Abstract
Zao pepper is a fermented type with a distinct flavor, and Zao pepper-based dishes are popular. However, in spontaneous fermentation (SF), the adverse microorganisms of Zao pepper are uncontrollable and alter the product quality. Lactic acid bacteria (LAB) inhibit the growth of harmful bacteria and endow a sour taste in SF. Therefore, the purpose of this study was to isolate autochthonic LAB from SF and through inoculated fermentation (IF) to improve Zao pepper quality. Four LAB strains were selected for probiotic experiments by sensory evaluation based on the flavor of Zao pepper in vitro. Intriguingly, Lactobacillus plantarum 5-1 showed superior safety and probiotics, with the fastest growth and acid-production rate. Moreover, the nitrite reductase viability of L. plantarum 5-1 reached 4.73 μmol/mL·h. The use of IF via L. plantarum 5-1 increased the total acid content 2-fold and reduced the nitrite content by 0.36-fold compared to SF in Zao pepper. In addition, IF improved the color and texture of Zao pepper. These results indicate that the autochthonic L. plantarum 5-1 improved the quality of Zao pepper, providing a new strategy for enhancing the stability of fermented pepper.
1. Introduction
Fermented chopped pepper is a cooking condiment widely used in food preparation [1]. Due to various raw materials and production methods, fermented chopped pepper includes Gochujang from Korea, Sriracha sauce from Thailand, and Zao pepper from China [2,3]. Guizhou is China’s largest pepper planting area [4], producing various fermented pepper products. Zao-pepper-based products and dishes are popular among these due to their unique pungent and tart flavor. Typically, Zao pepper is prepared with fresh chopped red line pepper (Capsicum annuum L.), ginger, garlic, and microorganisms in the surrounding environment [5]. Moreover, the total microbial flora of fermented pepper is uncontrollable in spontaneous fermentation (SF). The adverse microorganisms, including Mucor, Clostridium perfringen, and Salmonella enterica [6], generate stink [7], harmful substances [8], bloated packaging [9], and changes in product quality. Therefore, how to improve the quality and stability of fermented chili peppers has become an urgent problem to be solved.
In SF crushed red pepper, the microorganisms included Lactobacillus, Staphylococcus, and Streptococcus; among these, Lactobacillus is abundant, with 76.5% content at the end stages of fermentation [10]. In addition, lactic acid bacteria (LAB) produce antibacterial, organic acids, and ascorbic acid; these metabolites inhibit the pathogenic microorganisms and improve the product flavor and quality of SF foods [11,12]. A previous study has demonstrated that Lactobacillus plantarum can be separated from northeast sauerkraut through inoculated fermentation (IF) to increase the contents of organic acids, esters, and alcohols and reduce the nitrite level in sauerkraut, thereby enhancing the product quality and flavor [13]. LAB can also increase the sweetness and aroma of fermented chopped peppers (Hunan, China), as IF was found to increase the free amino acid content by 36.3% more than SF [14]. Owing to its importance in fermented food, LAB can be screened from traditional fermented pepper as a fermentation strain to improve product quality.
Although LAB are recognized as safe strains, safety issues, such as resistance-gene transfer, need to be elucidated. Hence, LAB were isolated from the natural environment; of these, seven strains consisted of nucleotide-transferase genes, and the level of antibiotic resistance exceeded that recommended by the European Food Safety Authority [15]. Therefore, safety and probiotic experiments should be conducted on strains isolated from traditional fermented foods before application. In addition, LAB isolated from traditional cheese in Northeast China were tested for antibiotic sensitivity, 1,1-diphenyl-2-picrylhydrazyl free radical scavenging rate, and hemolysis. The five strains of LAB were considered safe for use in food [16]. In addition, the probiotic ability of strains was evaluated with respect to their acidification, bacteriostasis, nitrite degradation, and intestinal epithelial adhesion abilities.
Accordingly, the purpose was to isolate and screen LAB from SF Zao pepper, thereafter utilizing inoculation fermentation to improve the quality of Zao pepper. Firstly, based on the flavor of Zao pepper samples, LAB were isolated and identified. Then, based on the sensory evaluation of IF Zao pepper from five aspects (spiciness, sourness, crispness, color, and smell), the Lactobacillus strain with the better flavor was selected. Secondly, we selected the fermentation strain with the best safety and probiotics based on the results of in vitro probiotic ability tests assessing growth, acid production, bacteriostatic effects, antibiotic sensitivity, automatic aggregation, and nitrite degradation abilities. Finally, the SF titratable total acidity (TA), nitrite content, chromaticity, and microstructure of the pepper tissue were compared with the IF Zao pepper strain. Therefore, this study found new strategies for improving the quality of fermented pepper.
2. Materials and Methods
2.1. Materials and Preparation of Zao Pepper
Ten types of Zao pepper samples were collected from Guiyang, Zunyi, and Liupanshui from the major pepper production zone of Guizhou Province in China. The main material red line peppers (Capsicum annuum L.) in Zao pepper were cultivated by our group in an open field in Guiyang. The auxiliary materials of Zao pepper consisted of ginger, garlic, Baijiu, sugar, and salt were purchased from Walmart (Guiyang, China).
Fresh red line pepper, ginger, and garlic were soaked individually in 8% brine for 10 min, washed twice with double-distilled water, and chopped into 5-cm pieces before use. All materials were placed into a sterile glass tank as follows: 85% chopped pepper, 2% crushed ginger, 2% crushed garlic, 2% Baijiu, 1% sugar, 6% salt, and finally inoculated with a 2% starter LAB culture. The prepared Zao pepper was stored at 30 °C for 30 days with samples taken every 5 days (on Days 1, 5, 10, 15, 20, 25, and 30).
2.2. Source and Culture of Strains
Lactobacillus brevis CICC 6239 and CICC 24450 were purchased from the China Center of Industrial Culture Collection. Lactobacillus fructivorans CGMCC 1.2427 was obtained from the China General Microbiological Culture Collection Center. These three strains of LAB served as control groups. The other LAB were isolated from 10 kinds of Zao pepper samples by our group (Table S1). In addition, L. plantarum 5-1 was deposited in the China Center for Type Culture Collection (CCTCC) (Wuhan, China) under the registration number CCTCC M 2022827. Staphylococcus aureus, Bacillus ceres, and Salmonella typhi were stored by our group. LAB and pathogenic bacteria were cultured at 37 °C for 24 h in the de Man, Rogosa, and Sharpe (MRS) medium (1% tryptone, 0.5% beef, 0.4% yeast extract, 0.2% C6H12O6·H2O, 0.01% Tween 80, 0.2% K2HPO4·3H2O, 0.5% CH3COONa·3H2O, 0.2% (NH4)2HC6H5O7, 0.02% MgSO4·7H2O, and 0.005% MnSO4·4H2O) and Luria–Bertani (LB) medium (1% tryptone, 0.5% yeast extract, and 1% NaCl), respectively.
2.3. Isolation and Physiochemical Analysis of LAB
Each Zao pepper sample (1 mL) was dissolved in 100 μL sterile physiological saline, the sample supernatant was diluted (10−3–10−6) in a gradient, spread on 2% calcium carbonate MRS agar plates, and cultured at 37 °C for 24 h [17]. The resulting colonies with clear circles on plates were selected for streaking; this process was repeated three times to obtain single colonies that were analyzed by Gram staining and catalase activity assay [18]. The colonies were identified by Guizhou Provincial Center for Disease Control and Prevention (GZCDC, Guiyang, China).
2.4. Sensory Evaluation of Zao Pepper
A total of 10 people were selected as qualified team members to taste samples (5 g) at 5-min intervals, during which the taste buds were washed with water [19]. The sensory evaluation was carried out from five aspects: spiciness, sourness, color, crispness, and smell (1 = very weak; 2 = weak; 3 = medium; 4 = strong; 5 = very strong).
2.5. Probiotic Capacity and Safety Testing
2.5.1. Determination of Growth and Acidification Capacity
LAB was cultured in MRS broth medium at 37 °C, 180 rpm for 24 h for testing growth and acidification capacity. LAB growth was measured by turbidity based on the absorbance at an optical density of 600 nm (OD600) [20]. The LAB absorbance and pH values were recorded at OD600 at 2-h intervals for 24 h to evaluate the growth and acidifying ability of each strain in MRS broth.
2.5.2. Determination of Antibiotic Sensitivity
The antibiotic susceptibility of LAB was determined using the agar plate diffusion method [21]. A volume of 100 μL LAB with an OD600 of 0.3 was spread on MRS agar plates. The antibiotic susceptibility tablets (amoxicillin, ampicillin, cephalexin, kanamycin, neomycin, gentamicin, streptomycin, tetracycline, chloramphenicol, clindamycin, erythromycin, vancomycin, tetracycline, and rifampicin) were placed on MRS agar plates, respectively. After incubation at 37 °C for 24 h, the diameter of the antibacterial circle (mm) around the drug-sensitive sheet was measured. The resistance and susceptibility were expressed according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [22].
2.5.3. Detection of Antibiotic Resistance Genes
The total DNA of the LAB strains was extracted using a genomic DNA purification kit (Omega Bio-Tek, GA, USA). Primers were synthesized based on the reported antibiotic genes in LAB. The extracted Lactobacillus genome served as the template, followed by amplification using the corresponding resistant primers to obtain PCR products that were verified by agarose gel electrophoresis. The primers are listed in Table S1.
2.5.4. Determination of Antimicrobial Activity
The bacteriostatic capacity of LAB isolates was determined by agar pore diffusion assay. S. aureus, S. typhi, and B. cereus were indicator pathogens. LAB cultured in MRS medium for 12 h were harvested by centrifugation at 10,000× g for 10 min to collect the supernatant, which was first neutralized to pH 5.00 with 1 mol/L NaOH and then treated at various temperatures (30 °C, 55 °C, 75 °C, 100 °C) for 15 min. Subsequently, these supernatants were filter-sterilized (0.22 μm) to obtain cell-free supernatant (CFS). The indicator microorganisms were grown in LB liquid medium for 12 h, washed twice in phosphate-buffered saline (PBS) (5000× g, 5 min), and resuspended at a concentration of OD600 0.3. The suspension was spread on LB agar medium, and then 50 μL of CFS was placed into the wells bored in agar plates. Fresh MRS broth was used as a control, incubated at 37 °C for 24 h. The bacteriostatic activity was determined by the diameter of the inhibition zone around the well [23,24].
2.5.5. Determination of Auto-Aggregation Activity
The protocol was as described previously with some modifications [25]. LAB were grown in MRS broth at 37 °C for 12 h. Fresh bacterial cells were collected, washed with sterile PBS, resuspended, and the OD600 value adjusted to 0.3. Then, the suspension was inoculated (1% v/v) into 5 mL of MRS broth medium and cultured at 37 °C for 1, 2, 3, and 4 h, until the OD600 was determined. The experiment was repeated three times, and the auto-aggregation was calculated as follows:
where A0 is the initial OD and A1 is the final OD.
2.5.6. Measure the Nitrite Reductase Activity
After overnight culture of LAB, the supernatant was collected by centrifugation at 10,000× g, 4 °C for 2 min, followed by use of the NiR Assay Kit (Solarbio, Beijing, China) to process the supernatant. Then, the supernatant nitrite reductase activity was computed by the measured absorbance at 540 nm.
2.6. Analysis of Physicochemical Characteristics of Zao Pepper
2.6.1. Analysis of pH and TA of Zao Pepper
An equivalent of 5 mL Zao pepper juice was measured on a pH meter (pHS-3C, Fangzhou Technology, Chengdu, China). The TA was assessed according to the national standard GB-12456-2021 [26].
2.6.2. Analysis of the Nitrite Content of Zao Pepper
The N-(1-naphthyl)-ethylenediamine dihydrochloride spectrophotometric method was used to determine the nitrite content in Zao pepper [27].
2.6.3. Analysis of the Color and Texture of Zao Pepper
The change in the color of Zao pepper was determined in terms of CIELAB parameters (L*, a*, and b*) on a colorimeter (Ultra scan Pro, Hunter Lab., Reston, VA, USA). An equivalent of 20 g samples was taken into the cuvette with illuminant D65, 10° observation, and a 50-mm path length cell [28]. The texture of Zao pepper was observed on the 5th and 30th days using a scanning electron microscope (SEM, S-3600N, Hitachi, Tokyo, Japan) and biomicroscope (BX53F, Olympus, Tokyo, Japan). The method has been modified slightly based on previous studies [29].
2.7. Statistical Analysis
The data are presented as the mean ± standard deviation (SD) and analyzed by SPSS 26.0 statistical software (SPSS Inc., Chicago, IL, USA). The means were assessed using Duncan’s test and a p-value <0.05 was considered statistically significant.
3. Results and Discussion
3.1. Strain Isolation and Identification
Based on the 10 kinds of Zao pepper samples, 26 strains were isolated using calcium carbonate MRS agar plates, which represent Gram-negative and catalase-positive strains. These 26 strains were identified through GZCDC, belonging to Lactobacillus, Pediococcus, and Weissella, respectively. The details of the strains are described in Table S2.
3.2. Sensory Evaluation of Zao Pepper
A total of 26 LAB strains were used to ferment peppers, and potential LAB was excavated based on the flavor evaluation index of the Zao pepper. According to Figure S2, all of the Zao pepper samples had a sour aroma. However, 8 types of Zao pepper samples produced a pungent odor, possibly due to the excessive amount of sulfur compounds produced by the strain during fermentation [30]. Additionally, 10 kinds of Zao pepper with a softened texture reduced the crispiness and decreased the Zao pepper scores (Table S3). Based on the order of scores, we selected L. plantarum 2-1, L. plantarum 5-1, L. alimentarius 19-2, and L. plantarum 21-1 for subsequent probiotic ability assessment (Figure 1).
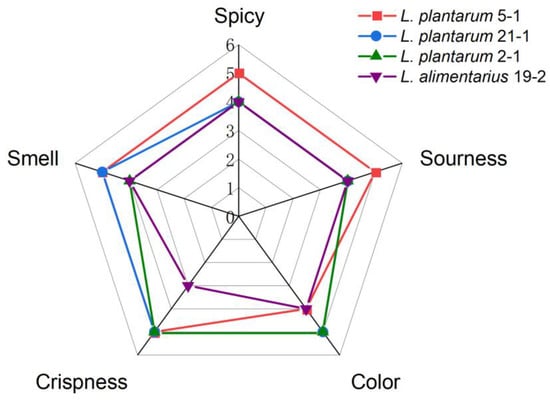
Figure 1.
Comparison of radar charts for sensory evaluation data of L. plantarum 2-1, L. plantarum 5-1, L. alimentarius 19-2, and L. plantarum 21-1 from Zao pepper. Each Zao pepper sample was fermented for 30 days.
3.3. Probiotic Ability and Safety of LAB
3.3.1. Growth and Acid-Producing Capacity
Fast growth and acid-production rates are the criteria for evaluating the probiotic ability of LAB; these parameters could shorten the food fermentation time [31]. The growth curve and acid-production curve of LAB are presented in Figure 2. According to the change in OD600, L. plantarum 2-1, L. plantarum 5-1, and L. plantarum 21-1 were the first to end the retardation phase at 2 h; the growth rate of L. plantarum 5-1 was the fastest with a stronger growth ability than the reference strain L. brevis CICC 6239. As shown in Figure 2B, L. plantarum 2-1 and L. plantarum 5-1 reduced the pH below 4.00 within 8 h. The pH of L. plantarum 5-1 pH changed rapidly, reaching a final pH of 3.31 at 24 h. Moreover, the acid-production rate of L. plantarum 5-1 was 10% higher than that reported for L. plantarum G83 [32].
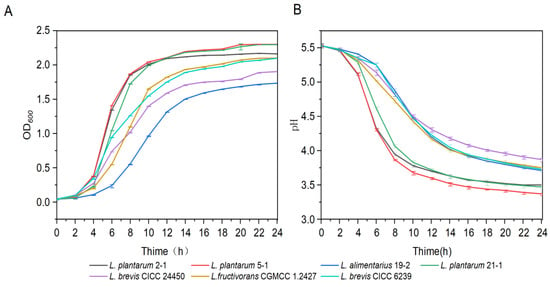
Figure 2.
Lactic acid bacteria (LAB) growth capacity (A) and pH value (B). Data are presented as the mean ± SD.
3.3.2. Antibiotic Susceptibility Experiment
Antibiotics, such as kanamycin, erythromycin, vancomycin, tetracycline, chloramphenicol, and gentamicin, are used to detect the antibiotic sensitivity of LAB [33]. The results in Table 1 indicate that the seven strains of LAB were resistant to vancomycin. Moreover, a high resistance to streptomycin and kanamycin was observed in six tested strains (85.7%). Lactobacillus may involve the risk of transferable resistance genes for aminoglycosides, penicillin, and tetracycline [34]. In order to determine the safety of antibiotic resistance in these strains, we used PCR to detect the presence of resistance genes (Figure S2). All strains harbored the vancomycin-resistance gene vanX, but it was chromosomally encoded and inaccessible [35]. Furthermore, except for L. plantarum 5-1, the kanamycin gene (aph3’’Ⅲ) was tested in the remaining six strains. The streptomycin gene amplicons (ant 6-Ⅰa) were also detected in L. plantarum 2-1 and L. fructivorans CGMCC 1.2427, whereas the neomycin gene (aph3’’Ⅰ) was detected only in L. plantarum 2-1. Another study has suggested that the resistance of Lactobacillus to vancomycin, kanamycin, gentamycin, streptomycin, and neomycin is intrinsic and could be attributed to the low cell membrane permeability of Lactobacilli to aminoglycosides [36,37]. Ultimately, L. plantarum 5-1 did not have the risk of antibiotic resistance and could be applied in the fermentation of food.

Table 1.
Antibiotic-resistance phenotype of LAB.
3.3.3. Antibacterial Activity
S. aureus, S. typhi, and B. ceres are used as indicator microorganisms since they are common foodborne pathogenic microorganisms in fermented foods, which produce enterotoxins and are detrimental to human health [38]. The antibacterial results are shown in Figure 3. As the temperature increased, the antibacterial activity of the control group (L. brevis CICC 24450, L. brevis CICC 6239, and L. fructivorans CGMCC 1.2427) decreased rapidly, but L. plantarum 5-1 still had a better antibacterial activity on S. aureus which can improve the safety and prolong the shelf life of products [39].
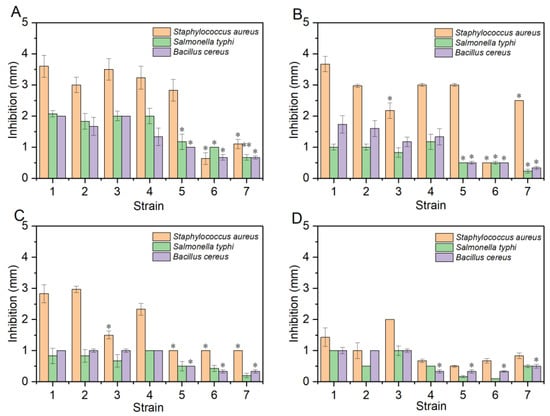
Figure 3.
Bacteriostatic ability of LAB cell-free supernatant (CFS) at different temperatures. (A) CFS was treated at 35 °C; (B) CFS was treated at 55 °C; (C) CFS was treated at 75 °C; (D) CFS was treated at 100 °C; 1: L. plantarum 5-1; 2: L. plantarum 2-1; 3: L. alimentarius 19-2; 4: L. plantarum 21-1; 5: L. brevis CICC 24450; 6: L. brevis CICC 6239; 7: L. fructivorans CGMCC 1.2427. Data are presented as the mean ± SD, * p < 0.05, ** p < 0.01.
3.3.4. Auto-Aggregation Capability
The adhesion and colonization capacity of probiotic strains in the gastrointestinal tract is associated with self-aggregation capacity [40]. In Figure 4A, compared to other strains, the L. plantarum 5-1 strain had the highest auto-aggregation ability, 68.68% at 4 h, which was higher than the previous Motey et al. study L. plantarum PA27 (67.2%) [41].
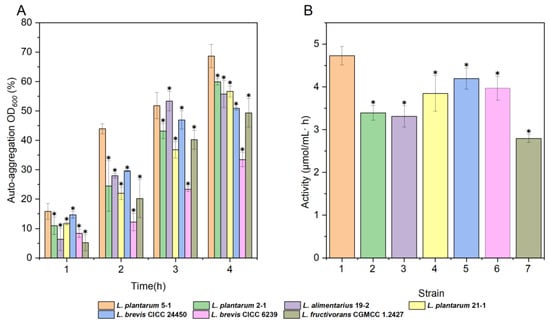
Figure 4.
Auto-aggregation capability (A) and nitrite reductase activity (B) of LAB. Data are presented as the mean ± SD, * p < 0.05.
3.3.5. Viability of Nitrite Reductase
The biodegradation reaction of nitrite involves a decline in enzymes. The nitrite is converted to NO or NH4+ through the nitrogen metabolism pathway of microorganisms, including Lactobacillus [42]. Accordingly, the ability of nitrite degradation was determined by assessing the activity of the strain nitrite reductase. The L. plantarum 5-1 nitrite reductase activity was significantly higher (p < 0.05) than in the other strains. As shown in Figure 4B, the nitrite reductase enzyme activity of L. plantarum 5-1 was 4.73 μmol/mL·h at 12 h, which is 12.9% higher than that of L. brevis CICC24450.
3.4. Physicochemical Properties of Zao Pepper
The content of pH and TA play a critical role in the quality of fermented food and are a key variable in determining the degree of fermentation [43]. As shown in Figure 5A, the pH value of inoculation L. plantarum 5-1 fermentation rapidly decreases by 28.4% on Day 5, producing a large number of acids. However, the pH value of SF shows an upward trend, indicating that SF produces acid slowly with a prolonged fermentation cycle. The content of TA was measured as the gradual acid accumulation due to fermentation over time in the samples: the acid-production rate and TA were 40.86% and 13.98 g/L, respectively (Figure 5B). Inoculated L. plantarum 5-1 fermentation produced a higher TA concentration than in sauerkraut, as reported previously (10.78 g/L) [44].
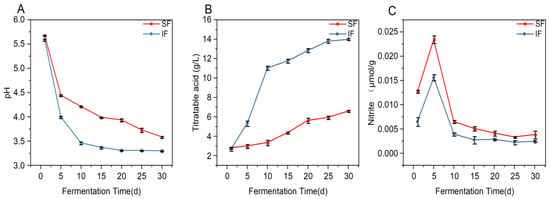
Figure 5.
Changes in pH (A), total acids (B), and nitrite content (C) of spontaneous fermentation (SF) and inoculated fermentation (IF) Zao pepper during fermentation. Data are presented as the mean ± SD.
In addition, the long-term intake of foods with a high nitrite content in humans increases the risk of cancer; nitrite content is considered a major indicator for assessing the safety of fermented food products [45]. In this study (Figure 5C), the nitrite content was reduced by 36.1% in inoculated L. plantarum 5-1 fermented Zao pepper compared to SF. Previous studies have shown that LAB inhibit the growth of nitrate-reducing bacteria, and the organic acids produced could degrade nitrite [46]. IF significantly reduces the fermentation time, and a shorter acidification process effectuates rapid fermentation, reducing commodity loss and production costs [47]. Thus, L. plantarum 5-1 promoted nitrite decomposed rapidly and inhibited the accumulation of nitrite.
3.5. Quality Changes of Zao Pepper
3.5.1. Color Analysis of Zao Pepper
The color of fermented Zao pepper was measured at different fermentation times using a colorimeter; L* indicates luminance, an increase in a* indicates a shift to red and vice versa to green. Similarly, an increase or decrease in b* values is associated with increasing and decreasing yellow and blue colors, respectively [48]. The Zao pepper color changes are shown in Table 2. After 30 days of fermentation, the L* and a* values of IF were 9.5% and 39.2% higher compared to SF, respectively. In both fermentations, the b* value decreased, of which SF declined significantly, which could be ascribed to the degradation of carotenoids and reduced oxidation–reduction potential in the pepper [49]. L. plantarum 5-1 exhibited a high antioxidant activity, which could be due to an increased vitamin C content by LAB fermentation in bell peppers [50]. Therefore, compared with SF Zao pepper, inoculated L. plantarum 5-1 maintains in the Zao pepper a brighter, more red and yellow color, which implies that the strain provides a positive effect on the color protection.

Table 2.
Color changes of Zao pepper during different fermentation periods.
3.5.2. Analysis of Texture Properties of Zao Pepper
The texture affects the sensory and market acceptance of foods, and suitable hardness could bring a crisp and refreshing taste. As shown in the Figure 6, the SF Zao pepper cell structure was loose (Figure 6B), the flesh structure had large, fractured gaps (Figure 6J), and the surfaces of the pepper were severely etched (Figure 6F), thus rendering the tissue of the Zao pepper soft. The decrease in hardness and fracturability is attributed to the disassembly of the cell wall pectic polysaccharides, especially the increase in water-soluble pectin content which can soften fruits and vegetables [51]. Similarly, the biofilm structure was complex, with bacilli and cocci, among which the proportion of cocci was high. Intriguingly, a large number of bacilli aggregated on the surface of IF Zao pepper (Figure 6G); presumably, LAB secreted extracellular polysaccharides, lipids, and proteins, which increased the adhesion and aggregation ability of the strain [52]. Moreover, the cell structure arrangement of IF Zao pepper was dense (Figure 6D), the flesh structure was complete (Figure 6L), and the degree of etching in the Zao pepper was shallow (Figure 6H), indicating that the tissue of the Zao pepper maintained its brittleness. Therefore, fermented Zao pepper inoculated with L. plantarum 5-1 has a better brittleness and mouthfeel than SF Zao pepper, protecting the fermented peppers’ texture.
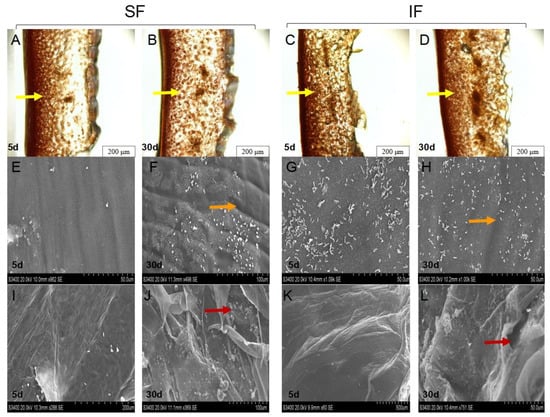
Figure 6.
Scanning electron microscopy images and tissue slices of Zao pepper. (A,B) are tissue slices from Days 5 and 30 of SF. (C,D) are tissue slices from Days 5 and 30 of IF. (E,F) are the skins of Zao peppers on Days 5 and 30 of SF. (G,H) are the skins of Zao peppers on days 5 and 30 of IF. (I,J) are Zao pepper sarcocarps on Days 5 and 30 of SF. (K,L) are Zao pepper sarcocarps on Days 5 and 30 of IF. Yellow, orange, and red arrows indicate the cell structure gaps, degree of surface etching of flesh, and structure fractured gaps in the Zao pepper, respectively.
4. Conclusions
In this study, 26 strains of LAB were isolated and identified from flavorful Zao pepper samples. Based on the IF Zao pepper sensory evaluation, 4 strains of LAB were selected for in vitro probiotic experiments. The results showed that L. plantarum 5-1 had the fastest growth and acid-production rates. In addition, compared to the other 6 strains, L. plantarum 5-1 had a better antibacterial stability, which inhibited the growth of S. aureus, B. cereus, and S. typhi after treatment at 100 °C. Moreover, only the presence of genes encoding vanX could be detected by amplifying 11 resistance genes. Additionally, L. plantarum 5-1 had an improved automatic aggregation ability and nitrite degradation ability at 68.68% and 4.73 μmol/mL·h, respectively. Therefore, it could be inferred that L. plantarum 5-1 has superior probiotic properties and safety, suitable for fermenting Zao pepper. Compared to SF, the inoculation of L. plantarum 5-1 during the fermentation of Zao pepper resulted in the pH, TA, and nitrite content decreasing rapidly, which shortened the fermentation time and improved the quality of Zao pepper. Interestingly, IF was more effective than SF in improving the bright red color and brittleness of the pepper. These findings indicated that inoculation with L. plantarum 5-1 might be a promising start for the fermentation of pepper, which can develop healthy and high-quality fermentation products for consumers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9060547/s1. Table S1: Primers for resistance genes; Table S2: Identification results of lactic acid bacteria (LAB); Table S3: Sensory evaluation score of Zao pepper with different LAB; Figure S1: Comparing radar charts for sensory evaluation data of 22 LAB from Zao pepper; Figure S2: Validation of the resistance gene. The picture shows the PCR amplification results: (A) vanX (454 bp); (B) aph3″III (292 bp); (C) ant 6-Ia (386 bp); (D) aph3″I (670 bp).
Author Contributions
Conceptualization, Y.C. and Y.W.; methodology, Y.C., Y.W. and C.L.; software, Y.C.; validation, Y.C.; investigation, Y.C. and S.Y.; resources, Y.W.; data curation, Y.C.; writing—original draft, Y.C.; writing—review and editing, Y.W. and C.L.; project administration, Y.W.; funding acquisition, Y.W., S.T., C.L. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Guizhou Engineering Technology Research Center for the Processing of Pepper Products, Qianjiaohe KV [2021] (006); the Guizhou Pepper Fermented Products Engineering and Technology Research Center; Qiankehe Platform Talent [2020] 2102; and several Guizhou Provincial Science and Technology Projects, namely Qian Ke He Zhi Cheng-ZK [2021] General 262, Qian Ke He Zhi Cheng-ZK [2022] General 172, Qian Ke He Zhi Cheng-ZK [2022] General 156, and Guizhou University Teganghezi [2023]15.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, J.; Wang, R.; Xiao, Q.; Liu, C.; Deng, F.; Zhou, H. SPME/GC-MS characterization of volatile compounds of Chinese traditional-chopped pepper during fermentation. Int. J. Food Prop. 2019, 22, 1863–1872. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Jang, D.-J.; Yang, H.J.; Chung, K.R. History of Korean gochu, gochujang, and kimchi. J. Ethn. Foods 2014, 1, 3–7. [Google Scholar] [CrossRef]
- Shah, N.N.; Singhal, R.S. 3—Fermented Fruits and Vegetables. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Sanromán, M.Á., Du, G., Soccol, C.R., Dussap, C.-G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 45–89. [Google Scholar]
- Chen, Z.; Geng, Y.; Wang, M.; Lv, D.; Huang, S.; Guan, Y.; Hu, Y. Relationship between microbial community and flavor profile during the fermentation of chopped red chili (Capsicum annuum L.). Food Biosci. 2022, 50, 102071. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Jiang, L.; Deng, F. Determination of fungal community diversity in fresh and traditional Chinese fermented pepper by pyrosequencing. FEMS Microbiol. Lett. 2016, 363, fnw273. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Zúñiga, C.; Worobo, R.W.; Padilla-Zakour, O.I.; Usaga, J. Fate of Spoilage and Pathogenic Microorganisms in Acidified Cold-Filled Hot Pepper Sauces. J. Food Prot. 2019, 82, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, J.; Chen, X.; Huang, L.; Zhang, X.; Gao, X. The Role of the Bacterial Community in Producing a Peculiar Smell in Chinese Fermented Sour Soup. Microorganisms 2020, 8, 1270. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Novais, C.; Peixe, L. Food-to-Humans Bacterial Transmission. Microbiol. Spectr. 2020, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, J.; Chen, L.; Hu, T.; Shi, L.; Wan, S.; Wang, M. Analysis and control of microbial gas production in fermented chili paste. J. Food Process. Pres. 2020, 44, e14806. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Xiao, Q.; Liu, C.; Jiang, L.; Deng, F.; Zhou, H. Analysis of bacterial diversity during fermentation of Chinese traditional fermented chopped pepper. Lett. Appl. Microbiol. 2019, 69, 346–352. [Google Scholar] [CrossRef]
- Hf, A.; Te, B.; Vs, C.; Mt, D.; Gt, E.; Tk, B.; Cm, E.; Sai, F.; Fz, G. Recent developments of lactic acid bacteria and their metabolites on foodborne pathogens and spoilage bacteria: Facts and gaps. Food Biosci. 2022, 47, 101741. [Google Scholar] [CrossRef]
- Rhee, S.J.; Lee, J.-E.; Lee, C.-H. Importance of lactic acid bacteria in Asian fermented foods. Microb. Cell Factories 2011, 10, S5. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Feng, K. Comparison of northeast sauerkraut fermentation between single lactic acid bacteria strains and traditional fermentation. Food Res. Int. 2020, 137, 109553. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H.; Ding, S.; Ye, M.; Jiang, L.; Wang, R. Changes in free amino acids of fermented minced peppers during natural and inoculated fermentation process based on HPLC-MS/MS. J. Food Sci. 2020, 85, 2803–2811. [Google Scholar] [CrossRef] [PubMed]
- Campedelli, I.; Mathur, H.; Salvetti, E.; Clarke, S.; Rea Mary, C.; Torriani, S.; Ross, R.P.; Hill, C.; O’Toole Paul, W. Genus-Wide Assessment of Antibiotic Resistance in Lactobacillus spp. Appl. Environ. Microbiol. 2018, 85, e01738-18. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cui, X.; Gu, S.; Yan, X.; Li, R.; Xia, S.; Chen, H.; Ge, J. Antioxidative and Probiotic Activities of Lactic Acid Bacteria Isolated from Traditional Artisanal Milk Cheese from Northeast China. Probiotics Antimicrob. Proteins 2019, 11, 1086–1099. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, Y.; Zhu, J.; Wen, R.; Chen, Q.; Kong, B. Technological characterization and flavor-producing potential of lactic acid bacteria isolated from traditional dry fermented sausages in northeast China. Food Microbiol. 2022, 106, 104059. [Google Scholar] [CrossRef]
- Kang, W.; Pan, L.; Peng, C.; Dong, L.; Cao, S.; Cheng, H.; Wang, Y.; Zhang, C.; Gu, R.; Wang, J.; et al. Isolation and characterization of lactic acid bacteria from human milk. J. Dairy Sci. 2020, 103, 9980–9991. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, J.; Zang, M.; Zhang, K.; Li, D.; Li, X. Flavor Profile Analysis of Instant and Traditional Lanzhou Beef Bouillons Using HS-SPME-GC/MS, Electronic Nose and Electronic Tongue. Bioengineering 2022, 9, 582. [Google Scholar] [CrossRef]
- Yang, E.; Fan, L.; Yan, J.; Jiang, Y.; Doucette, C.; Fillmore, S.; Walker, B. Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. AMB Express 2018, 8, 10. [Google Scholar] [CrossRef]
- Issaoui, K.E.; Khay, E.O.; Abrini, J.; Zinebi, S.; Amajoud, N.; Senhaji, N.S.; Abriouel, H. Molecular identification and antibiotic resistance of bacteriocinogenic lactic acid bacteria isolated from table olives. Arch. Microbiol. 2021, 203, 597–607. [Google Scholar] [CrossRef]
- M100-S24; Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2013.
- Das, P.; Khowala, S.; Biswas, S. In vitro probiotic characterization of Lactobacillus casei isolated from marine samples. LWT Food Sci. Technol. 2016, 73, 383–390. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Yang, H.; Bu, Y.; Yi, H.; Zhang, L.; Han, X.; Ai, L. Purification and Partial Characterization of Bacteriocin Lac-B23, a Novel Bacteriocin Production by Lactobacillus plantarum J23, Isolated From Chinese Traditional Fermented Milk. Front. Microbiol. 2018, 9, 2165. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, A.; Jahid, I.K. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties—ScienceDirect. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Bakyrbay, S.; Liu, L.; Tang, X.; Liu, Y. Microbiota Succession and Chemical Composition Involved in Lactic Acid Bacteria-Fermented Pickles. Fermentation 2023, 9, 330. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Jiang, A.; Xiu, Z.; Ji, Y.; Guan, Y.; Yang, X. Effect of salt concentration on quality of Chinese northeast sauerkraut fermented by Leuconostoc mesenteroides and Lactobacillus plantarum. Food Biosci. 2019, 30, 100421. [Google Scholar] [CrossRef]
- Cristea, E.; Ghendov-Mosanu, A.; Patras, A.; Socaciu, C.; Pintea, A.; Tudor, C.; Sturza, R. The Influence of Temperature, Storage Conditions, pH, and Ionic Strength on the Antioxidant Activity and Color Parameters of Rowan Berry Extracts. Molecules 2021, 26, 3786. [Google Scholar] [CrossRef]
- Lee, Y.; Wang, C. Morphological Change and Decreasing Transfer Rate of Biofilm-Featured Listeria monocytogenes EGDe. J. Food Prot. 2017, 80, 368–375. [Google Scholar] [CrossRef]
- An, Y.; Cai, X.; Cong, L.; Hu, Y.; Liu, R.; Xiong, S.; Hu, X. Quality Improvement of Zhayu, a Fermented Fish Product in China: Effects of Inoculated Fermentation with Three Kinds of Lactic Acid Bacteria. Foods 2022, 11, 2756. [Google Scholar] [CrossRef]
- Sauer, M.; Han, N.S. Lactic acid bacteria: Little helpers for many human tasks. Essays Biochem. 2021, 65, 163–171. [Google Scholar] [CrossRef]
- Wang, J.; Pu, Y.; Zeng, Y.; Chen, Y.; Zhao, W.; Niu, L.; Chen, B.; Yang, Z.; Wu, L.; Pan, K.; et al. Multi-functional Potential of Five Lactic Acid Bacteria Strains Derived from Giant Panda (Ailuropoda melanoleuca). Probiotics Antimicrob. Proteins 2022, 15, 668–681. [Google Scholar] [CrossRef]
- Mathur, S.; Singh, R. Antibiotic resistance in food lactic acid bacteria—A review. Int. J. Food Microbiol. 2005, 105, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Aquilanti, L.; Garofalo, C.; Osimani, A.; Silvestri, G.; Vignaroli, C.; Clementi, F. Isolation and Molecular Characterization of Antibiotic-Resistant Lactic Acid Bacteria from Poultry and Swine Meat Products. J. Food Prot. 2007, 70, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Ammor Mohammed, S.; Gueimonde, M.; Danielsen, M.; Zagorec, M.; van Hoek Angela, H.A.M.; de los Reyes-Gavilán Clara, G.; Mayo, B.; Margolles, A. Two Different Tetracycline Resistance Mechanisms, Plasmid-Carried tet(L) and Chromosomally Located Transposon-Associated tet(M), Coexist in Lactobacillus sakei Rits 9. Appl. Environ. Microb. 2008, 74, 1394–1401. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, A.; Iqbal, M.; Zhang, L.; Pan, H.; Liu, Z.; Li, J. Probiotic potential and safety assessment of Lactobacillus isolated from yaks. Microb. Pathog. 2020, 145, 104213. [Google Scholar] [CrossRef] [PubMed]
- Kirtzalidou, E.; Pramateftaki, P.; Kotsou, M.; Kyriacou, A. Screening for lactobacilli with probiotic properties in the infant gut microbiota. Anaerobe 2011, 17, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Toxins in Fermented Foods: Prevalence and Preventions—A Mini Review. Toxins 2019, 11, 4. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]
- Sornsenee, P.; Singkhamanan, K.; Sangkhathat, S.; Saengsuwan, P.; Romyasamit, C. Probiotic Properties of Lactobacillus Species Isolated from Fermented Palm Sap in Thailand. Probiotics Antimicrob. Proteins 2021, 13, 957–969. [Google Scholar] [CrossRef]
- Motey, G.A.; Johansen, P.G.; Owusu-Kwarteng, J.; Ofori, L.A.; Obiri-Danso, K.; Siegumfeldt, H.; Larsen, N.; Jespersen, L. Probiotic potential of Saccharomyces cerevisiae and Kluyveromyces marxianus isolated from West African spontaneously fermented cereal and milk products. Yeast 2020, 37, 403–412. [Google Scholar] [CrossRef]
- Wei, W.; Hu, X.; Yang, S.; Wang, K.; Zeng, C.; Hou, Z.; Cui, H.; Liu, S.; Zhu, L. Denitrifying halophilic archaea derived from salt dominate the degradation of nitrite in salted radish during pickling. Food Res. Int. 2022, 152, 110906. [Google Scholar] [CrossRef]
- Ye, Z.; Shang, Z.; Zhang, S.; Li, M.; Zhang, X.; Ren, H.; Hu, X.; Yi, J. Dynamic analysis of flavor properties and microbial communities in Chinese pickled chili pepper (Capsicum frutescens L.): A typical industrial-scale natural fermentation process. Food Res. Int. 2022, 153, 110952. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Ji, Y.; Guan, Y.; Feng, K. Microbial dynamics and volatilome profiles during the fermentation of Chinese northeast sauerkraut by Leuconostoc mesenteroides ORC 2 and Lactobacillus plantarum HBUAS 51041 under different salt concentrations. Food Res. Int. 2020, 130, 108926. [Google Scholar] [CrossRef] [PubMed]
- Motey, G.A.; Owusu-Kwarteng, J.; Obiri-Danso, K.; Ofori, L.A.; Ellis, W.O.; Jespersen, L. In vitro properties of potential probiotic lactic acid bacteria originating from Ghanaian indigenous fermented milk products. World J. Microbiol. Biotechnol. 2021, 37, 52. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, P.; Xie, Y.; Wang, X. Co-fermentation with Lactobacillus curvatus LAB26 and Pediococcus pentosaceus SWU73571 for improving quality and safety of sour meat. Meat Sci. 2020, 170, 108240. [Google Scholar] [CrossRef] [PubMed]
- Fadda, S.; López, C.; Vignolo, G. Role of lactic acid bacteria during meat conditioning and fermentation: Peptides generated as sensorial and hygienic biomarkers. Meat Sci. 2010, 86, 66–79. [Google Scholar] [CrossRef]
- Petchpoung, K.; Soiklom, S.; Siri-anusornsak, W.; Khlangsap, N.; Tara, A.; Maneeboon, T. Predicting antioxidant activity of wood vinegar using color and spectrophotometric parameters. MethodsX 2020, 7, 100783. [Google Scholar] [CrossRef]
- Carbonell, J.V.; Piñaga, F.; Yusá, V.; Peña, J.L. The dehydration of paprika with ambient and heated air and the kinetics of colour degradation during storage. J. Food Eng. 1986, 5, 179–193. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Witrowa-Rajchert, D.; Rybak, K.; Rolof, J.; Pobiega, K.; Woźniak, Ł.; Gramza-Michałowska, A. The Influence of Lactic Acid Fermentation on Selected Properties of Pickled Red, Yellow, and Green Bell Peppers. Molecules 2022, 27, 8637. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Y.; Ding, S.; Qin, Y.; Jiang, L.; Zhou, H.; Deng, F.; Wang, R. Changes in texture qualities and pectin characteristics of fermented minced pepper during natural and inoculated fermentation process. Int. J. Food Sci. Technol. 2021, 56, 6073–6085. [Google Scholar] [CrossRef]
- Clauss, M.; Tafin, U.F.; Bizzini, A.; Trampuz, A.; Ilchmann, T. Biofilm formation by staphylococci on fresh, fresh-frozen and processed human and bovine bone grafts. Eur. Cell Mater. 2013, 25, 159–166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).