Adaptive Laboratory Evolution of Bacillus subtilis 168 for Efficient Production of Surfactin Using NH4Cl as a Nitrogen Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Primers
2.2. Culture Media
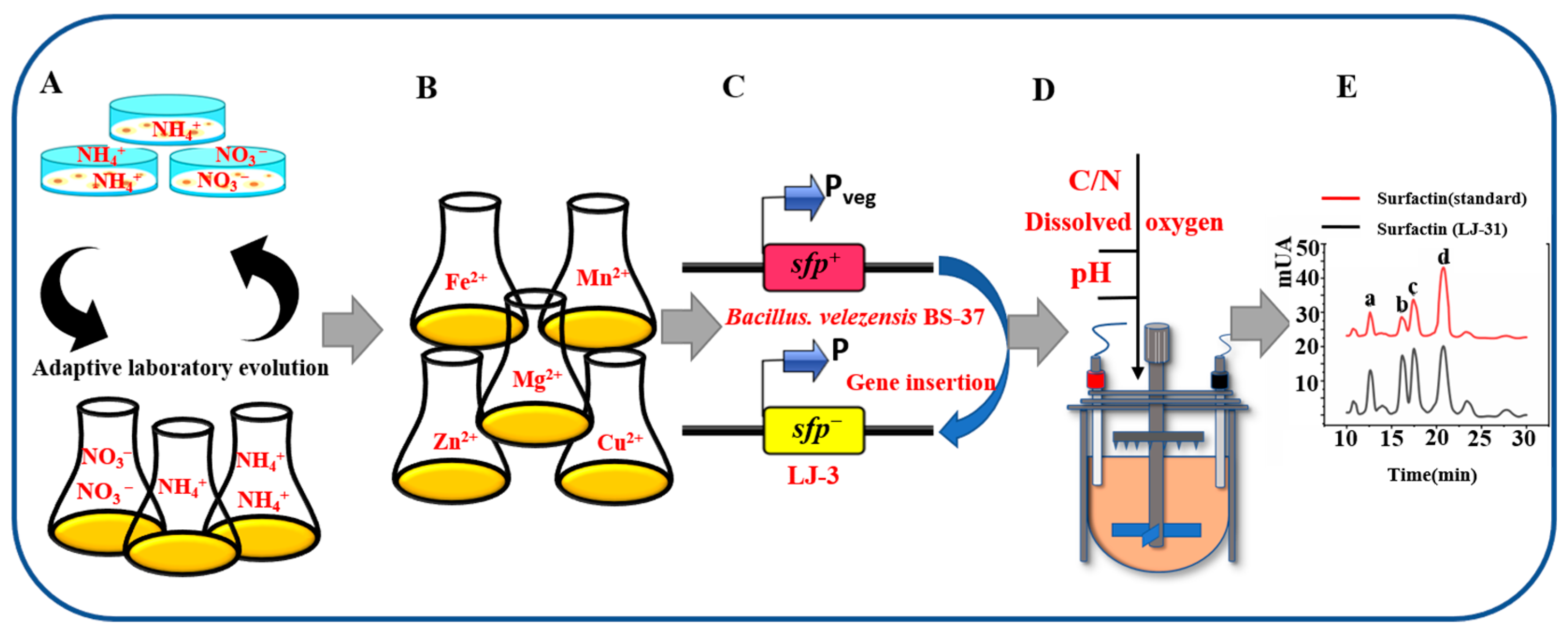
2.3. ALE of B. subtilis 168 and Optimization of Metal Ions in Synthetic Medium
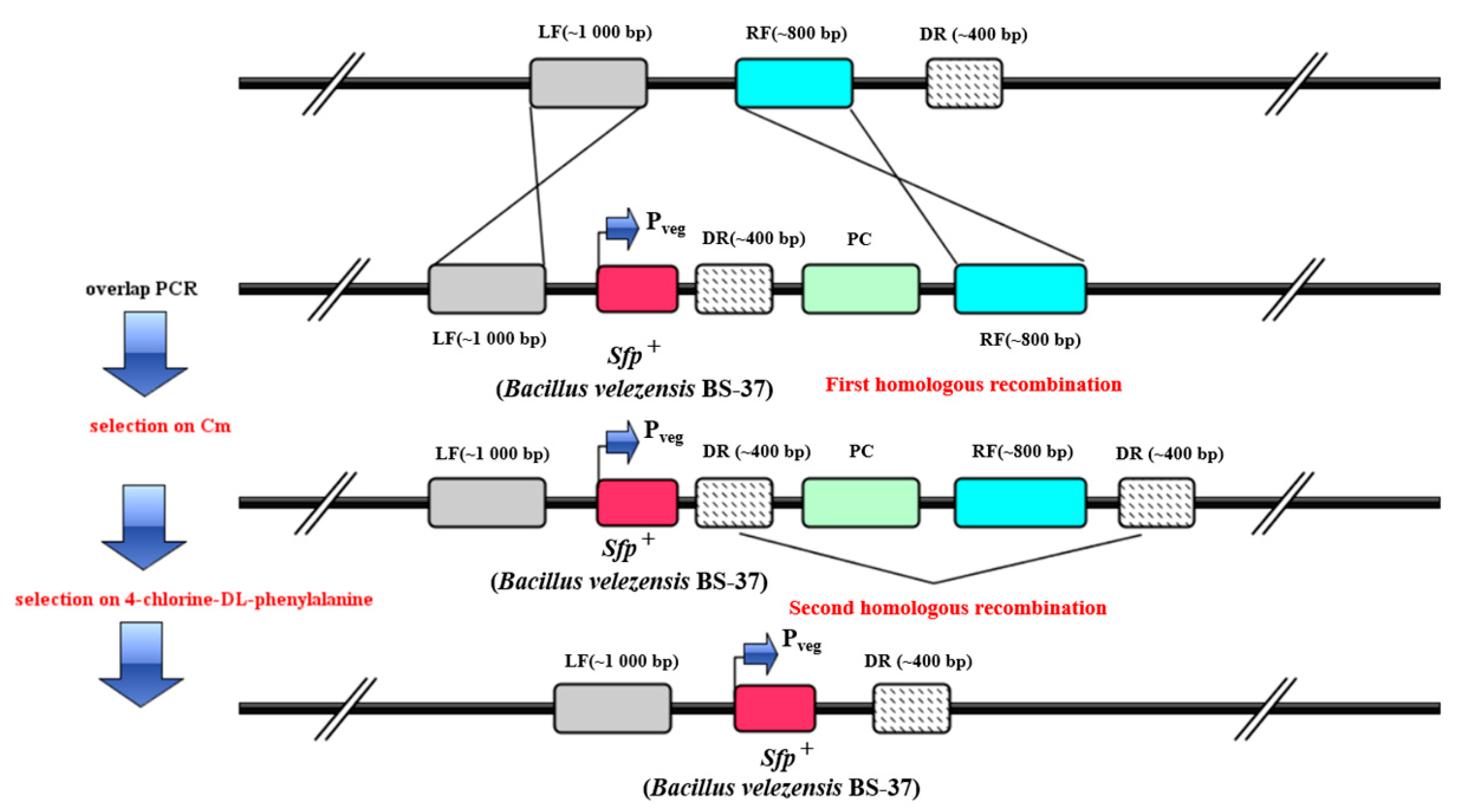
2.4. sfp+ Strain of B. subtilis 168 Variant (LJ-31)
2.5. Surfactin Production in Shake Flasks
2.6. Batch Fermentation for Surfactin Production in a 5L Bioreactor
2.7. Determination of Surfactin Concentration
2.8. Analysis
2.9. Calculation of Dynamic Parameters
3. Results and Discussion
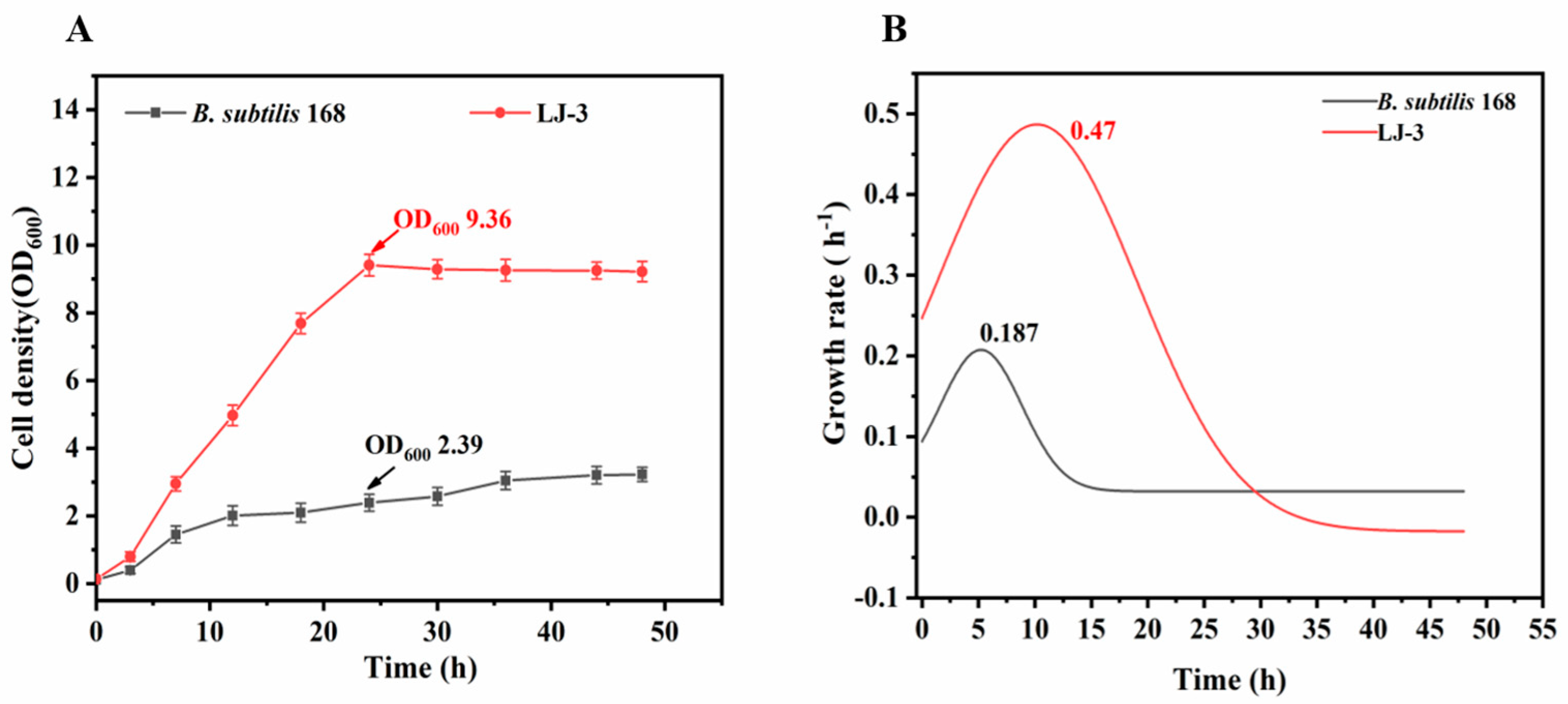
3.1. ALE of B. subtilis 168
3.2. Effect of Metal Ions on Growth of LJ-3
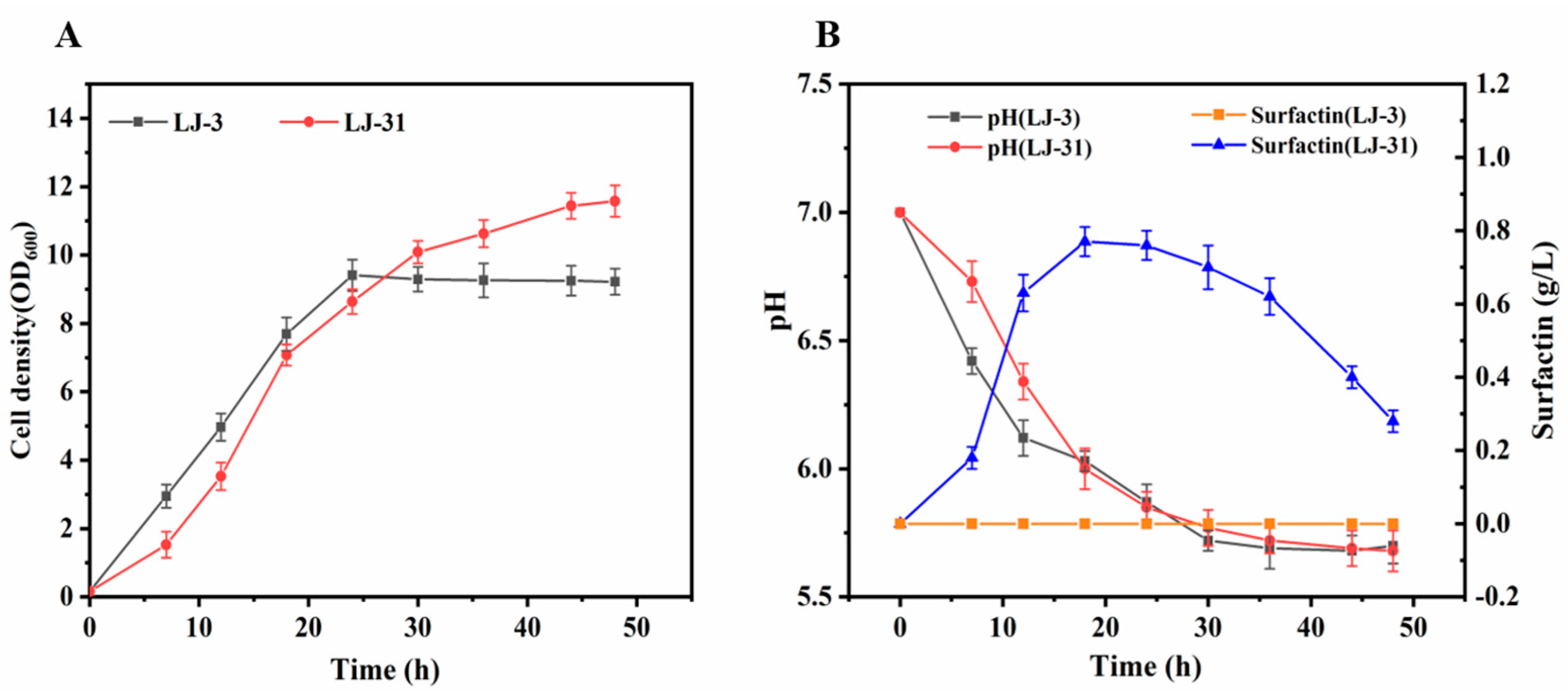
3.3. LJ-3 Was Used as Engineering Chassis to Produce Surfactin
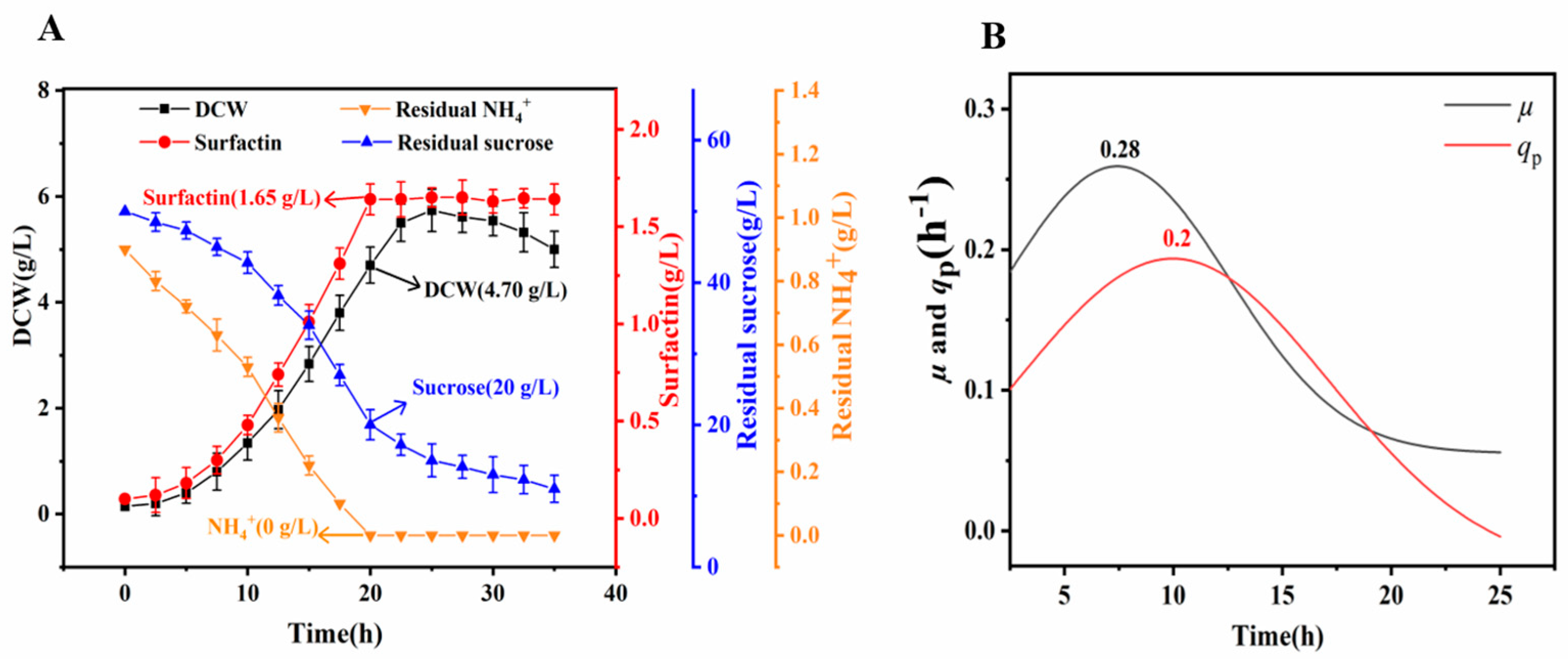
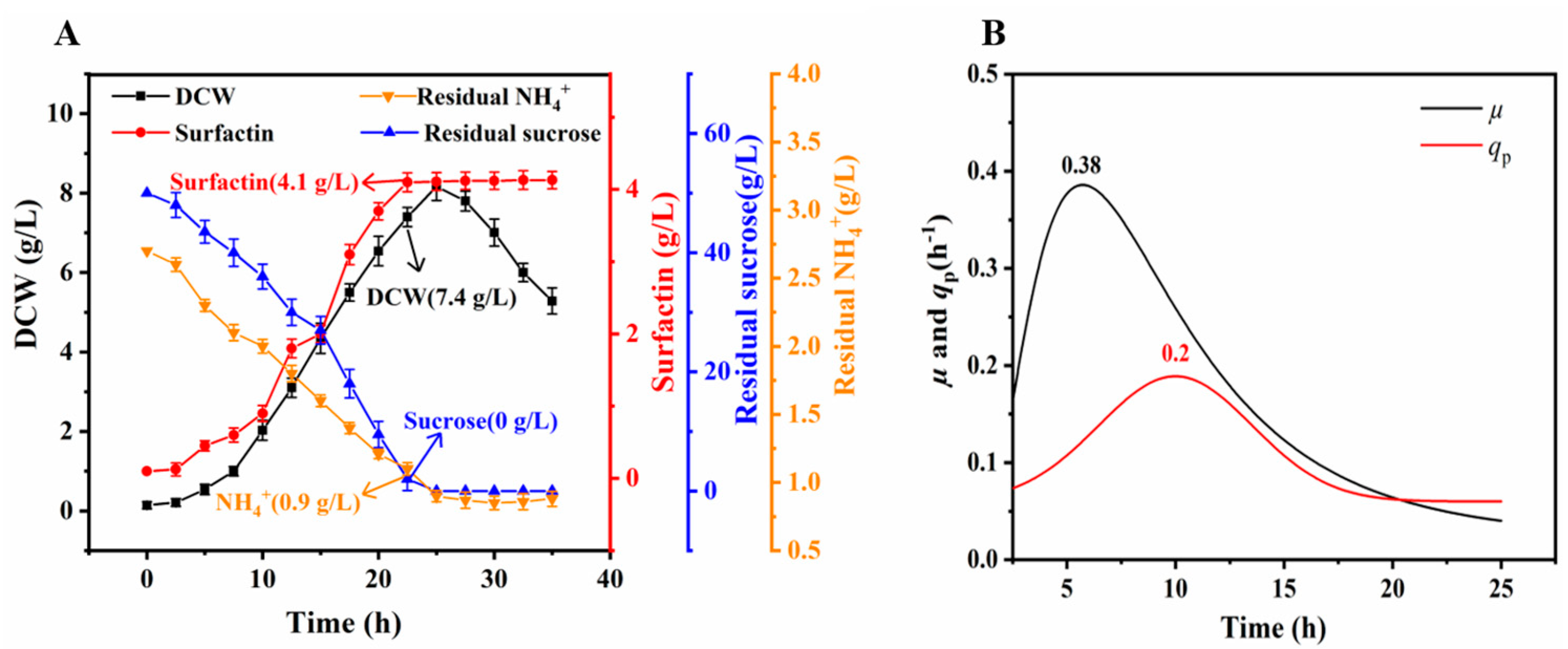
3.4. Production of Surfactin by LJ-31 in a Bioreactor
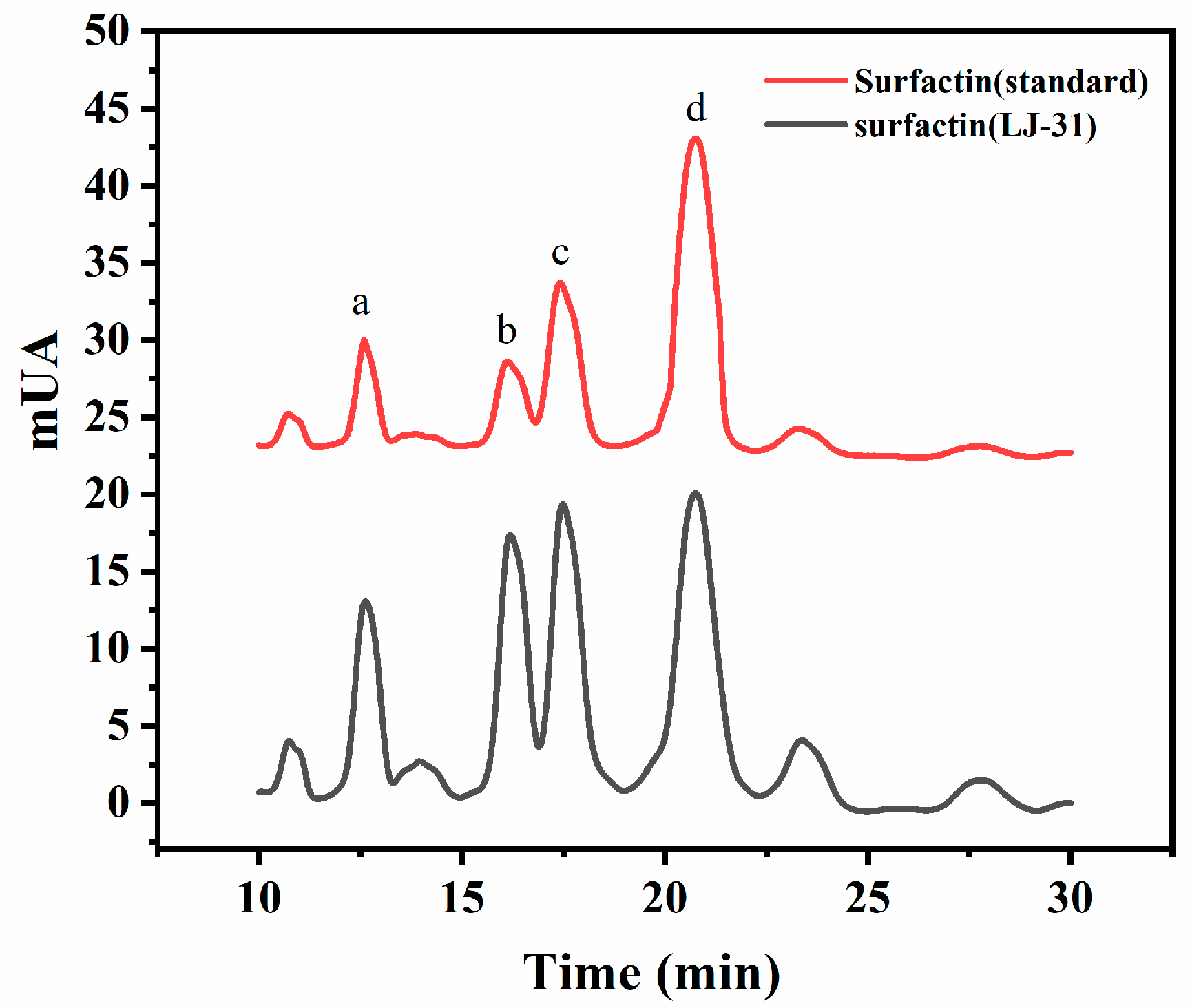
3.5. Composition of Surfactin Synthesized by LJ-31
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Westers, L.; Westers, H.; Quax, W.J. Bacillus subtilis as cell factory for pharmaceutical proteins: A biotechnological approach to optimize the host organism. Biochim. Biophys. Acta-Mol. Cell Res. 2004, 1694, 299–310. [Google Scholar] [CrossRef]
- He, L.L.; Liu, L.; Ban, R. Construction of a mutant Bacillus subtilis strain for high purity poly-gamma-glutamic acid production. Biotechnol. Lett. 2022, 44, 991–1000. [Google Scholar] [CrossRef]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- Rahman, F.B.; Sarkar, B.; Moni, R.; Rahman, M.S. Molecular genetics of surfactin and its effects on different sub-populations of Bacillus subtilis. Biotechnol. Rep. 2021, 32, e00686. [Google Scholar] [CrossRef]
- Li, S.S.; Huang, D.; Li, Y.; Wen, J.P.; Jia, X.Q. Rational improvement of the engineered isobutanol-producing Bacillus subtilis by elementary mode analysis. Microb. Cell Factories 2012, 11, 101. [Google Scholar] [CrossRef]
- Gu, Y.; Deng, J.Y.; Liu, Y.F.; Li, J.H.; Shin, H.D.; Du, G.C.; Chen, J.; Liu, L. Rewiring the glucose transportation and central metabolic pathways for overproduction of N-Acetylglucosamine in Bacillus subtilis. Biotechnol. J. 2017, 12, 1700020. [Google Scholar] [CrossRef]
- Mirouze, N.; Bidnenko, E.; Noirot, P.; Auger, S. Genome-wide mapping of TnrA-binding sites provides new insights into the TnrA regulon in Bacillus subtilis. Microbiologyopen 2015, 4, 423–435. [Google Scholar] [CrossRef]
- Bennett, A.F.; Hughes, B.S. Microbial experimental evolution. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R17–R25. [Google Scholar] [CrossRef]
- Sandberg, T.E.; Salazar, M.J.; Weng, L.L.; Palsson, B.O.; Feist, A.M. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metab. Eng. 2019, 56, 1–16. [Google Scholar] [CrossRef]
- Hu, F.X.; Cai, W.J.; Lin, J.Z.; Wang, W.D.; Li, S. Genetic engineering of the precursor supply pathway for the overproduction of the nC(14)-surfactin isoform with promising MEOR applications. Microb. Cell Factories 2021, 20, 96. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, J.Z.; Wang, W.D.; Huang, H.; Li, S. Production of surfactin isoforms by Bacillus subtilis BS-37 and its applicability to enhanced oil recovery under laboratory conditions. Biochem. Eng. J. 2015, 93, 31–37. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Shi, L.L.; Ye, B.; Feng, H.C.; Zhang, J.; Zhang, R.F.; Yan, X. pheS*, an effective host-genotype-independent counter-selectable marker for marker-free chromosome deletion in Bacillus amyloliquefaciens. Appl. Microbiol. Biotechnol. 2017, 101, 217–227. [Google Scholar] [CrossRef]
- Hu, F.X.; Liu, Y.Y.; Lin, J.Z.; Wang, W.D.; Li, S. Efficient production of surfactin from xylose-rich corncob hydrolysate using genetically modified Bacillus subtilis 168. Appl. Microbiol. Biotechnol. 2020, 104, 4017–4026. [Google Scholar] [CrossRef]
- Duarte-Delgado, D.; Narvaez-Cuenca, C.E.; Restrepo-Sanchez, L.P.; Kushalappa, A.; Mosquera-Vasquez, T. Development and validation of a liquid chromatographic method to quantify sucrose, glucose, and fructose in tubers of Solanum tuberosum Group Phureja. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2015, 975, 18–23. [Google Scholar] [CrossRef]
- Klausmann, P.; Hennemann, K.; Hoffmann, M.; Treinen, C.; Aschern, M.; Lilge, L.; Heravi, K.M.; Henkel, M.; Hausmann, R. Bacillus subtilis High Cell Density Fermentation Using a Sporulation-Deficient Strain for the Production of Surfactin. Appl. Microbiol. Biotechnol. 2021, 105, 4141–4151. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Xu, H.; Li, S.; Ying, H.J.; Ouyang, P.K. Enhanced welan gum production using a two-stage agitation speed control strategy in Alcaligenes sp. CGMCC2428. Bioprocess Biosyst. Eng. 2011, 34, 95–102. [Google Scholar] [CrossRef]
- Wu, Q.; Zhi, Y.; Xu, Y. Systematically engineering the biosynthesis of a green biosurfactant surfactin by Bacillus subtilis 168. Metab. Eng. 2019, 52, 87–97. [Google Scholar] [CrossRef]
- Detsch, C.; Stulke, J. Ammonium utilization in Bacillus subtilis: Transport and regulatory functions of NrgA and NrgB. Microbiology 2003, 149, 3289–3297. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Zhang, Y.Y.; Qiao, H.T.; He, J.T.; Yuan, Q.; Chen, X.N.; Fan, J.F. High-cell-density culture enhances the antimicrobial and freshness effects of Bacillus subtilis S1702 on table grapes (Vitis vinifera cv. Kyoho). Food Chem. 2019, 286, 541–549. [Google Scholar] [CrossRef]
- Zhou, Q.; Takenaka, S.; Murakami, S.; Seesuriyachan, P.; Kuntiya, A.; Aoki, K. Screening and characterization of bacteria that can utilize ammonium and nitrate ions simultaneously under controlled cultural conditions. J. Biosci. Bioeng. 2007, 103, 185–191. [Google Scholar] [CrossRef]
- Huang, X.F.; Liu, J.N.; Wang, Y.H.; Liu, J.; Lu, L.J. The positive effects of Mn2+ on nitrogen use and surfactin production by Bacillus subtilis ATCC 21332. Biotechnol. Biotechnol. Equip. 2015, 29, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.W.; Yang, Z.Y.; Kang, Z.H.; Yu, F.; Long, X.W. Enhanced surfactin fermentation via advanced repeated fed-batch fermentation with increased cell density stimulated by EDTA-Fe (II). Food Bioprod. Process. 2021, 127, 288–294. [Google Scholar] [CrossRef]
- Lau, C.K.Y.; Krewulak, K.D.; Vogel, H.J. Bacterial ferrous iron transport: The Feo system. FEMS Microbiol. Rev. 2016, 40, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Tanaka, E.; Nakano, Y. Purification and characterization on glutamine synthetase from Alkalophilic Bacillus No. 170. J. Gen. Appl. Microbiol. 2006, 41, 269–279. [Google Scholar] [CrossRef]
- Tanaka, E.; Kimura, K. Identification of amino acid residues modified by two ATP analogs in Bacillus subtilis glutamine synthetase. J. Biochem. 1991, 110, 780–784. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Hu, F.X.; Lin, J.Z.; Wang, W.D.; Li, S. Genome and transcriptome analysis of Bacillus velezensis BS-37, an efficient surfactin producer from glycerol, in response to d-/l-leucine. Microbiologyopen 2019, 8, e794. [Google Scholar] [CrossRef] [PubMed]
- Cosby, W.M.; Vollenbroich, D.; Lee, O.H.; Zuber, P. Altered srf expression in Bacillus subtilis resulting from changes in culture pH is dependent on the Spo0K oligopeptide permease and the ComQX system of extracellular control. J. Bacteriol. 1998, 180, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, H.M.; Wang, M.M.; Yang, H.; Shen, Z.Y. Enhanced biosynthesis and characterization of surfactin isoforms with engineered Bacillus subtilis through promoter replacement and Vitreoscilla hemoglobin co-expression. Process Biochem. 2018, 70, 36–44. [Google Scholar] [CrossRef]
- Yi, G.B.; Liu, Q.; Lin, J.Z.; Wang, W.D.; Huang, H.; Li, S. Repeated batch fermentation for surfactin production with immobilized Bacillus subtilis BS-37: Two-stage pH control and foam fractionation. J. Chem. Technol. Biotechnol. 2017, 92, 520–525. [Google Scholar] [CrossRef]
- Ghribi, D.; Ellouze-Chaabouni, S. Enhancement of Bacillus subtilis Lipopeptide Biosurfactants Production through Optimization of Medium Composition and Adequate Control of Aeration. Biotechnol. Res. Int. 2011, 2011, 653654. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ren, B.A.; Chen, M.; Wang, H.B.; Kokare, C.R.; Zhou, X.L.; Wang, J.D.; Dai, H.Q.; Song, F.H.; Liu, M. Production and characterization of a group of bioemulsifiers from the marine Bacillus velezensis strain H3. Appl. Microbiol. Biotechnol. 2010, 87, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Durval, I.J.B.; Resende, A.H.M.; Figueiredo, M.A.; Luna, J.M.; Rufino, R.D.; Sarubbo, L.A. Studies on Biosurfactants Produced using Bacillus cereus Isolated from Seawater with Biotechnological Potential for Marine Oil-Spill Bioremediation. J. Surfactants Deterg. 2019, 22, 349–363. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Xu, Q.; Jiang, L.; Huang, H.; Li, S. Polydiacetylene-Based High-Throughput Screen for Surfactin Producing Strains of Bacillus subtilis. PLoS ONE 2014, 9, e88207. [Google Scholar] [CrossRef] [PubMed]


| Names | Characteristics | References |
|---|---|---|
| Strains | ||
| B. subtilis 168 | start strain | This paper |
| B. subtilis 168 (Pveg-GFP) | B. subtilis 168 derivative, containing Pveg promoter (Strong promoter of Bacillus subtilis) | [10] |
| Bacillus. velezensis BS-37 | Wild-type, surfactin producer | [11] |
| LJ-3 | B. subtilis 168 variant | This study |
| LJ-31 | LJ-3 derivative, sfp+, Overexpression of the sfp gene derived from B. velezensis BS-37 was regulated by the promoter Pveg | This study |
| Plasmid | ||
| pNZT1-pheS* | pNZT1 with Pbc-pheS*-cat cassette | [12] |
| Primers | Primer Sequence (5′-3′) | Gene Fragment |
|---|---|---|
| LF-F | TTTGTGATTTTCAGCGTGATTGAAAACCT | LF |
| LF-R | CTGTGTAAGATAGATCTCTAGATCCTCCGTCTGCAAAAGATTGT | |
| Pveg-F | AGACGGAGGATCTAGAGATCTATCTTACACAGCATCACACTGG | Pveg |
| Pveg-R | ACTCCGTAAATCTTCATGTTTGTCCTCCTTATTAGTTAATCTACA TTTAT | |
| Sfp-F | AATAAGGAGGACAAACATGAAGATTTACGGAGTATATATGGACCGC | Sfp+ |
| Sfp-R | GCGCACTGAAAAGGAATTATAACAGCTCTTCATACGTTTTCATC TCAATC | |
| DR-F | GAGATGAAAACGTATGAAGAGCTGTTATAATTCCTTTTCAGTG CGCCTGC | DR |
| DR-R | TCATTTGTATACATACTTTAAAAATAGATTATCCGAAAGAAAAT CTATTA | |
| PC-F | TAATAGATTTTCTTTCGGATAATCTATTTTTAAAGTATGTATACA AATGA | PC |
| PC-R | ATAAATTCCGTAAATCTTCATTTATAAAAGCCAGTCATTAGGCCTATCTG | |
| RF-F | CCTAATGACTGGCTTTTATAAATGAAGATTTACGGAATTTATAT GGACCG | RF |
| RF-R | TCTCCTTGAGGCGATAGACCG |
| Conditions | Sucrose | NH4+ | DCW | Pmax | YP/S | YP/X | |
|---|---|---|---|---|---|---|---|
| (g/L) | (g/L) | (g/L) | (g/L) | (g/g) | (g/g) | ||
| pH 6.5 Oxygen supply (vvm): 0.06 | |||||||
| C/N (mol/mol) | 35 | 20 ± 1.2 | 0 | 4.70 ± 0.20 | 1.65 ± 0.05 | 0.055 | 0.35 |
| 17.51 | 7.6 ± 2.0 | 0.55 ± 0.18 | 6.62 ± 0.12 | 1.90 ± 0.04 | 0.045 | 0.29 | |
| 11.68 | 0 | 0.81 ± 0.12 | 8.24 ± 0.23 | 3.82 ± 0.06 | 0.076 | 0.46 | |
| 7 | 0 | 2.30 ± 0.18 | 10.72 ± 0.17 | 3.50 ± 0.07 | 0.070 | 0.33 | |
| pH 6.5 C/N (mol/mol): 11.68 | |||||||
| Oxygen supply (vvm) | 0.13 | 6.4 ± 1.5 | 1.29 ± 0.11 | 6.68 ± 0.21 | 1.60 ± 0.08 | 0.037 | 0.24 |
| 0.06 | 0 | 0.81 ± 0.12 | 8.24 ± 0.33 | 3.82 ± 0.06 | 0.076 | 0.29 | |
| 0.03 | 0 | 0.90 ± 0.10 | 7.40 ± 0.25 | 4.10 ± 0.04 | 0.082 | 0.55 | |
| 0.02 | 6.93 ± 1.3 | 1.27 ± 0.17 | 6.10 ± 0.21 | 2.55 ± 0.05 | 0.059 | 0.42 | |
| C/N (mol/mol): 11.68 Oxygen supply (vvm): 0.03 | |||||||
| pH | 6 | 1.50 ± 1.2 | 0.94 ± 0.12 | 8.11 ± 0.23 | 2.56 ± 0.05 | 0.053 | 0.32 |
| 6.5 | 0 | 0.90 ± 0.10 | 7.40 ± 0.35 | 4.10 ± 0.04 | 0.082 | 0.55 | |
| 7 | 0.17 ± 2.1 | 0.90 ± 0.15 | 8.29 ± 0.32 | 3.85 ± 0.06 | 0.077 | 0.46 | |
| 7.5 | 0.19 ± 1.9 | 0.70 ± 0.18 | 8.97 ± 0.26 | 3.70 ± 0.07 | 0.074 | 0.41 | |
| Process, Strains | Nitrogen Source | Biomass | Pmax | Reference |
|---|---|---|---|---|
| LJ-31 (B. subtilis 168 derivative bacteria) batch fermentation in bioreactor | NH4Cl | 7.4 g/L (DCW) | 4.1 (g/L) | This study |
| B. subtilis ATCC 21332 (commercial strain) batch fermentation in shake flasks | NaNO3, KNO3 | 10.2 (OD600) | 3.054 (g/L) | [22] |
| Bacillus subtilis SPB1 strain wild-type batch fermentation in bioreactor | Urea, NH4Cl, kerosene | 20 × 108 (cells/mL) | 4.922 (g/L) | [30] |
| Bacillus velezensis H3 (wild-type) batch fermentation in shake flasks | (NH4)2SO4 | — | 0.4–0.5 (g/L) | [31] |
| B. cereus BCS0 (wild-type) batch fermentation in shake flasks | Urea, NH4Cl, NaNO3 | — | 0.7–1.22 (g/L) | [32] |
| B. cereus BCS1 (wild-type) batch fermentation in shake flasks | Urea, NH4Cl, NaNO3 | — | 0.11–0.5 (g/L) | [32] |
| B. cereus BCS2 (wild-type) batch fermentation in shake flasks | Urea, NH4Cl, NaNO3 | — | 0.51–2.71 (g/L) | [32] |
| B. cereus BCS3 (wild-type) batch fermentation in shake flasks | Urea, NH4Cl, NaNO3 | — | 1.7–2.91 (g/L) | [32] |
| 168 (JABs24) and 3NA (JABs32) batch fermentation in bioreactor | (NH4)2SO4 | — | 2.56–2.68 (g/L) | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Tao, W.; Yue, S.; Yuan, Z.; Li, S. Adaptive Laboratory Evolution of Bacillus subtilis 168 for Efficient Production of Surfactin Using NH4Cl as a Nitrogen Source. Fermentation 2023, 9, 525. https://doi.org/10.3390/fermentation9060525
Li J, Tao W, Yue S, Yuan Z, Li S. Adaptive Laboratory Evolution of Bacillus subtilis 168 for Efficient Production of Surfactin Using NH4Cl as a Nitrogen Source. Fermentation. 2023; 9(6):525. https://doi.org/10.3390/fermentation9060525
Chicago/Turabian StyleLi, Jie, Weiyi Tao, Shenghui Yue, Zhangzhong Yuan, and Shuang Li. 2023. "Adaptive Laboratory Evolution of Bacillus subtilis 168 for Efficient Production of Surfactin Using NH4Cl as a Nitrogen Source" Fermentation 9, no. 6: 525. https://doi.org/10.3390/fermentation9060525
APA StyleLi, J., Tao, W., Yue, S., Yuan, Z., & Li, S. (2023). Adaptive Laboratory Evolution of Bacillus subtilis 168 for Efficient Production of Surfactin Using NH4Cl as a Nitrogen Source. Fermentation, 9(6), 525. https://doi.org/10.3390/fermentation9060525





