Comparison of Raspberry Ketone Production via Submerged Fermentation in Different Bioreactors
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Microorganism and Cultivation
2.3. Experimental Design
2.4. Flask Culture
2.5. Stirred-Tank Reactor
2.6. Panel Bioreactor
2.7. Fluidized Bed Reactor
2.8. HPLC and YSI Analysis
2.9. Other Characterizations
2.9.1. Biomass Determination
2.9.2. Pellet Morphology
3. Results and Discussion
3.1. Pellet Morphology and Biomass Distribution
3.2. Production of Raspberry Compounds and Glucose Uptake
3.3. Bioreactor Mixing Characteristics
3.4. Challenges and Prospects of Different Bioreactor Designs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amanullah, A.; Christensen, L.H.; Hansen, K.; Nienow, A.W.; Thomas, C.R. Dependence of morphology on agitation intensity in fed-batch cultures of Aspergillus oryzae and its implications for recombinant protein production. Biotechnol. Bioeng. 2002, 77, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Böker, A.; Fischer, M.; Berger, R.G. Raspberry Ketone from Submerged Cultured Cells of the Basidiomycete Nidula niveo-tomentosa. Biotechnol. Prog. 2001, 17, 568–572. [Google Scholar] [CrossRef]
- Barros, A.R.; Cavalcante de Amorim, E.L.; Reis, C.M.; Shida, G.M.; Silva, E.L. Biohydrogen production in anaerobic fluidized bed reactors: Effect of support material and hydraulic retention time. Int. J. Hydrogen Energy 2010, 35, 3379–3388. [Google Scholar] [CrossRef]
- Beekwilder, J.; van der Meer, I.M.; Sibbesen, O.; Broekgaarden, M.; Qvist, I.; Mikkelsen, J.D.; Hall, R.D. Microbial production of natural raspberry ketone. Biotechnol. J. 2007, 2, 1270–1279. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhong, H.; Zhu, H.; Wang, H.; Goh, K.-L.; Zhang, J.; Zheng, M. Efficient and sustainable preparation of cinnamic acid flavor esters by immobilized lipase microarray. LWT 2023, 173, 114322. [Google Scholar] [CrossRef]
- Cui, Y.Q.; van der Lans, R.G.J.M.; Luyben, K.C.A.M. Effect of agitation intensities on fungal morphology of submerged fermentation. Biotechnol. Bioeng. 1997, 55, 715–726. [Google Scholar] [CrossRef]
- dos Reis, C.M.; Silva, E.L. Effect of upflow velocity and hydraulic retention time in anaerobic fluidized-bed reactors used for hydrogen production. Chem. Eng. J. 2011, 172, 28–36. [Google Scholar] [CrossRef]
- Hakkinen, S.T.; Seppanen-Laakso, T.; Oksman-Caldentey, K.M.; Rischer, H. Bioconversion to Raspberry Ketone is Achieved by Several Non-related Plant Cell Cultures. Front. Plant Sci. 2015, 6, 1035. [Google Scholar] [CrossRef]
- Hamrouni, R.; Dupuy, N.; Karachurina, A.; Mitropoulou, G.; Kourkoutas, Y.; Molinet, J.; Maiga, Y.; Roussos, S. Biotechnological potential of Zymotis-2 bioreactor for the cultivation of filamentous fungi. Biotechnol. J. 2022, 17, e2100288. [Google Scholar] [CrossRef]
- Hernandez-Neuta, I.; Pereiro, I.; Ahlford, A.; Ferraro, D.; Zhang, Q.; Viovy, J.L.; Descroix, S.; Nilsson, M. Microfluidic magnetic fluidized bed for DNA analysis in continuous flow mode. Biosens. Bioelectron. 2017, 102, 531–539. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, H.J.; Xu, C.P.; Choi, J.W.; Yun, J.W. Effect of aeration and agitation on the production of mycelial biomass and exopolysaccharides in an enthomopathogenic fungus Paecilomyces sinclairii. Lett. Appl. Microbiol. 2003, 36, 321–326. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Waldron, K.; Kresnowati, P.; Danquah, M.K. Process challenges relating to hematopoietic stem cell cultivation in bioreactors. J. Ind. Microbiol. Biotechnol. 2011, 38, 761–767. [Google Scholar] [CrossRef]
- Krull, R.; Cordes, C.; Horn, H.; Kampen, I.; Kwade, A.; Neu, T.R.; Nörtemann, B. Morphology of Filamentous Fungi: Linking Cellular Biology to Process Engineering Using Aspergillus niger. In Biosystems Engineering II: Linking Cellular Networks and Bioprocesses; Wittmann, C., Krull, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–21. [Google Scholar]
- Li, S.; Li, F.; Zhu, X.; Liao, Q.; Chang, J.S.; Ho, S.H. Biohydrogen production from microalgae for environmental sustainability. Chemosphere 2022, 291, 132717. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, T.; Wu, M.; Chen, F.; Zhang, P.; Deng, Z.Y.; Luo, T. Potential metabolic activities of raspberry ketone. J. Food Biochem. 2022, 46, e14018. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically bound aroma precursors in fruits: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Wilkins, M.R. Process optimization and scale-up production of fungal aryl alcohol oxidase from genetically modified Aspergillus nidulans in stirred-tank bioreactor. Bioresour. Technol. 2020, 315, 123792. [Google Scholar] [CrossRef]
- Moore, S.J.; Hleba, Y.B.; Bischoff, S.; Bell, D.; Polizzi, K.M.; Freemont, P.S. Refactoring of a synthetic raspberry ketone pathway with EcoFlex. Microb. Cell Fact. 2021, 20, 116. [Google Scholar] [CrossRef]
- Moreira, M.T.; Sanromán, A.; Feijoo, G.; Lema, J.M. Control of pellet morphology of filamentous fungi in fluidized bed bioreactors by means of a pulsing flow. Application to Aspergillus niger and Phanerochaete chrysosporium. Enzym. Microb. Technol. 1996, 19, 261–266. [Google Scholar] [CrossRef]
- Osadolor, O.A.; Jabbari, M.; Nair, R.B.; Lennartsson, P.R.; Taherzadeh, M.J. Effect of media rheology and bioreactor hydrodynamics on filamentous fungi fermentation of lignocellulosic and starch-based substrates under pseudoplastic flow conditions. Bioresour. Technol. 2018, 263, 250–257. [Google Scholar] [CrossRef]
- Papoutsakis, E.T. Fluid-mechanical damage of animal cells in bioreactors. Trends Biotechnol. 1991, 9, 427–437. [Google Scholar] [CrossRef]
- Park, J.P.; Kim, Y.M.; Kim, S.W.; Hwang, H.J.; Cho, Y.J.; Lee, Y.S.; Song, C.H.; Yun, J.W. Effect of aeration rate on the mycelial morphology and exo-biopolymer production in Cordyceps militaris. Process Biochem. 2002, 37, 1257–1262. [Google Scholar] [CrossRef]
- Pereiro, I.; Bendali, A.; Tabnaoui, S.; Alexandre, L.; Srbova, J.; Bilkova, Z.; Deegan, S.; Joshi, L.; Viovy, J.L.; Malaquin, L.; et al. A new microfluidic approach for the one-step capture, amplification and label-free quantification of bacteria from raw samples. Chem. Sci. 2017, 8, 1329–1336. [Google Scholar] [CrossRef]
- Pereiro, I.; Tabnaoui, S.; Fermigier, M.; du Roure, O.; Descroix, S.; Viovy, J.L.; Malaquin, L. Magnetic fluidized bed for solid phase extraction in microfluidic systems. Lab Chip 2017, 17, 1603–1615. [Google Scholar] [CrossRef]
- Pleissner, D.; Kwan, T.H.; Lin, C.S. Fungal hydrolysis in submerged fermentation for food waste treatment and fermentation feedstock preparation. Bioresour. Technol. 2014, 158, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Porcel, E.M.; Casas López, J.L.; Sánchez Pérez, J.A.; Fernández Sevilla, J.M.; Chisti, Y. Effects of pellet morphology on broth rheology in fermentations of Aspergillus terreus. Biochem. Eng. J. 2005, 26, 139–144. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- SundarRajan, P.; Gopinath, K.P.; Greetham, D.; Antonysamy, A.J. A review on cleaner production of biofuel feedstock from integrated CO2 sequestration and wastewater treatment system. J. Clean. Prod. 2019, 210, 445–458. [Google Scholar] [CrossRef]
- Tan, N.; Ong, L.; Shukal, S.; Chen, X.; Zhang, C. High-Yield Biosynthesis of trans-Nerolidol from Sugar and Glycerol. J. Agric. Food Chem. 2023. [Google Scholar] [CrossRef]
- Taupp, D.E.; Nimtz, M.; Berger, R.G.; Zorn, H. Stress response of Nidula niveo-tomentosa to UV-A light. Mycologia 2008, 100, 529–538. [Google Scholar] [CrossRef]
- Taymaz-Nikerel, H.; de Mey, M.; Ras, C.; ten Pierick, A.; Seifar, R.M.; van Dam, J.C.; Heijnen, J.J.; van Gulik, W.M. Development and application of a differential method for reliable metabolome analysis in Escherichia coli. Anal. Biochem. 2009, 386, 9–19. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Zivkovic, V.; Zheng, M. Deacidification of high-acid rice bran oil by the tandem continuous-flow enzymatic reactors. Food Chem. 2022, 393, 133440. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J. The filamentous fungal pellet and forces driving its formation. Crit. Rev. Biotechnol. 2016, 36, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Goh, K.-L.; Ng, Y.L.; Chow, Y.; Wang, S.; Zivkovic, V. Process intensification in micro-fluidized bed systems: A review. Chem. Eng. Process. 2021, 164, 108397. [Google Scholar] [CrossRef]
- Zhang, Y.; Ng, Y.L.; Goh, K.-L.; Chow, Y.; Wang, S.; Zivkovic, V. Fluidization of fungal pellets in a 3D-printed micro-fluidized bed. Chem. Eng. Sci. 2021, 236, 116466. [Google Scholar] [CrossRef]
- Zorn, H.; Fischer-Zorn, M.; Berger, R.G. A labeling study to elucidate the biosynthesis of 4-(4-hydroxyphenyl)-butan-2-one (raspberry ketone) by Nidula niveo-tomentosa. Appl. Environ. Microbiol. 2003, 69, 367–372. [Google Scholar] [CrossRef] [PubMed]

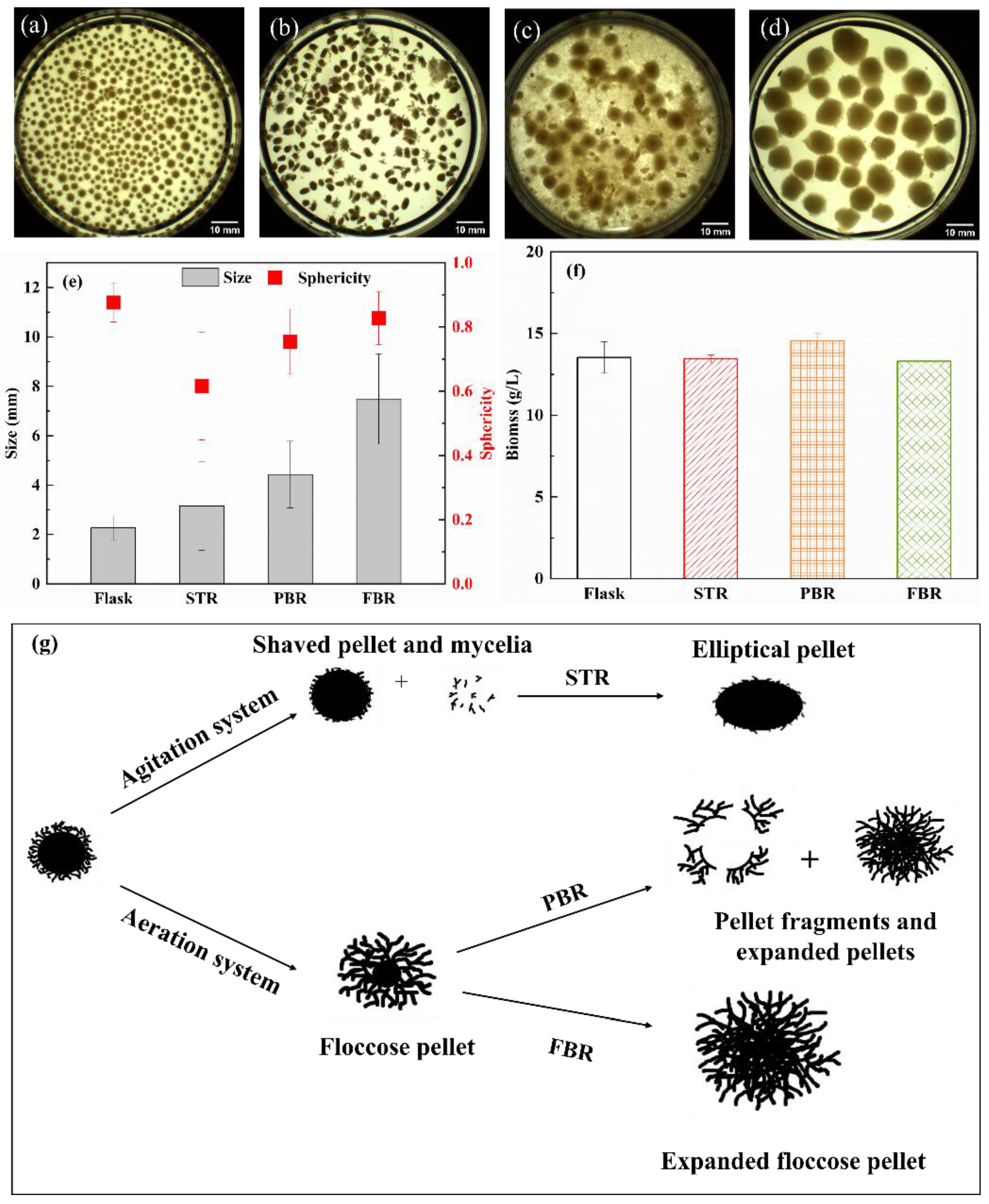
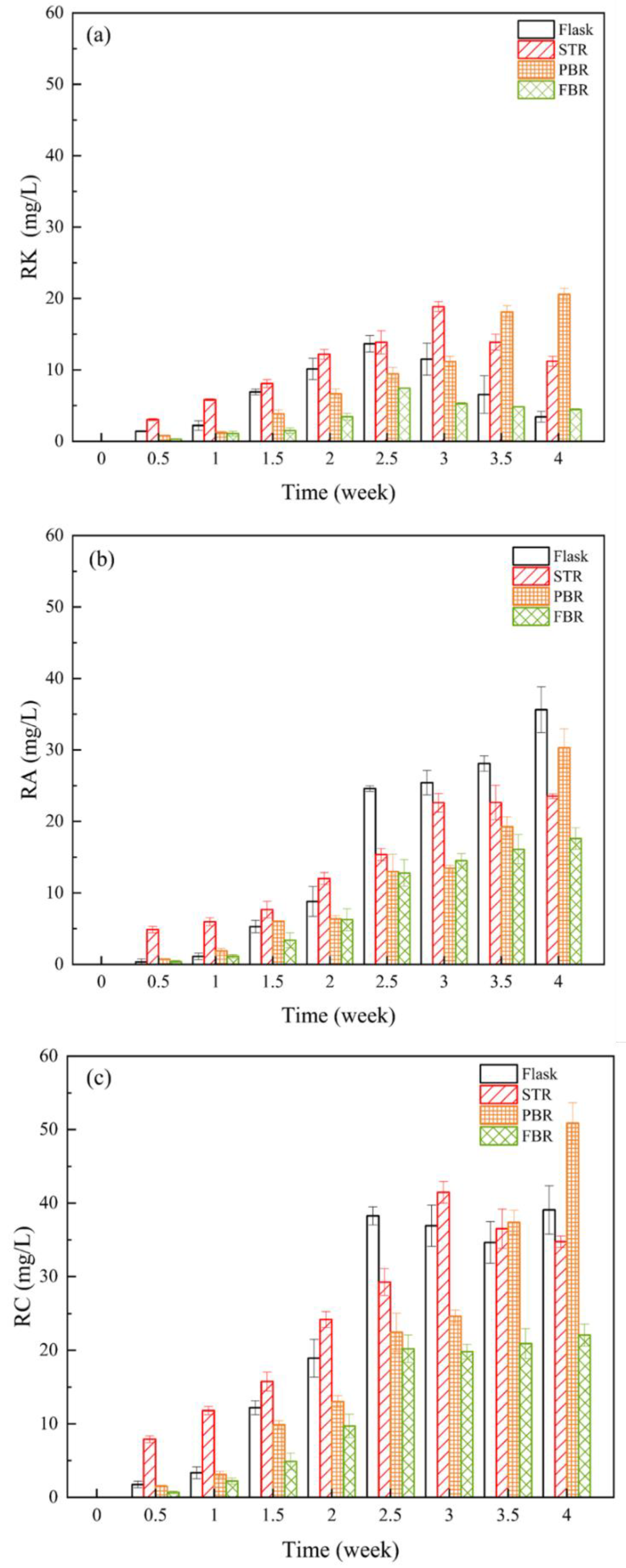
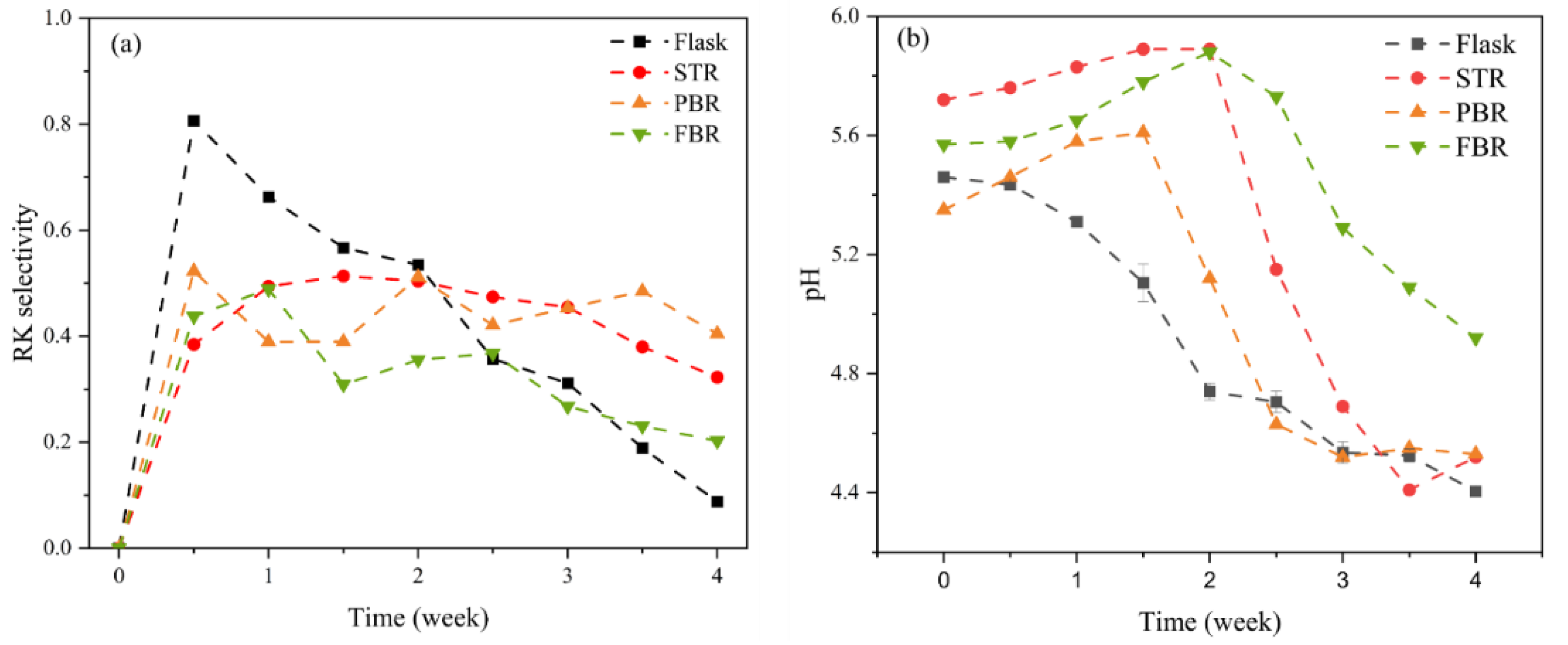

| Operating Conditions | Flask | STR | PBR | FBR |
|---|---|---|---|---|
| Cultivation volume (mL) | 48 | 750 | 400 | 1000 |
| Aeration rate (v.v.m) | / | 0.15 | 0.15 | 0.15 |
| Liquid flowrate (mL/s) | / | / | / | ~0.25 |
| Agitation rate (rpm) | / | 300 | 0–180 | / |
| UV-A radiation (h/day) | 10 | 10 | 10 | 10 |
| Yields | Flask | STR | PBR | FBR |
|---|---|---|---|---|
| Peak production point (week) | 2.5 | 3.0 | 4.0 | 2.5 |
| RK (mg/L) | 13.7 ± 1.2 | 18.9 ± 0.7 | 20.6 ± 0.7 | 7.4 ± 0.1 |
| RA (mg/L) | 24.6 ± 0.4 | 22.6 ± 1.3 | 30.3 ± 2.7 | 12.8 ± 1.9 |
| RC (mg/L) | 38.3 ± 1.2 | 41.5 ± 1.5 | 50.9 ± 2.8 | 20.2 ± 1.9 |
| RC YP/S of glucose (mg/g) | 7.1 ± 0.5 | 3.8 ± 0.2 | 2.5 ± 0.9 | 1.3 ± 0.1 |
| RC YP/S of phenylalanine (g/g) | 52.1 ± 0.1 | 68.1 ± 0.2 | 54.2 ± 2.8 | 49.8 ± 0.1 |
| RK Vol. prod.* | 0.78 | 0.90 | 0.74 | 0.42 |
| RC Vol. prod.* | 2.19 | 1.97 | 1.82 | 1.16 |
| RK selectivity | 0.36 ± 0.09 | 0.45 ± 0.05 | 0.40 ± 0.07 | 0.37 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Peterson, E.C.; Ng, Y.L.; Goh, K.-L.; Zivkovic, V.; Chow, Y. Comparison of Raspberry Ketone Production via Submerged Fermentation in Different Bioreactors. Fermentation 2023, 9, 546. https://doi.org/10.3390/fermentation9060546
Zhang Y, Peterson EC, Ng YL, Goh K-L, Zivkovic V, Chow Y. Comparison of Raspberry Ketone Production via Submerged Fermentation in Different Bioreactors. Fermentation. 2023; 9(6):546. https://doi.org/10.3390/fermentation9060546
Chicago/Turabian StyleZhang, Yi, Eric Charles Peterson, Yuen Ling Ng, Kheng-Lim Goh, Vladimir Zivkovic, and Yvonne Chow. 2023. "Comparison of Raspberry Ketone Production via Submerged Fermentation in Different Bioreactors" Fermentation 9, no. 6: 546. https://doi.org/10.3390/fermentation9060546
APA StyleZhang, Y., Peterson, E. C., Ng, Y. L., Goh, K.-L., Zivkovic, V., & Chow, Y. (2023). Comparison of Raspberry Ketone Production via Submerged Fermentation in Different Bioreactors. Fermentation, 9(6), 546. https://doi.org/10.3390/fermentation9060546








