitory compounds, such as ammonia and excess growth factors, are removed before being used in PHA production [71]. A broad range of microbes (as pure cultures or mixed-microbial cultures) have been used for PHA production [72].
Lee et al. [73] achieved a PHA content of more than 80% using pure strains of Alcaligens latus. Whereas Silva et al. [74] achieved an optimum PHA content of 71.3% using VFAs derived from fruit waste. Although pure cultures possess high PHA-producing yields, they require stringent bioprocess conditions, and this escalates the overall operational costs [71]. To circumvent this issue, mixed-microbial cultures are receiving widespread acceptance amongst scientists due to their ability to utilize a wide spectrum of carbon materials, their high accessibility, and their reduced operational PHA production costs [75]. The use of these inoculum sources could help in the advancement of PHA production, but a lot of hurdles need to be tackled before this bioprocess can reach industrialization. For instance, mixed-microbial cultures consist of other microbial populations which compete with PHA-producing cultures [76]. The use of mixed-cultures also results in low PHA yields due to the formation of undesirable by-products/reactions [76]. Consequently, innovative biotechnological strategies must be developed to inhibit the microbial populations/pathways that produce undesirable pathways.
5.2. Alternative Fuels
The application of acidogenic VFAs in alternative fuels (e.g., electricity, biogas, hydrogen, and biodiesel) has been widely accepted in the literature due to their chemical structure, high availability, and their significance in the field of circular bioeconomy. These building blocks will play an important role in the advancement of alternative fuels. Studies that have explored acidogenic-derived VFAs for the biosynthesis of biofuels are elaborated on in this section.
- Electricity—microbial fuel cells (MFCs) are bioelectrochemical reactors that use a wide variety of biofilm-forming microorganisms to harness chemical energy stored in organic wastes for electricity generation [77,78]. These reactor systems consist of an anodic compartment and a cathodic compartment, which are divided by a proton exchange membrane [79]. The anodic compartment is anaerobic so that biofilm-forming communities can effectively convert organic wastes into electrons, protons, and carbon dioxide [80]. These electrons and protons are then transferred into the aerobic cathodic compartment through the means of an electric circuit and proton exchange membrane. In the cathodic chamber, the protons and oxygen combine to generate water. Figure 2 demonstrates the working principles of MFCs with VFA as a model substrate [81]. Equations (1) and (2) show the reactions that occur in the anodic and cathodic chambers, with acetate as a carbon source [82].
CH3COO− + 2H2O → 2CO2 + 7H+ + 8e−
O2 + 4e– + 4H+ → 2H2O
- Different MFC designs are used for electricity generation, and these include single-chambered MFCs and two-chambered MFCs, the upflow MFC, and the stacked MFC. These have been detailed by Udama et al. [83] and Ramya and Kumar [81]. The use of acidogenic VFAs could provide many breakthroughs in MFC technology as these compounds can be directly used for bio-electricity generation without the need for pretreatments as opposed to other bioprocesses. Several authors have successfully demonstrated the production of electricity using waste-derived VFAs. For example, Mohanakrishna et al. [84] produced a power density of 111.76 mW/m2 using VFAs obtained from acidogenic fermentation. Asefi et al. [85] achieved an optimum power density of 422 mW/m2 using VFAs from food waste. Microbial characterization studies of the anodic chamber showed the prevalence of Geobacter species which are the predominant bioelectricity-producing microorganisms [85].
- The composition of VFAs in acidogenic effluents has been shown to have a considerable effect on the performance of MFCs during electricity generation. It was reported that the electrical current produced in an acetate fed-MFC was almost two times higher than the electrical current obtained in MFCs fed with other VFA types, resulting in high coulombic efficiency of 93% [86]. Similarly, the acetate-fed-MFC outperformed the butyrate- and propionate-fed MFCs [87]. The 16S ribosomal DNA sequencing results of the anodic biofilms showed the prevalence of Geobacter sp. [87]. It has been shown that acetate is an ideal substrate for electroactive microorganisms due to its biodegradability and stimulatory effects compared to other VFA compounds [88]. Research is ongoing to acquire a deeper understanding of the prevailing bio-electricity anodic biofilms, electron transfer mechanisms, MFC designs, and electrode types to advance this technology.
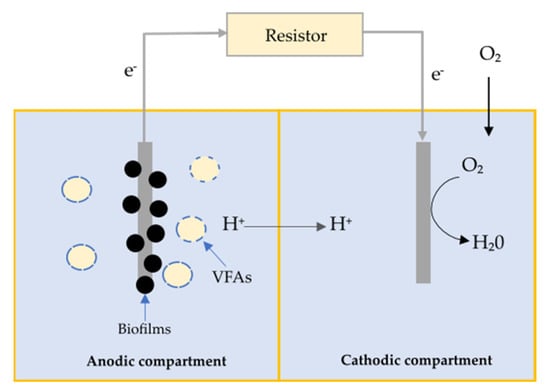
Figure 2.
An MFC bioreactor using VFA as a substrate [89].
- Biogas—biogas has received increasing attention over the past two decades due to its high methane content (30–80%), cost-competitiveness, and contribution to the bio-based economy [90]. Biogas occurs in the final step of the anaerobic process, where various bacterial and archaeal species use metabolites (VFAs and CO2) from the acidogenic and acetogenic stages as precursors for biogas formation [91]. Although various microorganisms have been reported in the biosynthesis of biogas, methanogenic species are the predominant species [90]. Biogas can be produced using a single-stage anaerobic process or a two-stage anaerobic process. Single-stage fermentations are not suitable because they generate low biogas content due to variations in the growth requirements of fermentative microorganisms [92]. Two-stage processes produce optimal biogas content because they cater to the growth conditions of different microbial communities. Acidogens are fast-growing species and require acidic pH alongside short HRTs, whereas methanogens are slow-growing species and proliferate under neutral pH and long HRTs [93]. The acidogenic step is used to convert the substrates into VFA-rich effluents, and these are later used by methane-producers in the second step [93]. The co-digestion of food waste and activated sludge was investigated in single-stage and two-stage anaerobic processes under mesophilic conditions. The two-stage process had a remarkable effect on methane content: increased from 61.2% to 70.1% [93]. Two-stage processes are appealing to scientific researchers because they eliminate the pretreatment steps, which are energy-intensive and expensive [94]. More studies are now focusing on biogas-upgrading technologies because these systems can produce high-purity methane (CH4 ≥90% v/v) that is applicable in natural gas pipelines [95].
- Hydrogen—VFAs from dark fermentation (DF) processes are also applicable in other hydrogen-producing technologies such as photo-fermentation (PF) [96], microalgal cultivation [97], electrohydrolysis [98], and microbial electrolysis cell (MEC) [99]. In photo-fermentation, purple non-sulfur bacteria use acidogenic compounds as substrates for hydrogen production [100]. Over the past few years, the coupling of DF and PF has been shown as an innovative approach that could augment the energetic gains in hydrogen production studies [101]. However, precautionary measures need to be applied because the main photosynthetic hydrogen producers, such as Rhodobacter sphaeroides, are fastidious and sensitive to certain growth conditions. For example, the acidogenic effluents consisting of VFAs must undergo vigorous pretreatments such as the removal of colloids, pH adjustment, dilution, and nutrient addition [102]. The types of VFAs found in the DF effluents also impact the growth of these photosynthetic species, as it has been reported that acetate- and propionate-rich effluents have a positive effect on hydrogen production yields compared to other VFA compounds [103]. Two-stage sequential batch processes of DK and PF can produce an overall H2 yield of 12 mols, as shown in Equations (3) and (4) [96]. This hybrid system holds a huge potential in the development of hydrogen-related technologies as some researchers have reported an optimal H2 yield of 10.21 mol H2/mol glucose and a VFA recovery of more than 90% [104,105]. Likewise, the use of microalgae is expanding and gaining recognition in hydrogen process development because these microorganisms are easily accessible and can be cultivated under various conditions [106]. Hybrid processes involving a microalgal consortium are therefore seen as a novel approach to harnessing clean hydrogen. Recent studies are focused on developing genetically engineered strains alongside optimal reactor designs to provide insights that could lead to the scalability of this process [107]. A coupled system of DF and the microalgal process was investigated for biohydrogen using food waste [108]. This integrated process generated a high biohydrogen yield of 133.66 mL/g substrate with almost 100% VFA consumption [108]. The energy conversion efficiency also increased from 10.14–24.06% [108].
DF: C6H12O6 + 2H2O → 2CH3COOH + 2CO2 + 4H2
PF: 2CH3COOH + 4H2O → 8H2 + 4CO2
- Electrohydrolysis of VFA is another option for producing renewable hydrogen, and this biotechnological route applies direct DC voltage into the VFA-rich effluents, causing the release of electrons from the metal electrodes (e.g., copper electrode), and these combine with protons to form hydrogen [109]. The mechanism of this technology is explained using Equations (5)–(7). This technology is advantageous as it is directly coupled in the anaerobic reactor to enable the endogenous (in-situ) production of hydrogen without the need for the pretreatment of effluents. Hydrogen production by electrohydrolysis of acidogenic VFAs was conducted by Tuna et al. [109]. It was observed that increasing the applied voltage from 1–3 V had a significant effect on H2 production [109]. However, studies that focus on the production of hydrogen by electrohydrolysis of waste-derived VFAs are still scarce in the literature. This implies that there are a lot of knowledge gaps in this area.
Cu → Cu2+ + 2e−
CH3COOH → CH3COO− + H+
2H+ + 2e− → CH3COO− + H+
- Hydrogen is also produced through the means of a MEC. The MEC is derived from the MFC but slightly differs as it requires an external electricity supply [110]. In the anodic compartment, the electrochemically-active bacteria breakdown the VFAs and release protons and electrons to generate hydrogen in the cathodic compartment [111]. It is only in recent years that the acidogenic VFAs were found to be valuable in MECs. These reactor systems were discovered in the early 2000s by scientists from Wageningen University (The Netherlands) and Penn State University (United States) [112] and have since gained considerable attention among researchers, with some studies reaching pilot-scale [113,114]. A diagram summarizing the use of acidogenic VFAs in other bioprocesses is shown in Figure 3.
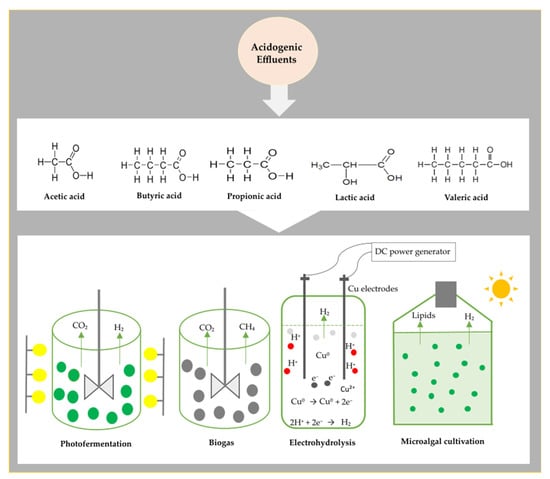 Figure 3. A schematic diagram illustrating the use of acidogenic VFAs in bioprocesses.
Figure 3. A schematic diagram illustrating the use of acidogenic VFAs in bioprocesses.

Table 1.
The different types of feedstocks used in VFA production studies.
Table 1.
The different types of feedstocks used in VFA production studies.
| Feedstock | Inoculum | Setpoint Conditions | VFA Production | VFA Composition | Reference |
| Food waste | Sludge | Temp = 35–55 °C, pH 5.0–6.0, FM = batch, process time = 5 days, | NA | Acetate, butyrate | [20] |
| Food waste | Sludge | Temp = 30 °C, pH = 6.0, FM = batch, process time = 15 days, | 0.908 g/g VSremoval | Acetate, butyrate | [115] |
| Food waste | Sewage sludge | Temp = 35 °C, pH = 10.0, FM = batch, process time = 35 days | NA | Acetic acid, propionate, butyrate, and valeric acid | [116] |
| Waste activated sludge | - | Temp = 35 °C, pH = 10.0, FM = batch, process time = 8 days | 4619.6 mg COD/L | - | [117] |
| Waste activated sludge | - | Temp = 35 °C, pH = 6.8, FM = batch, process time = 12 days | 327.8 mg COD/g VS | Acetic acid, butyric acid, valeric acid, and propionic acid | [118] |
| OFMSW | Digestate | Temp = 37 °C, pH = 7.0, FM = batch, process time = 180 days | 24.4 g CODVFA/L | Acetic acid, butyric acid, propionic acid, caproic acid, isobutyric acid, isocaproic acid, and isovaleric acid | [119] |
| Potato waste | Rumen fluid | Temp = 39 °C, pH = 6.95, FM = batch, process time = 2 days | NA | Acetic acid, propionic acid, butyric acid, iso-valeric acid, and valeric acid | [120] |
| Organic waste | Granular sludge | Temp = 55 °C, pH = 7.0, FM = batch, process time = 42 days | NA | Acetic acid, butyric acid | [121] |
| Cheese whey | Sludge | Temp = 30 °C, pH = 5.0–6.0, FM = batch, process time = 0.5 day | NA | Butyric acid, propionic acid, and valeric acid | [18] |
COD, chemical oxygen demand; FM, fermentation mode; HRT, hydraulic retention time; NA, not available/reported; OFMSW, organic fraction of municipal solid waste; OLR, organic loading rate; Temp, temperature; VS, volatile solids.
- Microbial lipids for biodiesel—biodiesel is an alternative fuel that has received enormous growth over the past two decades, and this technology has reached a pilot scale [122]. It is currently being used as a fuel blend in diesel engines [123]. The majority of the world’s biodiesel is synthesized using lipids extracted from edible crops such as rapeseed oil, sunflower oil, soybean oil, and palm oil [124]. However, this technological route is still being scrutinized by scientists as it disregards the concept of “circular bioeconomy through waste valorization”. The “food vs. fuel” debate has also reinvigorated scientists to search for other sources of feedstocks. It has been proposed that the waste-derived VFAs could play a crucial role in the advancement of biodiesel technologies as these compounds can be used by oleaginous species to produce lipids that are applicable in the transesterification process [125]. Lipids synthesized from waste-derived VFAs have similar fatty acid composition to other well-known biodiesel-producing feedstocks such as jatropha oil and soybean oil [126], making them an attractive feedstock that could compete with the existing carbon-based materials. It was recently reported that the two-stage batch process is the best strategy to obtain the highest lipid content (37% w/w) in microbial lipid synthesis using waste-derived VFAs [127]. Another study demonstrated the production of microbial lipids using VFAs that were obtained from wastepaper [128]. The biomass content achieved from VFAs derived from waste-office paper and the waste newspaper was 4.3 g/L and 2.9 g/L, respectively; the lipid content was 41.2% and 27.7% dry cell weight, respectively [128]. These scientific reports showcase a sustainable, ecologically-friendly, and economical approach to producing microbial lipids using waste-derived VFAs. These findings could serve as a basis upon which further development studies could be carried out in microbial lipid production using waste-derived VFAs.
5.3. Biobased Chemicals
The recent progress in the field of industrial microbiology has allowed researchers to obtain knowledge that is essential for the cultivation and engineering of microbial cell factories that are applicable in the synthesis of a wide array of bio-based chemicals. Due to their numerous applications in the food, chemical, and pharmaceutical industries, biobased chemicals such as lactic acid, succinic acid, and citric acid have gained attention in the field of bioeconomy as these compounds can be produced using microbial biorefineries [129]. Studies focusing on succinic acid and lactic acid revealed that these chemicals could be synthesized using VFAs acquired from organic residues such as OFSMW [130], lignocellulosic biomass [131], and industrial waste [99]. Bacterial strains such as Actinobacillus succinogenes and Lactobacillus rhamnosus are mostly used in the aforementioned bioprocesses. Likewise, the citric acid industry has been reliant on expensive sugars such as glucose and sucrose and therefore has struggled to develop a sustainable and economical process [132]. There is a paradigm shift in the production of these chemical additives (biobased VFAs) as scientists are now focusing on the exploitation of agro-industrial wastes such as apple pomace, brewer’s spent grain, and citrus waste [133].
5.4. Wastewater Treatment
Acidogenic VFAs have also been shown to play a role in environmental remediation processes targeting the removal of biological pollutants such as nitrogen and phosphorous in wastewater. Microbial processes such as aerobic nitrification and anoxic denitrification are used in the removal of nitrogen, whereas phosphorous is removed through the enhanced biological phosphorus removal technology under varying aerobic and anaerobic microenvironments [134]. Interestingly, studies have also shown the simultaneous removal of nitrogen and phosphorus in wastewater using an integrated approach known as the anaerobic-anoxic-aerobic process [135,136]. Due to the low availability of nutrients in wastewater, VFAs are usually supplied in excess, and these serve as a carbon source during the removal of biological pollutants. Ideally, the carbon to nitrogen requirements ranging from 5–10 mg COD/mg N should be used for the combined nitrification/denitrification process [137]. Although some studies are still dependent on the application of synthetic VFAs for the removal of biological pollutants in wastewater—this approach is expensive and also not sustainable [138]. The use of acidogenic VFAs is seen as an economical approach to removing pollutants in wastewater. A study that examined the use of acidogenic VFAs in nitrogen removal revealed a superior performance compared to synthetic VFAs [139]. This phenomenon could also be attributed to the synergistic interaction between these precursors and other constituents in wastewater [139]. Furthermore, the authors recorded a high phosphorus removal efficiency of up to 98% using waste-derived VFAs in comparison to synthetic acetate (71%) [139]. Sun et al. [140] observed an enhanced nitrogen removal efficiency of up to 57% upon the addition of VFAs obtained from the waste-activated sludge, and these results coincided with the local standards of pollutant removal for rural wastewater treatment plants in China [140]. Yang et al. [141] applied an innovative nitrogen removal technique known as Feammox using VFAs derived from waste-activated sludge. The technique focuses on the supplementation of Fe(III) compounds in anaerobic digesters. The addition of Fe2O3 and Fe(OH)3 not only improved the removal of nitrogen but also increased methane production and sludge solubilization [141]. Most importantly, these Fe(III) compounds induced Feammox to occur throughout the fermentation process [141]. The biological removal of nitrogen and phosphorous has also reached the pilot-scale [142]. The removal efficiencies in these biochemical studies varied from 80% to 90% [143], and these findings could provide breakthroughs in the use of VFAs as key nutrients in the removal of pollutants in wastewater.
5.5. Soil Amelioration
Apart from the ability of acidogenic fermentation processes to be integrated into a “microbial biorefinery framework” that leads to the production of other market-based products, it possesses other benefits that are embedded in a circular bioeconomy. The fermented liquid slurries/digestates are applicable in sustainable agricultural practices as these acidogenic residues are characterized by diverse micro-nutrients (e.g., Na, Fe, Ca, Mg, Zn, and Cu), macro-nutrients (e.g., N, P, and K), and other organic constituents which are well-suited for soil amelioration/fertility approaches without altering the soil’s indigenous microbiota, natural pH, salinization, and moisture content as opposed to chemical fertilizers [144]. A study by Peng and Pivato [145] showed that these digestates could serve as biofertilizers owing to their high composition of NPK, which have bio-stimulatory effects on plants and soil properties. Interestingly, the authors conducted a chemical characterization study and observed that the heavy metal content in the digestates was below the EU’s regulatory standards, suggesting that they will not pose any harm when used for agricultural purposes. Some studies have stressed the need to pretreat the digestates prior to being used as soil ameliorants as they might contain some toxic compounds and gases that will pollute the environment; pretreatment methods such as dilution, aeration, and air drying are widely used as these regimes are affordable [146].
5.6. Nutritional Compounds
In addition to all the abovementioned high-value added compounds, it has recently been shown that the diverse microbial assemblages (in mixed consortia) present in anaerobically fermented slurries consist of industrially-relevant compounds such as single-cell protein—this is protein-rich biomass that is produced by some acidogenic species and other microorganisms that synergistically interacts with each other to improve the performance of the anaerobic digestion process. The technology of SCP is advantageous as it does not cause environmental pollution, does not require a lot of land and water as opposed to animal- and plant-based protein, and is cheap. Zhang et al. [147] successfully produced SCP using seven different yeast strains fed with anaerobically-digested slurry due to its high concentration of NH4+-N. The authors also observed that acetic acid was an ideal pH regulator than HCL, and this favored the growth of Nectaromyces rattus [147]. Xu et al. [148] demonstrated a novel approach of simultaneously producing SCP and biogas upgrading (CH4, H2, and CO2) using a biobased electrosynthesis process. In the context of acidogenesis, the VFA-rich effluents could be used as cheap substrates for biogas and SCP. Although this technology is still in its infancy, it has the potential to provide new solutions for sustainable protein production, alternative fuels, and waste valorization, as these thematic topics are aligned with the UN’s Sustainable Development Goals. All the valuable products that can be produced from the VFAs are outlined in Figure 4.
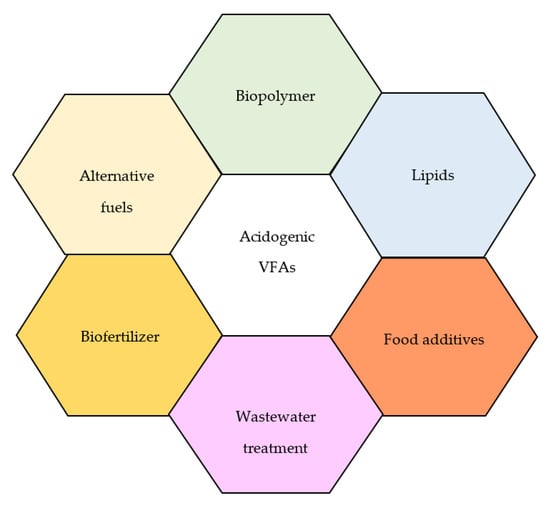
Figure 4.
Shows the possible applications of acidogenic VFAs.
6. Recent Progress, Technical Barriers, and Future Outlook in Microbial Biorefineries
The Case of Acidogenic-Based Microbial Systems
Studies that focus on acidogenic microbial biorefineries are still limited in the literature as the majority of commercial microbial biorefineries (>188 out of 200 in the EU alone, in 2017) are considered “first-generation” biorefinery facilities because they solely rely on edible crops such as sugarcane, corn, and oils to synthesize a wide variety of chemicals and fuels [149]. Nonetheless, the skepticism surrounding the use of edible crops for commercial products has reinvigorated scientists to search for suitable alternatives. Consequently, there has been an upsurge of interest in the development of “second-generation” biorefineries to avert this issue.
It is only in recent years that researchers started paying attention to acidogenic-derived VFAs due to their chemical structure and applicability, and it was proposed that these building blocks could be integrated into microbial biorefineries [150]. A pilot-scale anaerobic baffled reactor was recently used for the treatment of wastewater [151]. The synthesized acidogenic VFAs were later used as a carbon source for the organic removal of organic pollutants in wastewater [151]. In another pilot study, these chemical precursors were strategically produced so that they could also be used as a carbon source for the biological removal of nitrogen and phosphorous in wastewater, as corroborated in Section 5.4 [152]. Acidogenic microbial biorefineries are also tailored toward the valorization/beneficiation of organic waste, whereby multiple value-added products can be produced. For example, a pilot-scale demonstration study was conducted to produce chemical precursors (VFAs) using food waste [153]. In this work, the concentration of VFAs increased from 8419–15,048 mg COD/L upon elevating the pH from 4.5 to 6.5. The anaerobic reactor consisted mainly of Clostridium, Proteiniphilum, and Sporanaerobacter species which maximized the bioconversion of food waste into VFAs [153]. Furthermore, a techno-economic assessment showed that the biosynthesis of VFAs using cellulosic primary sludge as a carbon source contributed to a higher net benefit as compared to only biogas production [153]. Similarly, it was observed in a pilot-scale study that the primary sludge consisting of cellulosic waste was suitable for the enrichment of acidogenic VFAs [154]. In this study, a maximum VFA yield of 2.57 kg COD/m3 d was achieved at pH 9.0 under mesophilic conditions [154]. Elsewhere, a two-stage pilot-scale process was used in the enrichment of VFAs using OFSMW under mesophilic fermentation [119].
New research directions are also being explored in this field. For instance, pilot-scale studies involving the simultaneous production of VFAs, hydrogen, and methane (referred to as one-pot fermentation) are now being reported in the literature [155]. The VFAs serve as carbon sources for microbial communities contained within the bioreactor during biohythane production. This one-pot fermentation process has shown superior performance, as some scientists have reported more than 80% of biogas content and 90% of COD removal during the anaerobic process [155]. In addition, companies such as DuPont have pioneered the biotechnology of cellulosic ethanol production from genetically engineered strains via a fermentation approach known as consolidated bioprocessing (CBP) [156]. CBP is similar to one-pot fermentation as it combines saccharification and fermentation in a single-step process that is catalyzed by microorganisms [157]. In this way, a microbial consortium capable of biomass deconstruction converts the cellulose into VFAs and subsequently uses these for ethanol production [157]. This fermentation method offers a cost-effective process as it eradicates the various substrate pretreatment steps and requires low-energy inputs [157]. A pilot-scale facility that was developed by DuPont produced more than 30 million gallons of ethanol from corn stover from an engineered strain of Z. mobilis, and efforts are underway to improve the physiological and metabolic traits of this bacterium [158]. Other existing pilot-scale demonstrations of the biorefinery-based projects that can be integrated with acidogenic fermentations are shown in Table 2.
It is evident that microbial biorefineries will play a pivotal role in decarbonizing the current energy systems, as shown by these pilot-scale investigations. Moreover, bio-based technologies will serve as a key to fast-tracking the establishment of alternative fuels in industrialized nations as such nations aim to diversity their energy mix to include at least 30% of renewable resources in the energy supply and around 10% of renewables in the transport sector by 2030 as part of the UN’s Sustainable Development Energy Goals [159].

Table 2.
Pilot-scale demonstrations of microbial biorefinery-based plants [160].
Table 2.
Pilot-scale demonstrations of microbial biorefinery-based plants [160].
| Company | Country | Feedstock | Product(s) | Capacity (Tons) |
|---|---|---|---|---|
| Borregaard | Norway | Woody plants | Cellulose, biovanillin, ethanol | 250,000 |
| Alco Bio Fuel | Belgium | Corn and wheat | Ethanol, protein-rich feed, electricity, liquid CO2 | NA |
| Tereos | Czech Republic | Molasses | Ethanol | 200,000 to 1,000,000 |
| Novozymes | Denmark | Corn | Ethanol | 25,000 |
| Pannonia Bio | Hungary | Corn | Ethanol, distillers’ grain, corn oil | 85,000 |
| Essentica | Bulgaria | Grain | Ethanol | NA |
| Enocell Mill | Finland | Wood | Pulp | 490,000 |
| Lantmännen | Sweden | Grain | Ethanol | 200,000 |
| Abengoa Bioenergy | France | Corn | Ethanol, biodiesel | NA |
| Abengoa Bioenergy | France | Corn | Ethanol, biodiesel | NA |
| Crop Energies | Germany | Sugarcane | Ethanol | 750,000 |
NA, not available.
7. Economic Aspects Relating to Biohydrogen and Its Associated Products
While there is a plethora of studies that prove that it is economically feasible to produce bioH2 from various feedstocks, including wastewaters [161,162,163,164], the economic studies elucidating the synthesis of high-value-added products using bioH2 effluents as a carbon source are still scarce in the literature. Only a few studies have been undertaken in order to ascertain the economic aspects relating to the use of acidogenic VFAs in other bioprocesses via the biorefinery strategy. For instance, a techno-economic evaluation study by Ladakis et al. [165] showed that it was economically viable to produce succinic acid, lipids, proteins, and biopolymers using acidogenic effluents via a biorefinery approach. Kwan et al. [166] showed that it was profitable to produce lactic acid, lactide, and poly-lactic acid from acidogenic VFAs derived. All these biobased products were economically viable, with lactic acid having a higher net profit of >USD 2 million per annum and the shortest payback period of 5 years. Mahmod et al. [167] conducted an economic profitability assessment of a two-stage anaerobic process involving bioH2 and BioCH4 using palm oil mill effluent. The results showed a payback period of 8 years, a return-on-investment rate of 19%, and a net profit of more than USD 400,000. Therefore, these preliminary studies show the importance of integrating bioH2 within the circular bioeconomy, as this can result in the production of a wide spectrum of valuable products. These studies coincide with the literature, as 50% of the overall costs are associated with the feedstock.
In addition, the circular bioeconomy is seen as a novel approach that could be used to unlock new value chains for economic growth and industrial expansion while fulfilling the United Nations’ Development Goals, which relate to climate change, food insecurity, energy insecurity, resource depletion, and other anthropogenic issues [168,169,170,171,172,173,174,175,176]. This can also promote a synergistic/beneficial phenomenon known as “industrial symbiosis”, whereby the unwanted biowaste streams produced by biomass sectors are used as a valuable feedstock by biomass valorization industries. This will, in turn, reduce the financial burden incurred by industries regarding the management of their biowastes.
8. Conclusions and Suggestions for Future Research
Research that is tailored toward renewable and sustainable energy has experienced significant growth over the last two decades as the global community aims to reduce the high dependency on fossil-based technologies. Microbial biorefineries will play a significant role in the advancement of a circular bioeconomy due to their ability to convert different biomass feedstocks, particularly organic residues, into multiple value-added compounds such as biofuels and biochemicals. The acidogenic-based microbial refineries also hold a huge potential in this field, as confirmed by the pilot-scale demonstrations. These things considered, VFAs from bio-H2 will assist in alleviating the high production costs in microbial-oriented biorefineries as these building blocks are seen as waste and can be directly used as feedstocks in other microbial biorefineries. Studies that focus on the beneficiation of biohydrogen-spent effluents are scarce in the literature, especially in the form of a review article. It is hoped that this work will serve as the solid foundation upon which further research can be undertaken in the field of microbial biorefineries targeting the synthesis of valuable compounds such as clean fuels, chemicals, and polymers, as shown in this work.
This review demonstrates the use of biomass-derived/acidogenic VFAs as building blocks or chemical precursors for the biosynthesis of industrially-relevant products (biofuels and platforms chemicals), as shown in Section 5. It explicates the key setpoint parameters that affect the recovery/extraction of these compounds in acidogenic fermentations. The papers also explore the recent advances that have been accomplished using acidogenic VFAs, particularly the development of pilot-scale acidogenic-based microbial systems. As mentioned earlier, the use of these precursors is new in microbial biorefineries as most of these systems still rely on edible crops or solid wastes. Therefore, there are many knowledge gaps in this research field, and some of the following recommendations are proposed for future studies:
- It is imperative to acquire deeper insights into the various microbial assemblages when using mixed cultures during the acidogenic biohydrogen fermentations, as this will help in the cultivation of the dominant VFA-producing biocatalysts.
- It is crucial to understand the nutrient-rich substrates alongside the optimal bioprocess conditions (especially the synergistic or antagonistic interactions of these setpoint variables), as this will lead to the optimal VFA recovery.
- Conducting pilot-scale studies using acidogenic VFAs as a sole carbon source will also assist in ascertaining the most suitable process dynamics.
- Acidogenic microbial-based biorefineries could be implemented with newly developed biochemical engineering tools, such as consolidated bioprocessing, in-vitro synthetic biology, and novel enzymes, in order to create biosynthetic pathways that will allow the biomanufacturing of multiple bio-based compounds.
Author Contributions
Conceptualization, P.T.S.; writing—original draft preparation, P.T.S.; writing—review and editing, O.E. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Council for Scientific and Industrial Research—Biorefinery Industry Development Facility (CSIR-BIDF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramchuran, S.O.; O’Brien, F.; Dube, N.; Ramdas, V. An overview of green processes and technologies, biobased chemicals and products for industrial applications. Curr. Opin. Green Sustain. Chem. 2023, 41, 100832. [Google Scholar] [CrossRef]
- Kora, E.; Patrinou, V.; Antonopoulou, G.; Ntaikou, I.; Tekerlekopoulou, A.G.; Lyberatos, G. Dark fermentation of expired fruit juices for biohydrogen production followed by treatment and biotechnological exploitation of effluents towards bioplastics and microbial lipids. Biochem. Eng. J. 2023, 195, 108901. [Google Scholar] [CrossRef]
- Pereira, A.S.; Lopes, M.; Duarte, M.S.; Alves, M.M.; Belo, I. Integrated bioprocess of microbial lipids production in Yarrowia lipolytica using food-waste derived volatile fatty acids. Ren. Energy 2023, 202, 1470–1478. [Google Scholar] [CrossRef]
- Sarkar, O.; Matsakas, L.; Rova, U.; Christakopoulos, P. Ultrasound-controlled acidogenic valorization of wastewater for biohydrogen and volatile fatty acids production: Microbial community profiling. iScience 2023, 26, 106519. [Google Scholar] [CrossRef] [PubMed]
- Perez-Almada, D.; Galán-Martín, Á.; del Mar Contreras, M.; Castro, E. Integrated techno-economic and environmental assessment of biorefineries: Review and future research directions. Sustain. Energy Fuels 2023, in press. [Google Scholar] [CrossRef]
- Lee, H.; Sohn, Y.J.; Jeon, S.; Yang, H.; Son, J.; Kim, Y.J.; Park, S.J. Sugarcane wastes as microbial feedstocks: A review of the biorefinery framework from resource recovery to production of value-added products. Bioresour. Technol. 2023, 376, 128879. [Google Scholar] [CrossRef]
- Quaid, T.; Reza, T. COSMO-RS predictive screening of type 5 hydrophobic deep eutectic solvents for selective platform chemicals absorption. J. Mol. Liq. 2023, 382, 121918. [Google Scholar] [CrossRef]
- Mineo, A.; Cosenza, A.; Mannina, G. Sewage sludge acidogenic fermentation for organic resource recovery towards carbon neutrality: An experimental survey testing the headspace influence. Bioresour. Technol. 2023, 367, 128217. [Google Scholar] [CrossRef]
- Tabibian, S.S.; Sharifzadeh, M. Statistical and analytical investigation of methanol applications, production technologies, value-chain and economy with a special focus on renewable methanol. Ren. Sustain. Energy Rev. 2023, 179, 113281. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; González-Fernández, C.; Greses, S.; Kennes, C.; Otero-Logilde, N.; Veiga, M.C.; Bolzonella, D.; Müller, B.; Passoth, V. Production of short-chain fatty acids (SCFAs) as chemicals or substrates for microbes to obtain biochemicals. Biotechnol. Biofuels 2023, 16, 96. [Google Scholar] [CrossRef]
- BCC Research, 2019. Oleochemical Fatty Acids: Global Markets to 2023. Available online: https://www.bccresearch.com/market-research/chemicals/oleochemical-fatty-acids-global-markets.html (accessed on 20 April 2022).
- Turon, V.; Trably, E.; Fouilland, E.; Steyer, J.-P. Potentialities of dark fermentation effluents as substrates for microalgae growth: A review. Process Biochem. 2016, 51, 1843–1854. [Google Scholar] [CrossRef]
- Rama, C.; Rani, P.; Kumar, A. Recent developments in biohydrogen production from wastewater: A review. Biocatal. Biotrans. 2023, 1–18, in press. [Google Scholar] [CrossRef]
- Badawi, E.Y.; Elkharsa, R.A.; Abdelfattah, E.A. Value proposition of bio-hydrogen production from different biomass sources. Energy Nexus 2023, 10, 100194. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Sillero, L.; Forster-Carneiro, T.; Solera, R.; Perez, M. Determination of Anaerobic Co-fermentation of Brewery Wastewater and Brewer’s Spent Grains for Bio-hydrogen Production. Bioenergy Res. 2023, 16, 1073–1083. [Google Scholar] [CrossRef]
- Dahiya, S.; Lingam, Y.; Venkata Mohan, S. Understanding acidogenesis towards green hydrogen and volatile fatty acid production—Critical analysis and circular economy perspective. Chem. Eng. J. 2023, 464, 141550. [Google Scholar] [CrossRef]
- Sanchez-Ledesma, L.M.; Ramírez-Malule, H.; Rodríguez-Victoria, J.A. Volatile Fatty Acids Production by Acidogenic Fermentation of Wastewater: A Bibliometric Analysis. Sustainability 2023, 15, 2370. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Wang, Z.; Wu, D.; Xing, T.; Kong, X.; Sun, Y. Impact of pH, temperature, and hydraulic residence time on the acidogenic fermentation of fruit and vegetable waste and microbial community analysis. J. Chem. Technol. Biotechnol. 2023, 98, 819–828. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, D.; Du, J.; Zhou, B.; Wang, D.; Liu, X.; Yan, C.; Liang, J.; Zhou, L. Enhancing propionic acid production in the acidogenic fermentation of food waste facilitated by a fungal mash under neutral pH. J. Environ. Manag. 2023, 327, 116901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, M.; Mou, H.; An, Z.; Fu, H.; Su, X.; Chen, C.; Chen, J.; Lin, H.; Sun, F. Comparation of mesophilic and thermophilic anaerobic co-digestion of food waste and waste activated sludge driven by biochar derived from kitchen waste. J. Clean. Product. 2023, 408, 137123. [Google Scholar] [CrossRef]
- Jiang, M.; Qiao, W.; Wang, Y.; Zou, T.; Lin, M.; Dong, R. Balancing acidogenesis and methanogenesis metabolism in thermophilic anaerobic digestion of food waste under a high loading rate. Sci. Total Environ. 2022, 824, 153867. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Sun, Y.; Li, Y. Bioaugmentation improves batch psychrophilic anaerobic co-digestion of cattle manure and corn straw. Bioresour. Technol. 2022, 343, 126118. [Google Scholar] [CrossRef] [PubMed]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Sanjaya, A.; Mondylaksita, K.; Millati, R.; Budhijanto, W. Evaluation of volatile fatty acids (VFAs) production in thermophilic and mesophilic anaerobic digestion of oil palm empty fruit bunch (OPEFB). Conf. Ser. Earth Environ. Sci. 2022, 963, 012049. [Google Scholar] [CrossRef]
- Forrest, A.K.; Hernandez, J.; Holtzapple, M.T. Effects of temperature and pretreatment conditions on mixed-acid fermentation of water hyacinths using a mixed culture of thermophilic microorganisms. Bioresour. Technol. 2010, 101, 7510–7515. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luo, G.; Wang, W.; He, Y.; Zhang, R.; Liu, G. The effects of pH and temperature on the acetate production and microbial community compositions by syngas fermentation. Fuel 2018, 224, 537–544. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, R.; Liu, F.; Yong, X.; Wu, X.; Zheng, T.; Jiang, M.; Jia, H. Biogas production and microbial community shift through neutral pH control during the anaerobic digestion of pig manure. Bioresour. Technol. 2016, 217, 44–49. [Google Scholar] [CrossRef]
- Kumari, S.; Das, D. Improvement of biohydrogen production using acidogenic culture. Int. J. Hydrogen Energy 2017, 42, 4083–4094. [Google Scholar] [CrossRef]
- Marra, L.M.; de Oliveira-Longatti, S.M.; Soares, C.R.; de Lima, J.M.; Olivares, F.L.; Moreira, F.M. Initial pH of medium affects organic acids production but do not affect phosphate solubilization. Braz. J. Microbiol. 2015, 46, 367–375. [Google Scholar] [CrossRef]
- Luo, G.; Jing, Y.; Lin, Y.; Zhang, S.; An, D. A novel concept for syngas biomethanation by two-stage process: Focusing on the selective conversion of syngas to acetate. Sci. Total Environ. 2018, 645, 194–1200. [Google Scholar] [CrossRef]
- Yin, D.M.; Mahboubi, A.; Wainaina, S.; Qiao, W.; Taherzadeh, M.J. The effect of mono- and multiple fermentation parameters on volatile fatty acids (VFAs) production from chicken manure via anaerobic digestion. Bioresour. Technol. 2021, 330, 124992. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Kiela, P.R.; Salamon, A.; Barberán, A.; Chen, Y.; Yang, F.; Błaszczyk, M.K.; Sikora, A. Dynamics of dark fermentation microbial communities in the light of lactate and butyrate production. Microbiome 2021, 9, 158. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef]
- Hao, T.; Xiao, Y.; Varjani, S. Transiting from the inhibited steady-state to the steady-state through the ammonium bicarbonate mediation in the anaerobic digestion of low-C/N-ratio food wastes. Bioresour. Technol. 2022, 351, 127046. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, H.; Zhang, L.; Yang, M.; Fu, B.; Liu, H. Novel insight into the relationship between organic substrate composition and volatile fatty acids distribution in acidogenic co-fermentation. Biotechnol. Biofuels 2017, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, L.; Loh, K.C. Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresour. Bioprocess 2021, 8, 68. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Chen, Y.; Du, G.; Chen, J. Effects of organic matter and initial carbon-nitrogen ratio on the bioconversion of volatile fatty acids from sewage sludge. J Chem. Technol. Biotechnol. 2008, 83, 1049–1055. [Google Scholar] [CrossRef]
- Paranjpe, A.; Saxena, S.; Jain, P. Biogas yield using single and two stage anaerobic digestion: An experimental approach. Energy Sustain. Develop. 2023, 74, 6–19. [Google Scholar] [CrossRef]
- Franceschi, F.F.; Acosta-González, A.; Vega, L.T.; Gomez, M.F. Improving dry anaerobic methane production from OFMSW by co-digestion with grass waste and pretreatment with white rot fungi. Energy Sustain. Dev. 2023, 74, 6–19. [Google Scholar] [CrossRef]
- Zou, X.; Mengjiao, G.; Mohammed, A.; Liu, Y. Responses of various carbon to nitrogen ratios to microbial communities, kinetics, and nitrogen metabolic pathways in aerobic granular sludge reactor. Bioresour. Technol. 2023, 367, 128225. [Google Scholar] [CrossRef]
- De Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Selecting fermentation products for food waste valorisation with HRT and OLR as the key operational parameters. Waste Manag. 2021, 127, 80–89. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, B.J.; Jeong, C.M.; Choi, J.D.; Ahn, Y.H.; Chang, H.N. Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour. Technol. 2008, 99, 7866–7874. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Farouk, R.Y.; Mostafa, E.; Wang, Y. Evaluation of hydrogen and volatile fatty acids production system from food waste. Biomass Conv. Bioref. 2023, 13, 5253–5259. [Google Scholar] [CrossRef]
- Tsegaye, D.; Khan, M.; Leta, S. Optimization of Operating Parameters for Two-Phase Anaerobic Digestion Treating Slaughterhouse Wastewater for Biogas Production: Focus on Hydrolytic—Acidogenic Phase. Sustainability 2023, 15, 5544. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Visvanathan, C.; Abeynayaka, A. Effect of organic loading rate on VFA production, organic matter removal and microbial activity of a two-stage thermophilic anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 5353–5360. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar] [CrossRef] [PubMed]
- Ünyay, H.; Yılmaz, F.; Başar, I.A.; Perendeci, N.A.; Çoban, I.; Şahinkaya, E. Effects of organic loading rate on methane production from switchgrass in batch and semi-continuous stirred tank reactor system. Biomass Bioenergy 2022, 156, 106306. [Google Scholar] [CrossRef]
- Zou, X.; Wang, Y.; Dai, Y.; Zhou, S.; Wang, B.; Li, Y.; Li, J. Batch and semi-continuous experiments examining the sludge mesophilic anaerobic digestive performance with different varieties of rice straw. Bioresour. Technol. 2022, 346, 126651. [Google Scholar] [CrossRef]
- Yakaboylu, O.; Albrecht, I.; Harinck, J.; Smit, K.G.; Georgios-Archimidis, T.; Marcello, M.; de Jong, W. Supercritical water gasification of biomass in fluidized bed: First results and experiences obtained from TU Delft/Gensos semi-pilot scale setup. Biomass Bioenergy 2018, 111, 330–342. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Selvam, A.; Wong, J.W. Hydrolysis-acidogenesis of food waste in solid-liquid-separating continuous stirred tank reactor (SLS-CSTR) for volatile organic acid production. Bioresour. Technol. 2016, 200, 366–373. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Rohini, K.B.; Gunasekaran, M.; Gokulakrishnan, K.; Kumar, G.; Banu, J.R. Prediction of effective substrate concentration and its impact on biogas production using Artificial Neural Networks in Hybrid Upflow anaerobic Sludge Blanket reactor for treating landfill leachate. Fuel 2022, 313, 122697. [Google Scholar] [CrossRef]
- Safari, M.; Tondro, H.; Zilouei, H. Biohydrogen production from diluted-acid hydrolysate of rice straw in a continuous anaerobic packed bed biofilm reactor. Int. J. Hydrogen Energy 2022, 47, 5879–5890. [Google Scholar] [CrossRef]
- Hanvajanawong, K.; Suyamud, B.; Suwannasilp, B.B.; Lohwacharin, J.; Visvanathan, C. Unravelling capability of two-stage thermophilic anaerobic membrane bioreactors for high organic loading wastewater: Effect of support media addition and irreversible fouling. Bioresour. Technol. 2022, 348, 126725. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, K.; Terpou, A.; Treu, L.; Kougias, P.G.; Kornaros, M. Thermophilic anaerobic digestion of olive mill wastewater in an upflow packed bed reactor: Evaluation of 16S rRNA amplicon sequencing for microbial analysis. J. Environ. Manag. 2022, 301, 113853. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Becarelli, S.; Pecorini, I.; Di Gregorio, S.; Iannelli, R. Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste in Plug-Flow Reactors: Focus on Bacterial Community Metabolic Pathways. Water 2022, 14, 195. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Gassara, F.; Brar, S.K. Nanoparticles production and role in biotransformation. J. Nanosci. Nanotechnol. 2011, 11, 899–918. [Google Scholar] [CrossRef]
- Montalvo, S.; Vielma, S.; Borja, R.; Huiliñir, C.; Guerrero, L. Increase in biogas production in anaerobic sludge digestion by combining aerobic hydrolysis and addition of metallic wastes. Renew. Energy 2018, 123, 541–548. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Shei, S.-H. Heavy metal effects on fermentative hydrogen production using natural mixed microflora. Int. J. Hydrogen Energy 2008, 33, 587–593. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Li, H.; Shi, B.; Xu, Y.; Liu, J.; Zhang, D.; Tang, J.; Hou, P. Effect of temperature on fermentative VFAs production from waste sludge stimulated by riboflavin and the shifts of microbial community. Water Sci. Technol. 2022, 85, 1191–1201. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Shahbaz, A.; Hussain, N.; Saleem, M.Z.; Saeed, M.U.; Bilal, M.; Iqbal, H.M.N. Nanoparticles as stimulants for efficient generation of biofuels and renewables. Fuel 2022, 319, 123724. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, H. Use of magnetic nanoparticles to enhance bioethanol production in syngas fermentation. Bioresour. Technol. 2016, 204, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Bian, Y.; Dong, L.; Zheng, X.; Chen, Y. Long-term effects of copper nanoparticles on volatile fatty acids production from sludge fermentation: Roles of copper species and bacterial community structure. Bioresour. Technol. 2022, 348, 126789. [Google Scholar] [CrossRef]
- Pramanik, N. A tool for biomedical application: Synthesis and modification of polyhydroxyalkanoates. Sustain. Chem. Pharm. 2023, 32, 101041. [Google Scholar] [CrossRef]
- Alves, A.A.; Siqueira, E.C.; Barros, M.P.S.; Silva, P.E.C.; Houllou, L.M. Polyhydroxyalkanoates: A review of microbial production and technology application. Int. J. Environ. Sci. Technol. 2023, 20, 3409–3420. [Google Scholar] [CrossRef]
- Rajvanshi, J.; Sogani, M.; Kumar, A.; Arora, S.; Syed, Z.; Sonu, K.; Gupta, N.S.; Kalra, A. Perceiving biobased plastics as an alternative and innovative solution to combat plastic pollution for a circular economy. Sci. Tot. Environ. 2023, 874, 162441. [Google Scholar] [CrossRef]
- Marreiros, B.C.; Carvalheira, M.; Henriques, C.; Pequito, D.; Nguyen, Y.; Solstad, R.G.; Eksteen, J.J.; Reis, M.A.M. Pilot-scale valorisation of salmon peptone into polyhydroxyalkanoates by mixed microbial cultures under conditions of high ammonia concentration. J. Environ. Chem. Eng. 2023, 11, 110100. [Google Scholar] [CrossRef]
- Zhou, W.; Bergsma, S.; Colpa, D.I.; Euverink, G.-J.W. Polyhydroxyalkanoates (PHAs) synthesis and degradation by microbes and applications towards a circular economy. J. Environ. Manag. 2023, 341, 118033. [Google Scholar] [CrossRef]
- Montiel-Corona, V.; Buitrón, G. Polyhydroxyalkanoates from organic waste streams using purple non-sulfur bacteria. Bioresour. Technol. 2021, 323, 124610. [Google Scholar] [CrossRef]
- Carlozzi, P.; Giovannelli, A.; Traversi, M.L.; Touloupakis, E. Poly(3-hydroxybutyrate) bioproduction in a two-step sequential process using wastewater. J. Water Process Eng. 2021, 39, 101700. [Google Scholar] [CrossRef]
- Cavaliere, C.; Capriotti, A.L.; Cerrato, A.; Lorini, L.; Montone, C.M.; Valentino, F.; Laganà, A.; Majone, M. Identification and Quantification of Polycyclic Aromatic Hydrocarbons in Polyhydroxyalkanoates Produced from Mixed Microbial Cultures and Municipal Organic Wastes at Pilot Scale. Molecules 2021, 26, 539. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, J.-I.; Han, K.; Song, J.Y. Removal of endotoxin during purification of poly(3-hydroxybutyrate) from gram-negative bacteria. Appl. Environ. Microbiol. 1999, 65, 2762–2764. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Matos, M.; Pereira, B.; Ralo, C.; Pequito, D.; Marques, N.; Carvalho, G.; Reis, M.A.M. An integrated process for mixed culture production of 3-hydroxyhexanoate-rich polyhydroxyalkanoates from fruit waste. Chem. Eng. J. 2022, 427, 131908. [Google Scholar] [CrossRef]
- Saratale, G.R.; Cho, S.K.; Saratale, D.G.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, N.R.; Kumar, G.; Kim, S.D.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Singh Patel, S.K.; Shanmugam, R.; Lee, J.K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef] [PubMed]
- Meylani, V.; Surahman, E.; Fudholi, A.; Almalki, W.H.; Ilyas, N.; Sayyed, R.Z. Biodiversity in microbial fuel cells: Review of a promising technology for wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 109503. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Zhang, Y.; Guo, Q.; Hu, T.; Xiao, H.; Lu, W.; Jia, J. Progress on anodic modification materials and future development directions in microbial fuel cells. J. Power Sources 2023, 556, 232486. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ng, K.H.; Papadopoulos, A.M.; Le, A.T.; Kumar, S.; Hadiyanto, H.; Pham, V.V. Microbial fuel cells for bioelectricity production from waste as sustainable prospect of future energy sector. Chemosphere 2022, 287, 132285. [Google Scholar] [CrossRef]
- Greenman, J.; Mendis, B.A.; Gajda, I.; Ieropoulos, I.A. Microbial fuel cell compared to a chemostat. Chemosphere 2022, 296, 133967. [Google Scholar] [CrossRef]
- Ramya, M.; Kumar, S.P. A review on recent advancements in bioenergy production using microbial fuel cells. Chemosphere 2022, 288, 132512. [Google Scholar] [CrossRef]
- Mohyudin, S.; Farooq, R.; Jubeen, F.; Rasheed, T.; Fatima, M.; Sher, F. Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ. Res. 2022, 204, 112387. [Google Scholar] [CrossRef]
- Uduma, R.C.; Oguzie, K.L.; Chijioke, C.F.; Ogbulie, T.E.; Oguzie, E.E. Bioelectrochemical technologies for simultaneous treatment of dye wastewater and electricity generation: A review. Int. J. Environ. Sci. Technol. 2023, in press. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Mohan, S.V.; Sarma, P.N. Utilizing acid-rich effluents of fermentative hydrogen production process as substrate for harnessing bioelectricity: An integrative approach. Int. J. Hydrogen Energy 2010, 35, 3440–3449. [Google Scholar] [CrossRef]
- Asefi, B.; Li, S.-L.; Moreno, H.A.; Sanchez-Torres, V.; Hu, A.; Li, J.; Yu, C.-P. Characterization of electricity production and microbial community of food waste-fed microbial fuel cells. Process Saf. Environ. Protect. 2019, 125, 83–91. [Google Scholar] [CrossRef]
- Freguia, S.; The, E.H.; Boon, N.; Leung, K.M.; Keller, J.; Rabaey, K. Microbial fuel cells operating on mixed fatty acids. Bioresour. Technol. 2010, 101, 1233–1238. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.J.; Lee, J.W.; Kim, K.Y.; Kim, I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Khater, D.Z.; El-Khatib, K.M.; Hassan, H.M. Microbial diversity structure in acetate single chamber microbial fuel cell for electricity generation. J. Genet. Eng. Biotechnol. 2017, 15, 127–137. [Google Scholar] [CrossRef]
- Escapa, A.; Mateos, R.; Martinez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Ren. Sustain. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
- Gkotsis, P.; Kougias, P.; Mitrakas, M.; Zouboulis, A. Biogas upgrading technologies—Recent advances in membrane-based processes. Int. J. Hydrogen Energy 2023, 48, 3965–3993. [Google Scholar] [CrossRef]
- Kougias, P.G.; Angelidaki, I. Biogas and its opportunities-A review. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Kornaros, M. Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies 2021, 14, 5423. [Google Scholar] [CrossRef]
- Baldi, F.; Pecorini, I.; Iannelli, R. Comparison of single-stage and two-stage anaerobic co-digestion of food waste and activated sludge for hydrogen and methane production. Renew. Energy 2019, 143, 1755–1765. [Google Scholar] [CrossRef]
- Sasidhar, K.B.; Kumar, P.S.; Xiao, L. A critical review on the two-stage biohythane production and its viability as a renewable fuel. Fuel 2022, 317, 123449. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Rao, R.; Basak, N. Process optimization and mathematical modelling of photo-fermentative hydrogen production from dark fermentative cheese whey effluent by Rhodobacter sphaeroides O.U.001 in 2-L cylindrical bioreactor. Biomass Conv. Bioref. 2023, 13, 3929–3952. [Google Scholar] [CrossRef]
- Chang, H.; Feng, H.; Wang, R.; Zhang, X.; Wang, J.; Li, C.; Zhang, Y.; Li, L.; Ho, S.-H. Enhanced energy recovery from landfill leachate by linking light and dark bio-reactions: Underlying synergistic effects of dual microalgal interaction. Water Res. 2023, 231, 119578. [Google Scholar] [CrossRef]
- Moreno, R.; Escapa, A.; Cara, J.; Carracedo, B.; Gómez, X. A two-stage process for hydrogen production from cheese whey: Integration of dark fermentation and biocatalyzed electrolysis. Int. J. Hydrogen Energy 2015, 40, 168–175. [Google Scholar] [CrossRef]
- Phan, T.P.; Ta, Q.T.H.; Nguyen, P.K.T. Maximizing performance of microbial electrolysis cell fed with dark fermentation effluent from water hyacinth. Int. J. Hydrogen Energy 2023, 48, 5447–5462. [Google Scholar] [CrossRef]
- Attia, Y.A.; Samer, M.; Moselhy, M.A.; Arisha, A.H.; Abdelqader, A.A.; Abdelsalam, E.E. Influence of laser photoactivated graphitic carbon nitride nanosheets and nickel nanoparticles on purple non-sulfur bacteria for biohydrogen production from biomass. J. Clean. Prod. 2021, 299, 126898. [Google Scholar] [CrossRef]
- Brar, K.K.; Cortez, A.A.; Pellegrini, V.O.A.; Amulya, K.; Polikarpov, I.; Magdouli, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K.; Brar, S.K. An overview on progress, advances, and future outlook for biohydrogen production technology. Int. J. Hydrogen Energy 2022, 47, 37264–37281. [Google Scholar] [CrossRef]
- Mirza, S.S.; Qazi, J.I.; Liang, Y.; Chen, S. Growth characteristics and photofermentative biohydrogen production potential of purple non sulfur bacteria from sugar cane bagasse. Fuel 2019, 255, 115805. [Google Scholar] [CrossRef]
- Uyar, B.; Eroglu, I.; Yücel, M.; Gündüz, U. Photofermentative hydrogen production from volatile fatty acids present in dark fermentation effluents. Int. J. Hydrogen Energy 2009, 34, 4517–4523. [Google Scholar] [CrossRef]
- Su, H.; Cheng, J.; Zhou, J.; Song, W.; Cen, K. Combination of dark- and photo-fermentation to enhance hydrogen production and energy conversion efficiency. Int. J. Hydrogen Energy 2009, 34, 8846–8853. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Waste-to-energy nexus for circular economy and environmental protection: Recent trends in hydrogen energy. Sci. Total Environ. 2020, 713, 136633. [Google Scholar] [CrossRef]
- Mutanda, T.; Naidoo, D.; Bwapwa, J.K.; Anandraj, A. Biotechnological Applications of Microalgal Oleaginous Compounds: Current Trends on Microalgal Bioprocessing of Products. Front. Energy Res. 2020, 17, 1–21. [Google Scholar] [CrossRef]
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production-A review. Bioresour. Technol. 2017, 243, 1194–1206. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Kong, F.; Zhao, L.; Ma, J.; Ren, N.-Q. Favorable energy conversion efficiency of coupling dark fermentation and microalgae production from food wastes. Energy Conv. Manag. 2018, 165, 156–162. [Google Scholar] [CrossRef]
- Tuna, E.; Kargi, F.; Argun, H. Hydrogen gas production by electrohydrolysis of volatile fatty acid (VFA) containing dark fermentation effluent. Int. J. Hydrogen Energy 2009, 34, 262–269. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Hua, T.; Li, S.; Li, F.; Zhou, Q.; Ondon, B.S. Microbial electrolysis cell as an emerging versatile technology: A review on its potential application, advance and challenge. J. Chem. Technol. Biotechnol. 2009, 94, 1697–1711. [Google Scholar] [CrossRef]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically assisted microbial production of hydrogen from acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a pilot scale microbial electrolysis cell fed on domestic wastewater at ambient temperatures for a 12 month period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Reid, R.; Hussain, A.; An, J.; Lee, H.S. Hydrogen peroxide production in a pilot-scale microbial electrolysis cell. Biotechnol. Rep. 2018, 19, e00276. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wu, M.; Jiang, Y.; Wang, H. Effects of different temperatures and pH values on volatile fatty acids production during codigestion of food waste and thermal-hydrolysed sewage sludge and subsequent volatile fatty acids for polyhydroxyalkanoates production. Bioresour. Technol. 2021, 333, 125149. [Google Scholar] [CrossRef]
- Pang, J.; He, J.; Ma, Y.; Pan, X.; Zheng, Y.; Yu, H.; Yan, Z.; Nan, J. Enhancing volatile fatty acids production from waste activated sludge by a novel cation-exchange resin assistant strategy. J. Clean. Prod. 2021, 278, 123236. [Google Scholar] [CrossRef]
- Xin, X.; She, Y.; Hong, J. Insights into microbial interaction profiles contributing to volatile fatty acids production via acidogenic fermentation of waste activated sludge assisted by calcium oxide pretreatment. Bioresour. Technol. 2021, 320, 124287. [Google Scholar] [CrossRef]
- Valentino, F.; Munarin, G.; Biasiolo, M.; Cavinato, C.; Bolzonella, D.; Pavan, P. Enhancing volatile fatty acids (VFA) production from food waste in a two-phases pilot-scale anaerobic digestion process. J. Environ. Chem. Eng. 2021, 9, 106062. [Google Scholar] [CrossRef]
- Pourbayramian, R.; Abdi-Benemar, H.; Seifdavati, J.; Greiner, R.; Elghandour, M.M.M.Y.; Salem, A.Z.M. Bioconversion of potato waste by rumen fluid from slaughterhouses to produce a potential feed additive rich in volatile fatty acids for farm animals. J. Clean. Prod. 2021, 280, 124411. [Google Scholar] [CrossRef]
- Weide, T.; Brügging, E.; Wetter, C.; Ierardi, A.; Wichern, M. Use of organic waste for biohydrogen production and volatile fatty acids via dark fermentation and further processing to methane. Int. J. Hydrogen Energy 2019, 44, 24110–24125. [Google Scholar] [CrossRef]
- Abderrahim, B.; Diaz, Y.; Martinez, M.; Aracil, J. Pilot plant studies of biodiesel production using Brassica carinata as raw material. Catal. Today 2005, 106, 193–196. [Google Scholar]
- Soccol, C.R.; Dalmas Neto, C.J.; Soccol, V.T.; Sydney, E.B.; da Costa, E.S.F.; Medeiros, A.B.P.; Vandenberghe, L.P.S. Pilot scale biodiesel production from microbial oil of Rhodosporidium toruloides DEBB 5533 using sugarcane juice: Performance in diesel engine and preliminary economic study. Bioresour. Technol. 2017, 223, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.C.; Kuroki, A.; Dai, Y.; Tong, Y.W. Microbial biodiesel production from industrial organic wastes by oleaginous microorganisms: Current status and prospects. J. Hazard Mat. 2021, 402, 123543. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.D.; Kim, N.; Kang, J. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.S.; Lopes, M.; Miranda, S.M.; Belo, I. Bio-oil production for biodiesel industry by Yarrowia lipolytica from volatile fatty acids in two-stage batch culture. Appl. Microbiol. Biotechnol. 2022, 106, 2869–2881. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N.; Fernandez-Castane, A.; Oleskowicz-Popiel, P. Production of microbial lipids utilizing volatile fatty acids derived from wastepaper: A biorefinery approach for biodiesel production. Fuel 2020, 276, 118087. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef]
- Stylianou, E.; Pateraki, C.; Ladakis, D.; Cruz-Fernández, M.; Latorre-Sánchez, M.; Coll, C.; Koutinas, A. Evaluation of organic fractions of municipal solid waste as renewable feedstock for succinic acid production. Biotechnol. Biofuels 2020, 13, 72. [Google Scholar] [CrossRef]
- Yankov, D. Fermentative Lactic Acid Production from Lignocellulosic Feedstocks: From Source to Purified Product. Front. Chem. 2022, 10, 823005. [Google Scholar] [CrossRef]
- Dhillon, S.G.; Brar, K.S.; Verma, M.; Tyagi, R.D. Recent Advances in Citric Acid Bio-Production and Recovery. Food Biop. Technol. 2011, 4, 505–529. [Google Scholar] [CrossRef]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Zhang, P.; Ye, J.; Liang, J.; Nabi, M.; Zhou, Z.; Tao, X.; Chen, N.; Sun, K.; et al. Biological nutrient removal and recovery from solid and liquid livestock manure: Recent advance and perspective. Bioresour. Technol. 2020, 301, 122823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, Y.; Chen, Y. Advanced nitrogen and phosphorus removal in A2O-BAF system treating low carbon-to-nitrogen ratio domestic wastewater. Front. Environ. Sci. Eng. China 2011, 5, 474. [Google Scholar] [CrossRef]
- Liu, S.; Daigger, G.T.; Liu, B.; Zhao, W.; Liu, J. Enhanced performance of simultaneous carbon, nitrogen and phosphorus removal from municipal wastewater in an anaerobic-aerobic-anoxic sequencing batch reactor (AOA-SBR) system by alternating the cycle times. Bioresour. Technol. 2020, 301, 122750. [Google Scholar] [CrossRef]
- Zhu, G.; Peng, Y.; Li, B.; Guo, J.; Yang, Q.; Wang, S. Biological removal of nitrogen from wastewater. Rev. Environ. Cont. Toxicol. 2008, 192, 159–195. [Google Scholar]
- Wang, J.; Li, Z.; Wang, Q.; Lei, Z.; Yuan, T.; Shimizu, K.; Zhang, Z.; Adachi, Y.; Lee, D.J.; Chen, R. Achieving stably enhanced biological phosphorus removal from aerobic granular sludge system via phosphorus rich liquid extraction during anaerobic period. Bioresour. Technol. 2022, 346, 126439. [Google Scholar] [CrossRef]
- Lim, S.-J.; Choi, D.W.; Lee, W.G.; Kwon, S.; Chang, H.N. Volatile fatty acids production from food wastes and its application to biological nutrient removal. Bioprocess Eng. 2000, 22, 543–545. [Google Scholar] [CrossRef]
- Sun, J.; Song, J.; Fang, W.; Cao, H. Enhanced nitrogen removal upon the addition of volatile fatty acids from activated sludge by combining calcium peroxide and low-thermal pretreatments. J. Environ. Sci. 2021, 108, 145–151. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Y.; Zhao, H.; Peng, H. Nitrogen removal during anaerobic digestion of wasted activated sludge under supplementing Fe(III) compounds. Chem. Eng. J. 2018, 332, 711–716. [Google Scholar] [CrossRef]
- Stewart, R.D.; Bashar, R.; Amstadt, C.; Uribe-Santos, G.A.; McMahon, K.D.; Seib, M.; Noguera, D.R. Pilot-scale comparison of biological nutrient removal (BNR) using intermittent and continuous ammonia-based low dissolved oxygen aeration control systems. Water Sci. Technol. 2022, 85, 578–590. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.Y.; Ryu, H.D.; Min, K.K.; Lee, S.I. Long term operation of pilot-scale biological nutrient removal process in treating municipal wastewater. Bioresour. Technol. 2009, 100, 3180–3184. [Google Scholar] [CrossRef]
- Al-Mallahi, J.; Ishii, K. Attempts to alleviate inhibitory factors of anaerobic digestate for enhanced microalgae cultivation and nutrients removal: A review. J. Environ. Manag. 2022, 304, 114266. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Pivato, A. Sustainable Management of Digestate from the Organic Fraction of Municipal Solid Waste and Food Waste Under the Concepts of Back to Earth Alternatives and Circular Economy. Waste Biomass Val. 2019, 10, 465–481. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.K.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: A way forward through waste valorization approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, P.; Chen, Y.C.; Cao, Q.; Liu, X.F.; Li, D. The production of single cell protein from biogas slurry with high ammonia-nitrogen content by screened Nectaromyces rattus. Poult. Sci. 2021, 100, 101334. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhao, D.; Zhu, X.; Su, Y.; Angelidaki, I.; Zhang, Y. Biogas upgrading and valorization to single-cell protein in a bioinorganic electrosynthesis system. Chem. Eng. J. 2021, 426, 131837. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends Biotechnol. 2019, 37, 231–234. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Sravan, S.J.; Chatterjee, S.; Sarkar, O.; Venkata Mohan, V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, Z.; Chen, M.; Xiong, D. Pilot-Scale Anaerobic Treatment of Printing and Dyeing Wastewater and Performance Prediction Based on Support Vector Regression. Fermentation 2022, 8, 99. [Google Scholar] [CrossRef]
- Liu, H.; Han, P.; Liu, H.; Zhou, G.; Fu, B.; Zheng, Z. Full-scale production of VFAs from sewage sludge by anaerobic alkaline fermentation to improve biological nutrients removal in domestic wastewater. Bioresour. Technol. 2018, 260, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Tu, W.; Wu, M.; Zhang, Z.; Wang, H. Pilot-scale fermentation of urban food waste for volatile fatty acids production: The importance of pH. Bioresour. Technol. 2021, 332, 125116. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, C.; Conca, V.; Eusebi, A.L.; Frison, N.; Fatone, F. Sieving of municipal wastewater and recovery of bio-based volatile fatty acids at pilot scale. Water Res. 2020, 174, 115633. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-W.; Chung, J. Bioproduction of hydrogen from food waste by pilot-scale combined hydrogen/methane fermentation. Int. J. Hydrogen Energy 2020, 35, 11746–11755. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Gokhale, D.V. Lignocellulosic biomass: Hurdles and challenges in its valorization. Appl. Microbiol. Biotechnol. 2019, 103, 9305–9320. [Google Scholar] [CrossRef]
- Maleki, F.; Changizian, M.; Zolfaghari, N.; Rajaei, S.; Noghabi, K.A.; Zahiri, H.S. Consolidated bioprocessing for bioethanol production by metabolically engineered Bacillus subtilis strains. Sci. Rep. 2021, 11, 13731. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, X.; Li, Y.; Jiang, X.; Liu, L.; Wang, H. New technologies provide more metabolic engineering strategies for bioethanol production in Zymomonas mobilis. Appl. Microbiol. Biotechnol. 2019, 103, 2087–2099. [Google Scholar] [CrossRef]
- Hughes, S.R.; Jones, M.A. Green Energy to Sustainability: Strategies for Global Industries; Chapter 7; Wiley: Hoboken, NJ, USA, 2020; p. 145. [Google Scholar]
- The Bio-Based Industries Consortium. 2017. Available online: https://biconsortium.eu/news/mapping-european-biorefineries (accessed on 20 April 2022).
- Li, Y.-C.; Liu, Y.-F.; Chang, P.-L.; Hsu, C.-W.; Lin, P.-J.; Wu, S.-Y. Techno-economic evaluation of biohydrogen production from wastewater and agricultural waste. Int. J. Hydrogen Energy 2012, 37, 15704–15710. [Google Scholar] [CrossRef]
- Mutsvene, B.; Chetty, M.; Kumari, S.; Bux, F. Biohydrogen production from brewery wastewater in an Anaerobic baffled reactor. A preliminary techno-economic evaluation. S. Afr. J. Chem. Eng. 2023, 43, 9–23. [Google Scholar] [CrossRef]
- Han, W.; Liu, Z.; Fang, J.; Huang, J.; Zhao, H.; Li, Y. Techno-economic analysis of dark fermentative hydrogen production from molasses in a continuous mixed immobilized sludge reactor. J. Clean. Product. 2016, 127, 567–572. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Liu, Z.; Tang, J. Techno-economic evaluation of a combined bioprocess for fermentative hydrogen production from food waste. Bioresour. Technol. 2016, 202, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ladakis, D.; Stylianou, E.; Ioannidou, S.-M.; Koutinas, A.; Pateraki, C. Biorefinery development, techno-economic evaluation and environmental impact analysis for the conversion of the organic fraction of municipal solid waste into succinic acid and value-added fractions. Bioresour. Technol. 2022, 354, 127172. [Google Scholar] [CrossRef]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Techno-economic analysis of a food waste valorisation process for lactic acid, lactide and poly(lactic acid) production. J. Clean. Product. 2018, 181, 72–87. [Google Scholar] [CrossRef]
- Mahmod, S.S.; Jahim, J.; Abdul, P.M.; Luthfi, A.A.I.; Takriff, M.S. Techno-economic analysis of two-stage anaerobic system for biohydrogen and biomethane production from palm oil mill effluent. J. Environ. Chem. Eng. 2021, 9, 105679. [Google Scholar] [CrossRef]
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen production through dark fermentation: Recent trends and advances in transition to a circular bioeconomy. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Mahata, C.; Dhar, S.; Ray, S.; Das, D. Biohydrogen production from starchy wastewater in upflow anaerobic sludge blanket (UASB) reactor: Possibilities toward circular bioeconomy. Environ. Technol. Innov. 2023, 30, 103044. [Google Scholar] [CrossRef]
- Machineni, L.; Deepanraj, B.; Chew, K.W.; Rao, A.G. Biohydrogen production from lignocellulosic feedstock: Abiotic and biotic methods. Ren. Sustain. Energy. Rev. 2023, 182, 113344. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Rashid, R.; Hashim, Z.; Meng, C.C.; Lun, C.K.; Jumaatuden, D.M.H.; Yasin, N.A.; Jati, A.; Hassim, M. M Economic study on biohydrogen production from liquid pineapple waste. Clean Technol. Environ. Pol. 2023, 25, 703–716. [Google Scholar] [CrossRef]
- Goria, K.; Singh, H.M.; Singh, A.; Kothari, R.; Tyagi, V.V. Insights into biohydrogen production from algal biomass: Challenges, recent advancements and future directions. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Goveas, L.C.; Nayak, S.; Kumar, P.S.; Vinayagam, R.; Selvaraj, R.; Rangasamy, G. Recent advances in fermentative biohydrogen production. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Rene, E.R.; Khanongnuch, R.; Race, M.; Pugazhendhi, A. Eco-technologies for waste to energy conversion: Applying the concepts of cleaner production, circular economy, and biorefinery. Clean Technol. Environ. Pol. 2023, 25, 311–312. [Google Scholar] [CrossRef]
- Vinayak, V.; Sirotiya, V.; Khandelwal, P.; Rai, A.; Jadhav, D.A.; Pugazhendhi, A.; Schoefs, B.; Marchand, J.; Chae, K.-J. Recent trends in engineering algae for biohydrogen production: State of art strategies. Fuel 2023, 348, 128636. [Google Scholar] [CrossRef]
- Yang, E.; Chon, K.; Kim, K.Y.; Le, G.T.; Nguyen, H.Y.; Le, T.T.; Nguyen, H.T.; Jae, M.R.; Ahmad, I.; Oh, S.E.; et al. Pretreatments of lignocellulosic and algal biomasses for sustainable biohydrogen production: Recent progress, carbon neutrality, and circular economy. Bioresour. Technol. 2023, 369, 128380. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).