Abstract
In recent years, the development of non-dairy probiotic beverages has been stimulated due to the increase in the number of people with milk protein allergies, lactose intolerance, and those that are vegetarian and vegan eating. These functional foods have a number of health benefits, combining properties of plant matrices and probiotic effects. However, a major challenge in formulating these beverages is the limited number of adapted microbial strains with probiotic phenotype that promote desirable sensory characteristics, besides remaining viable in the final product for long periods. Therefore, this review aimed to provide an overview of the production of traditional non-dairy fermented beverages produced in the world and to show the biotechnological potential of these foods as a source of strains presenting a probiotic phenotype. In addition, the latest developments on the role of lactic acid bacteria, Bifidobacterium, and yeast species in the development of new probiotic beverages from the fermentation of fruit and cereal are discussed. Finally, some aspects related to food safety issues are shown.
1. Introduction
Functional foods are foods that provide health benefits in addition to basic nutrition. Regular consumption of probiotic products can be beneficial for improving gut health, enhancing immune function, and reducing inflammation, thus making them a popular choice for functional foods [1,2]. It is speculated that probiotic products account for about 60 to 70% of the total functional food market [3,4]. This success has been achieved mainly due to the development of dairy foods, such as fermented milk, ice cream, cheese, yogurt, milk powder, and other products containing probiotics [4,5]. The most recent definition of probiotics, as established by the International Scientific Association for Probiotics and Prebiotics (ISAPP), is “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”. This definition underscores the significance of consuming a sufficient quantity of live microorganisms and the requirement for scientific evidence supporting their health-promoting effects.
The increasing number of cases of lactose intolerance and milk protein (casein) allergy, coupled with a change in eating habits (i.e., vegetarian or vegan diet) of a significant portion of the population, has stimulated dairy companies to develop and add new non-dairy functional foods in their portfolios [6,7,8,9,10]. In this context, fermented beverages produced from fruits, cereals, and vegetables have received attention from researchers due to the possibility of offering a food rich in minerals, vitamins, proteins, antioxidants, and dietary fiber [11,12,13]. However, there are still several challenges to overcome, as there is little information about the fermentative processes of these food matrices compared to the dairy industry [3]. On the other hand, a wide variety of traditional/regional fermented non-dairy substrate-based foods exist worldwide, but their commercialization is limited due to the lack of standardized manufacturing methodology and processes [14,15]. Thus, an alternative to aid in the development of new products for the food industry is to understand the microbial diversity and processes involved in the production of traditional non-dairy fermented beverages [16]. In general, these rudimentary foods are a rich source of lactic acid bacteria (LAB), acetic acid bacteria (AAB), and yeasts that may exhibit probiotic properties [17,18]. As they are well adapted to plant substrates, microorganisms obtained from spontaneous fermentations may also confer some technological advantages over commercial strains, as a large proportion of probiotics used in the industry have been isolated from the human gastrointestinal tract and may not adapt to the plant matrix [4].
From a technological point of view, one of the major challenges in using probiotic strains to formulate non-dairy fermented beverages is to ensure the viability of these microorganisms for long periods. Several factors such as storage temperature, pH, oxygen concentration, and the presence of other microbial groups can influence the survival of these microorganisms [15,19]. Thus, fruit juices have been suggested as an ideal substrate for the development of probiotic beverages because they contain high contents of sugars and nutrients that are important for the growth and maintenance of cell viability. In addition, fruit juices generally exhibit greater acceptance by consumers due to their pleasant flavors and aromas [14,19,20,21]. On the other hand, cereals also exhibit interesting characteristics because, in addition to supporting microbial growth, they contain carbohydrates that protect cells against the extreme conditions of the gastrointestinal tract, making them good vectors for probiotic delivery [22].
Therefore, the present review focused on encouraging researchers and the food industry to explore the biotechnological potential of non-dairy fermented beverages as a source of microorganisms with probiotic potential. In addition, the positive impact of consuming probiotic beverages produced from the fermentation of fruit and cereal juices on human health is briefly shown.
2. Fermented Products
It is speculated that traditional fermented non-dairy beverages have been produced by humans since around 7000 B.C. [23]. These foods are known to confer several health benefits due to their biological properties that include anti-inflammatory, antioxidant, antimicrobial, immunomodulatory, antihypertensive, and antidiabetic effects [11,15,24,25]. These health effects are associated with the presence of viable beneficial microorganisms (probiotics) [1], their metabolites and cellular fragments (postbiotics) [26], and non-digestible fibers (prebiotics) [27]. Therefore, the subsequent topics of this section deal with the production method of some fermented non-dairy beverages produced from cereals, fruits, and vegetables that can be used as a source for prospecting new strains with probiotic potential.
2.1. Chhang and Jau Chhang
Chhang and jau chhang are fermented alcoholic beverages produced by the native peoples inhabiting the Himalayan belt in India [28]. These beverages are described as sweet and smooth and are prepared using a starter culture called “phab” and their respective substrates; rice is used to produce chhang and barley is used for the production of jau chhang. However, other substrates such as grains of wheat and grapes can replace rice and barley, respectively [29,30]. In general, the production of chhang and jau chhang can be divided into four main steps: (i) boiling the grains, (ii) adding the starter culture “phab”, (iii) fermentation, and (iv) extraction and filtration.
Before carrying out the fermentation, it is necessary to prepare the substrate. Initially, the grains and water are transferred to a large container where the cooking takes place using on average a ratio of 2:1 substrate water. This process must be performed on low heat and is finished after the grains absorb most of the water. After cooking, the excess water is removed by spreading the grains on a cloth. This mass is then mixed at regular intervals until it reaches a temperature of 28–32 °C. The starter culture (i.e., phab) is obtained locally in tablet form and must be ground and spread evenly over the cooked grains. After inoculation, this mass is transferred to a cloth bag and placed on a straw mound with a stone on top to keep the high temperature. Usually, this process can take up to two days during the summer but can require up to seven in the winter. To determine the end point of growth of the starter culture, native peoples use a combination of the moisture of the grain and the smell of the fermentation [29,30].
To perform fermentation, the contents of the bag are transferred to a clay jar and capped with a stone wrapped in a clean cloth. These conditions create an environment with low oxygen concentration favoring the growth of microorganisms with fermentative metabolism, mainly LAB and yeast [29]. The work conducted by Thakur. et al. [29] used culture-dependent methods to evaluate microbial growth during the fermentative process of chhang and jau chhang. At the end of the fermentation process, the number of LAB was in the vicinity of 1.7 × 104 CFU/g in chhang and 2.9 × 104 CFU/g in jau chhang, while yeast showed a population of 3.5 × 104 CFU/g for chhang, 1 × 105 CFU/g for jau chhang. In general, fermentation requires around 7–10 days and the completion of this process is determined almost exclusively by an elderly woman through tasting the fermented grains. After completing this stage, the fermented contents are removed from the clay jar and transferred to a cylindrical wooden drum containing a volume of water capable of covering the beans for extraction. This process is carried out for a period between 2 and 5 h, and the longer the extraction time, the higher the alcohol content of the beverage. Then, the liquid fraction is retained from the drum and filtered to obtain chhang. This process can be repeated about four times. The initial filtrates have a higher alcohol content, and based on this characteristic and taste, some mixtures are made between the different filtrates, resulting in a final beverage that contains between 5 and 7% alcohol and a pH between 3.6 and 3.8. Finally, chhang should be consumed soon after preparation and should not be stored [29,30,31,32].
2.2. Tarubá
Tarubá is a low-alcohol beverage produced from cassava by the Indigenous people of the Sateré-Mawé tribe, on the border of the states of Amazonas and Pará in Brazil. To produce this beverage, the cassava must be washed in running water, peeled, grated, and transferred to a traditional Indigenous instrument called tipiti, which works as a kind of press to separate the liquid fraction from the solid. After this process, a wet flour is obtained that must be sifted and baked for about 30 min, resulting in something similar to a cookie known as beiju. Then, beiju is transferred to a wooden tray and covered with candiúba (Trema micrantha) and/or banana (Musa spp.) leaves moistened with water and left for about 12 days for the fermentative process to occur [16,33]. As this is a process carried out in the presence of oxygen, high ethanol production is not observed, but generally, after 8 days of fermentation, low concentrations (about 0.25 g/kg) of alcohol are detected [34]. Regarding the microbiota involved in this process, Ramos et al. [34] showed that AAB, LAB, and yeast are the predominant microorganisms that have an important role in starch hydrolysis, and no production of organic acids and volatile compounds that impact the sensory profile of the beverage occur. However, a population of 4.6 log CFU/g enterobacteria was identified after 12 days of fermentation, and the authors associated this increase with the environmental and hygienic conditions of the process [34]. Finally, the fermented mass is diluted in water and filtered, obtaining tarubá. This beverage is often consumed as a daily tonic by Indigenous peoples [16,33,34].
2.3. Chicha
Chinchas are a large group of traditional alcoholic beverages produced by the Indigenous communities inhabiting the Andes and some lowland regions of Ecuador, Brazil, Bolivia, Colombia, Peru, and Argentina for over 3000 years [35,36]. This kind of beverage can be produced from various substrates that include corn, rice, cassava, peanuts, and even fruits [35,37]. Traditionally, chicha production begins with the chewing of the substrate by Indigenous women and children resulting in the transfer of amylolytic enzymes for fermentation. The presence of these enzymes along with the microorganisms in the saliva may favor the hydrolysis of the starch into fermentable sugars and thus accelerate the fermentative process [38]. However, other strategies for starch breakdown such as the malting (germination) process of corn kernels or a pre-fermentation step involving heated water can also be used.
For instance, in Ecuador, different types of chichas can be found, such as chicha de jora, chicha de mandioca, and chicha de yamor (also known as seven-grains chicha) [36]. Chicha de jora is produced from yellow corn and is most common in this region. The preparation of this beverage begins with the transfer and incubation of the corn kernels in a container of water for about 13 days. During this period, there is intense metabolic activity within the kernels, and complex carbohydrates are converted into simple sugars. After this process, the beans are dried in the sun to stop the biochemical reactions. The dried grains are then ground into flour that is mixed with water and transferred to a container where spontaneous fermentation occurs for approximately 5 days [35,36,39]. The alcohol content of chicha can vary greatly from 0.8% to 13.2%, but a large part of the beverage types presents values lower than 5.8% [37]. In addition, it is important to note that some herbs and spices can also be added as an alternative to modulating the flavor of the beverage [37].
Regarding the microbial groups involved in this process, several genera of yeasts (e.g., Saccharomyces, Torulaspora, Pichia, Candida, and others) have been reported. However, Torulaspora delbrueckii and Saccharomyces cerevisiae have been the main species identified by culture-dependent and culture-independent methods [37,38]. Among the prokaryotes, Lactiplantibacillus plantarum (former Lactobacillus plantarum), Leuconostoc, Streptococcus, and Weissella are the main representatives of LAB. Other genera such as Bacillus, Klebsiella, Enterobacter, Staphylococcus, and Micrococcus have also been reported. Among AAB, only the genus Acetobacter spp. has been found in chicha de jora [38,40,41,42,43].
2.4. Apple Cider
Apple cider is a fermented beverage consumed almost everywhere in the world. However, there are several types of ciders on the market, as each country has a traditional method of production. For example, British cider is produced from inoculated fermentations with commercial yeasts, resulting in a fast process and a beverage with higher alcohol content. On the other hand, French cider is produced from spontaneous fermentations without modern additives or treatment. Due to this characteristic, French cider tends to have fruity aromas and flavors in addition to the lower alcohol content [44]. In general, the production of this beverage starts with the washing and separation of defective and rotten apples. The remaining fruit is crushed and ground into small pieces producing a pulp. In the French cider preparation process, the pulp is oxidized for up to 5 h and pressed. Fermentation is carried out by indigenous microbiota from the fruits themselves, with the yeast Saccharomyces being commonly reported as the predominant group at the beginning of the fermentation process. However, other genera including Candida, Pichia, Hanseniaspora, and Metschnikowia are also reported [44,45,46]. Among the bacterial community, heterofermentative LAB species such as Secundilactobacillus collinoides (former L. collinoides), S. paracollinoides (former L. paracollinoides), Limosilactobacillus fermentum (former L. fermentum), Lentilactobacillus buchneri (former L. buchneri), Lentilactobacillus (former L. hilgardii), Lentilactobacillus diolivorans (former L. diolivorans), Paucilactobacillus suebicus (former L. suebicus) and, L. plantarum are frequently identified [44]. In general, cider fermentation is carried out with light to moderate agitation, and this process can take from 1 to 3 months. After this period, a clarification step is performed, and this process can be performed by centrifugation, filtration, or sedimentation. Finally, the cider is bottled, and carbonation or yeast can be added to trigger the second fermentation in the bottle itself [44].
2.5. Water Kefir
Water kefir grains are used in alternative substrates, such as vegetables, fruits, and molasses, to produce functional beverages with distinct sensory characteristics [47,48]. These beverages are described as acidic, refreshing, mild in carbon dioxide, and low in acetic acid and alcohol [49]. Water kefir grains are formed mostly by a matrix of a dextran exopolysaccharide. Associated with this matrix is a complex and high microbial diversity, composed mainly of yeasts (e.g., S. cerevisiae and Dekkera bruxellensis), LAB (e.g., Lacticaseibacillus casei (former L. casei), Lactiplantibacillus pentosus (former L. pentosus), L. plantarum, L. hilgardii) and AAB (e.g., Acetobacter lovaniensis and A. fabarum). However, this microbiota shows large variations between kefir grains grown in different regions, and microbial succession during the fermentative process is still unclear [50]. Currently, beverage production from water kefir fermentation is performed almost exclusively at household levels using the backslopping technique (i.e., kefir grains are recovered from one fermentation and inoculated into a new fermentation), as the grains are delivered from person to person [51]. From an industrial point of view, the kefir fermentation process is difficult, as this process has a low reproducibility rate with many microbial species involved in this fermentation, resulting in an unstable microbiota [51].
2.6. Kombucha
Kombucha is a traditional beverage produced from the fermentation of green or black tea (Camellia sinensis) that has an acidic and slightly sweet flavor [52]. The tea fermentation process is carried out by a cooperative microbial community of yeasts and bacteria, which are embedded in a cellulose biofilm known as the SCOBY (Symbiotic Colony of Bacteria and Yeasts) [53]. In general, the production of this beverage starts with the preparation of tea, and then sugar is added (e.g., mainly sucrose), which serves as the substrate for the SCOBY [53]. The main bacterial species found in kombucha include Gluconacetobacter xylinus (former Acetobacter xylinum) and Gluconacetobacter hansenii (former Acetobacter hansenii). The primary yeast species found are Saccharomyces cerevisiae and Brettanomyces bruxellensis, while the less frequently found LAB consist mainly of the Lactobacillus genus (e.g., Lactobacillus brevis and Lactobacillus plantarum). Other bacterial genera such as Bifidobacterium, Enterococcus and Propionibacterium are also reported [53,54,55]. Regarding the fermentative parameters for kombucha production, the literature shows significant variation. For instance, Watawana et al. [56] suggest that the process be carried out from 3 to 60 days at room temperature, while Jayabalan et al. [57] suggest using temperatures between 20 and 22 °C for 7 to 10 days. However, work conducted Neffe-Skocińska et al. [58] showed that the ideal conditions for kombucha production are 10 days of fermentation at 25 °C. These parameters resulted in a microbiologically stable product, high sensory quality, and increased acid production, including pro-health glucuronic acid. To obtain comprehensive information regarding the microbiological and physicochemical aspects of kombucha, readers are advised to refer to reviews authored by Miranda et al. [52], Coelho et al. [53], Laavanya et al. [59], Mousavi et al. [60], and Kapp et al. [61].
3. Microbial Diversity
3.1. Lactic Acid Bacteria
Non-dairy fermented beverages present rich diversity of bacteria, yeasts, and fungi species (Table 1 and Table 2). Especially in traditional beverages, open system fermentation practice increases microorganism group multiplicity due to the use of raw materials, the surrounding environment and human contact [62]. Among the bacterial groups that inhabit these beverages, LAB broadly predominate in fermentation process, playing fundamental role in final product. They comprise more than half of bacterial species reported in this study, which are divided into two major clades (low-GþC-content Firmicutes phylum and high-GþC-content Bifidobacterium), low GþC content occurring in the taxonomic genera Lactobacillus, Leuconostoc, Pediococcus, Lactococcus, Enterococcus, Weissella, and Oenococcus. Lactobacillus is the most recurrent genera in the non-dairy beverages, with L. plantarum and L fermentum as the most frequent species, present in jau, chhang, chicha, tarubá and water kefir grains. Other less common species include Lacticaseibacillus pantheris (former L. pantheris), Lacticaseibacillus manihotivorans (former L. manihotivorans), Paucilactobacillus vaccinostercus (former L. vaccinostercus), Ligilactobacillus ruminis (former L. ruminis), Lactobacillus amylophilus (former L. amylophilus), Loigolactobacillus backii (former L. backii), Ligilactobacillus salivarius (former L. salivarius), Liquorilactobacillus nagelii (L. nagelii), S. paracollinoides, and L. hilgardii. The most frequent genera after Lactobacillus are Leuconostoc and Weissela. In apple cider, for example, Leuconostoc is the unique LAB member [45]. More rare LAB species can also dominate diversity in non-dairy beverages as is the case for Pediococcus pentosaceus in the chhang beverage [29]. The occurrence of some species may be associated with the type of substrate used, for example, as is the presence of L. mesenteroides in cassava-based beverages of distinct countries, in chicha from Ecuador and in tarubá from Brazil and L. casei and Lacticaseibacillus paracasei (former L. paracasei) in corn-based beverages. Bifidobacterium, apparently, is a genus noticed in beverages that contains a matrix carriage, such as the SCOBY (Symbiotic Culture of Bacteria and Yeasts) in kombucha, and grains in water kefir. These matrices present anaerobic and microaerobic environments, favoring species of the Bifidobacterium genus [63].
LAB are normally associated to dairy-based beverages as they are highly efficient lactose consumers; however, some LAB can hydrolyze starch as the primary carbon source, and convert it into numerous products, especially lactic acid and other organic acids, in a single fermentation step [64]. It occurs on barley, rice, wheat, cassava and corn-based fermentations. These LAB possess amylolytic activity [65,66], constituting the so-called ALAB group. Several ALAB strains have been isolated from different foods, environments, and regions, for example, L. plantarum from cassava, fish, and rice; L. fermentum from maize sourdoughs; Lactococcus lactis from pickled yams; and P. ethanolidurans from pickles [67]. Other species known for ALAB trace include L. manihotivorans, Amylolactobacillus amylophilus (former L. amylophilus), Lactobacillus amylolyticus, and Lactobacillus amylovorus. These strains act as natural food preservers, and they perform partial hydrolysis, make foods more digestible and contribute to flavor and taste [68]. In addition, they decrease matrix viscosity and produce maltoligosaccharides, which is an indicator of starch hydrolysis [69]. Amylases responsible for this process include mainly cytoplasmic α-amylase and extracellular amylopullulanase. Genome sequencing of ALAB found some key genes shared on these enzymes, for example, amyA, which encodes for an extracellular α-amylase and is shared among ALABs [70].
3.2. Other Bacterial Groups
Members of AAB (e.g., Acetobacter, Gluconobacter, and Komagataeibacter) were also present in non-dairy beverages, especially in apple cider, kombucha and kefir. AAB mainly oxidize ethanol content to acetic acid, promoting a sour taste for the beverages, and they have many applications in the food and biomedical industries as they produce gluconic acid, L-sorbose and bacterial cellulose [71]. Besides AAB, there are plenty of other groups present in these beverages. S. salivarius and S. mutans were found in chicha beer in Ecuador, and their origin is human oral microbiota. They were able to grow and persist in cassava mush [38]. Also externally inoculated, the species Bacillus amyloliquefaciens, C. maltaromaticum, Enterobacter sp., and Serratia sp. were acquired by the environment during chicha preparation [38]. Surprisingly, some might contribute positively to the fermentation process. B. amyloliquefaciens, for example, present in chhang, chicha, tarubá and water kefir grains, is found in food, plants, animals and soil, and has shown probiotic and prebiotic potential. It can synthetize bioactive compounds such as peptides and exopolysaccharides; antimicrobial compounds; hydrolyze insoluble proteins, carbohydrates, fibers, hemicellulose, and lignin [72].


Table 1.
List of the main bacteria identified by culture-dependent and culture-independent methods in non-dairy fermented beverages.
Table 1.
List of the main bacteria identified by culture-dependent and culture-independent methods in non-dairy fermented beverages.
| Beverage | Microorganisms | Substrate | Identification Method | Country | References |
|---|---|---|---|---|---|
| Jau Chhang | L. plantarum SAA 595; P. pentosaceus SAA 599; Serratia sp. SAA 601 | Barley/grape | rDNA gene sequencing CD | India | [29] |
| Chhang | P. pentosaceus SAA 599; B. amyloliquefaciens SAA 610 | Rice/wheat | rDNA gene sequencing CD | India | [29] |
| Chicha | L. mesenteroides; L. fermentum; S. mutans; L. lactis; S. salivarius | Chewed cassava | Next-generation sequencing | Ecuador | [73] |
| L. fermentum; S. salivarius; | Mushed cassava | ||||
| L. casei; L. mesenteroides; L. plantarum; L. parabuchneri; L. paracasei; L. pantheris | Corn | ||||
| Chicha | E. asburiae; E. cancerogenus; K. ascorbate; L. brevis *; L. camelliae *; L. delbrueckii; L. fermentum; L. manihotivorans; L. plantarum; Lactobacillus sp.; P. vaccinostercus; L. lactis; Lactococcus sp.; L. lactis; Serratia sp.; S. oralis; S. parasanguinis; S. pneumoniae; S. salivarius; S. thermophilus; S. vestibularis; W. cibaria; W. confusa; W. paramesenteroides; Weissella sp. | Chewed cassava | Next-generation sequencing | Ecuador | [73] |
| Chicha | B. amyloliquefaciens; L. brevis; L. fermentum; Lactococcus sp.; L. citreum; L. lactis; S. gallolyticus; S. oralis; S. parasanguinis; S. pasteurianus; S. pneumoniae; S. salivarius; S. thermophilus; S. vestibularis; W. confusa | Mushed cassava | Next-generation sequencing | Ecuador | [73] |
| Chicha | C. maltaromaticum; Fructobacillus sp., G. intermedius; L. brevis; L. camelliae; L. casei; S. harbinensis *; L. parabuchneri; L. paracasei; S. paracollinoides; L. plantarum; Lactococcus sp.; L. lactis; Leuconostoc sp.; O. kitaharae; W. cibaria; W. confuse; Weissella sp. | Corn | Next-generation sequencing | Ecuador | [73] |
| Tarubá | L. plantarum; L. brevis; L. mesenteroides; L. lactis; P. pentosaceus; B. subtilis; B. amyloliquefaciens; B. licheniformis; Bacillus sp.; A. orientalis; C. terrae; O. intermedium | Cassava | rDNA gene sequencing CD PCR–DGGE analysis | Brazil | [34] |
| Apple cider | Leuconostoc sp.; L. pseudomesenteroides; Gluconobacter sp.; Rahnella; A. malorum; G. oxydans; Gluconobacter cerinus; K. saccharivorans; R. inusitata | Apple | rDNA gene sequencing CD | China | [45] |
| Kombucha | Gluconobacter sp.; Lyngbya sp.; Bifidobacterium sp.; Enterobacter sp.; Weissella sp.; Lactobacillus sp. Leuconostoc sp. | black tea | Next-generation sequencing | India | [55] |
| Water kefir grains | L. ruminis; B. methanolicus; Lactococcus sp. 1JSPR7; A. persici; A. amylophilus; Lactococcus sp. 1JSPR7; Marinilactibacillus sp. 15R; A. xylosoxidans; L. buchneri; P.pentosaceus; M. plutonius; E. faecium; M. plutonius; K. accharivorans; S. aureus; Marinilactibacillus sp. 15R; S. sobrinus; P. synxantha; Marinilactibacillus sp. 15R; L. sakei *; E. faecium; B. amyloliquefaciens; B. thuringiensis; L. backii; A. persici; L. agilis *; L. fermentum | Sugar | Next-generation sequencing | Turkiye | [74] |
* Levilactobacillus brevis (former Lactobacillus brevis); Lacticaseibacillus camelliae (former Lactobacillus camelliae); Schleiferilactobacillus harbinensis (former Lactobacillus harbinensis); Latilactobacillus sakei (former Lactobacillus sakei); Ligilactobacillus agilis (former Lactobacillus agilis). CD = Culture-dependent method.
3.3. Yeasts and Filamentous Fungi
Non-dairy beverages are as rich in fungi species as they are rich in bacteria content. Of all fungi groups presented in Table 2, more than 80% are yeasts, which have the greatest impact and relevance in the fermentation process. S. cerevisiae is unquestionably the most frequent species, as it is present in all beverages except for tarubá. It is the most disseminated and commercially ethanologenic yeast [75]. Yeasts obtain energy by sugar converting them into alcohol, and do not demand much for their growth compared to other microorganism groups. They are able to ferment a vast range of sugars (e.g., glucose, fructose, sucrose, maltose and maltotriose) [75]. Beverages such as kefir, kombucha and apple cider normally have readily fermentable sugars; on the other hand, cassava, corn, rice and barley-based beverages require a pre-hydrolysis of the cereal starches before fermentation by yeasts [76]. Besides S. cerevisiae, S. bayanus is present in chicha [38] and in apple cider [46], also representing the large group of Saccharomyces.
In addition, these beverages harbor plenty of non-Saccharomyces yeasts, especially Candida (C. tropicalis; C. oleophila; C. zeylanoides; C. rugosa; C. ethanolica; C. stellimalicola; C. tropicalis; C. parapsilosis and C. glabrata), Hanseniaspora (H. uvarum; H. guilliermondii; H. opuntiae; H. valbyensis; H. osmophila; H. guilliermondii; H. meyeri and H. vineae) and Pichia (P. kudriavzevii; P. burton; P. fermentans; P. kluyveri; P. fermentans; P. exigua; P. guilliermondii; P. anômala and P. mexicana). Other functional but minor genera include Torulaspora, Rhodotorula, Yarrowia, Wickerhamomyces, Lachancea, Kluyveromyces, Starmera and Dekkera. Non-Saccharomyces contribute positively to sensory characteristics of the beverages through their metabolic activity [75], promoting complex flavor and taste in the final product. However, some groups present a dual effect. Candida, for example, is associated with production of key metabolite and different enzymes; however, C. glabrata and C. parapsilosis are known to cause human infection [77]. Filamentous fungi are present to a lesser extent, mainly in the genera Penicillium, Aspergillus and Fusarium, but also Eremothecium, Mucor, Hyphopichia, Cryptococcus and Galactomyces. These classes might play a significant role in the fermentation processes producing essential enzymes (e.g., α-amylase, amylo glucosidase, cellulase, β-galactosidase, hemicellulase, invertase, lipase, maltase, pectinase, and proteases) [78]. However, they can release undesirable and even toxic metabolites such as mycotoxins (e.g., aflatoxins, alternariol monomethyl ether, deoxynivalenol, fumonisins, and ochratoxin A), posing a risk to human health. Chicha contains Wallemia muriae, a filamentous fungi specie that has been reported to relate tonhuman health problems along with other species of Wallemia genera, causing allergological diseases and subcutaneous/cutaneous infections [79]. In this way, food-borne pathogenic fungi should be monitored in traditional non-dairy beverages.


Table 2.
List of the main yeasts and fungi identified by culture-dependent and culture-independent methods in non-dairy fermented beverages.
Table 2.
List of the main yeasts and fungi identified by culture-dependent and culture-independent methods in non-dairy fermented beverages.
| Beverage | Microorganisms | Substrate | Identification Method | Country | References |
|---|---|---|---|---|---|
| Jau Chhang | C. tropicalis SAA 613; S. cerevisiae SAA 620 | Barley/grape | rDNA gene sequencing CD | India | [29] |
| Chhang | S. cerevisiae SAA 616 | Rice/wheat | rDNA gene sequencing CD | India | [29] |
| Chicha | S. cerevisiae; P. citrinum; D. hansenii; H. uvarum; W. muriae; Wallemia sp.; Aspergillus sp.; P. kudriavzevii; A. versicolor; P. burtonii; H. burtonii; Cyberlindneras; Pichia sp.; S. bayanus; Galactomyces sp.; P. fermentans | Chewed cassava/Mushed cassava | Next-generation sequencing | Ecuador | [55] |
| Chicha | H. guilliermondii; H. opuntiae; H. uvarum; Hanseniaspora sp.; T. delbrueckii; Candida sp.; S. cerevisiae; P. kluyveri; P. kudriavzevii; R. mucilaginosa; R. slooffiae; Cryptococcus sp.; Y. lipolytica; W. anomalus | Rice/oat/grape/mixture of seven corn varieties | rDNA gene sequencing CD | Ecuador | [80] |
| Chicha | Acremonium sp.; Cladosporium sp.; Fusarium sp.; Mucor sp.; Penicillium sp.; Peyronellaea sp.; C. oleophila; C. zeylanoides; C. magnus; D. hansenii; G. candidum; H. uvarum; K. lactis; K. marxianus; M. caribbica; M. guilliermondii; P. fermentans; Pichia sp. NRRL Y-17803; R. mucilaginosa; S. cerevisiae; T. domesticum; W. anomalus | Corn | rDNA gene sequencing CD Next-generation sequencing | Argentina | [41] |
| Tarubá | P. exigua; H. uvarum; C. rugosa; T. delbrueckii; C. tropicalis; P. kudriavzevii; W. anomalus; C. ethanolica; P. manshurica | Cassava | rDNA gene sequencing CD PCR–DGGE analysis | Brazil | [34] |
| Apple cider | Saccharomyces sp.; Hanseniaspora sp.; Torulaspora sp. | Apple | rDNA gene sequencing CD | China | [45] |
| Apple cider | H. valbyensis; H. uvarum; H. osmophila; M. pulcherrima; P. guilliermondii; S. bayanus; S. cerevisiae | Apple | PCR-RFLP | Spain | [46] |
| Pineapple wine | H. guilliermondii; P. anomala; M. guilliermondii; H. uvarum; W. anomalus; M. guilliermondii; H. opuntiae; H. uvarum; | Pineapple | rDNA gene sequencing CD | Angola | [81] |
| Kombucha | C. stellimalicola; C. tropicalis; C. parapsilosis; L. thermotolerans; L. fermentati; L. kluyveri; E. cymbalariae; E. ashbyi; K. marxianus; D. hansenii; P. mexicana; M.caribbica; M. guilliermondii; Z. californica; S. cerevisiae; S. fibuligera; H. uvarum; H. meyeri; H. vineae; M. ingelheimense; S. lactativora; K. telluris; K. exigua; S. amethionina; S. caribaea | black tea | Next-generation sequencing | India | [55] |
| Water kefir grains | P. kudriavzevii; S. cerevisiae; E. cymbalariae; C. glabrata; O. parapolymorpha; T. terrestris; T. phaffii; F. oxysporum; S. lignohabitans | Sugar | Next-generation sequencing | Turkiye | [74] |
CD = culture-dependent method.
4. Beneficial Effects
Clinical trials have demonstrated that regular consumption of probiotics is associated with health benefits [82], in addition to helping in the treatment of metabolic disorders such as obesity, metabolic syndromes and type 2 diabetes, among others [83]. Considering the main probiotic properties, antioxidant [84,85], antimicrobial [86], anti-inflammatory [87] and anticancer effects stand out [88]. Products with antioxidant activity can act to attenuate oxidative stress, inflammation and/or conditions associated with the presence of reactive oxygen species (ROS), such as cancer, Alzheimer’s and Parkinson’s [89] diseases, or even act in the modulation of gut microbiota, in addition to producing substances capable of inactivating toxins, contributing to detoxification processes [90,91,92] (Figure 1).
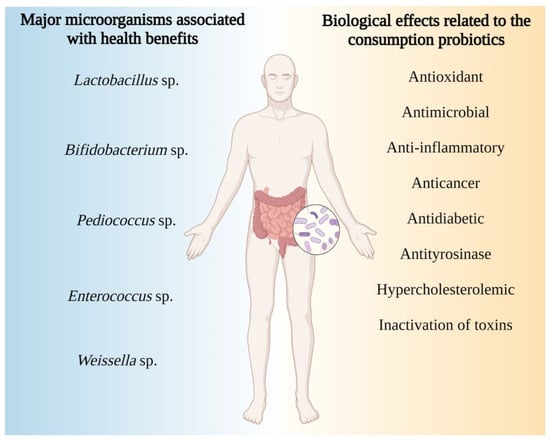
Figure 1.
Relationship of the intake of different probiotic species with their bioactive properties. This figure was created using BioRender (https://biorender.com/).
The regular ingestion of probiotic strains can play an important role in prevention and/or treatment of some types of cancer [93,94]. For example, regular consumption of Lactobacillus sp. and Bifidobacterium sp. was able to significantly reduce the release of pro-inflammatory cytokines, tumor necrosis factor-α and different interleukins (IL-6, IL-10, IL-12, IL-17A, IL-17C and IL-22) directly involved in the acute phase of inflammatory responses [95,96]. In post-surgical colorectal cancer patients, Lactobacillus acidophilus, L. lactis, L. casei, Bifidobacterium longum, Bifidobacterium bifidum, and Bifidobacterium americana showed promising results [95]. Lipopolysaccharides, exopolysaccharides, short-chain fatty acids and proteins correspond to the main components in probiotics related to anticancer activity [96].
Probiotic species of Lactobacillus sp. may reinforce the integrity of the intestinal epithelial tissue, strengthening the barrier of intercellular regions of necrotizing enterocolitis, an important inflammatory disease [97,98]. Among the probiotic species, Lacticaseibacillus rhamnosus (former L. rhamnosus) was the most efficient in protection as it was able to significantly reduce membrane permeability after pre-treatment of epithelial cells [97]. According to the authors, the results suggest that these strains strengthened the intestinal barrier, with consequent improvement in gastrointestinal health. Promising applications of strains of Lactobacillus delbrueckii, L. plantarum, L. acidophilus, L. casei, Bifidobacterium brevis and B. longum have also been demonstrated in previous studies. Thus, the use of probiotics corresponds to a therapeutic strategy capable of contributing to the improvement in condition of patients with gut inflammatory diseases [99].
Among other activities, probiotic strains can also inhibit the growth of undesirable microorganisms through the production of bacteriocins, peroxides, and organic acids [100,101]. Junnarkar et al. [84] demonstrated that the pathogens Klebsiella pneumoniae, Staphylococcus epidermidis, Escherichia coli, Enterobacter cloaceae and Citrobacter freundii controlled by Lactobacillus sp., Enterococcus sp., and Weissella sp., Pediococcus acidilactici, P. pentosaceus, L. plantarum, L. lactis, and Enterococcus faecium are regularly referenced as effective bacteriocinogenic species against foodborne pathogens [86] that, in addition, positively modulate the intestinal microbiota [102].
Finally, a combination of probiotic strains L. acidophilus and B. bifidum was able to reduce the serum levels of total cholesterol, HDL-cholesterol and LDL-cholesterol in hypercholesterolemic patients after 6 weeks of treatment [83]. The effect was mainly associated with cholesterol uptake by growing microbial cells, therefore reducing cholesterol levels in host cells [83,103].
5. Novel Non-Dairy Probiotic Beverages
Considering the growing demand for probiotic non-dairy foods and beverages, new research has been carried out to select promising probiotic strains for industrial application. The LAB group includes those most used as probiotics by the traditional fermented food industry [17,104], with emphasis on Lactobacillus [21,85,105,106,107] and Pediococcus [108,109,110]. In addition to LAB, Bifidobacterium spp. are commonly used in the preparation of non-dairy probiotic beverages [111,112,113,114,115]. Among the yeasts, Saccharomyces sp. [38,116,117] and Pichia sp. [38,118,119] stand out. The main microbial genera used in the production of non-dairy fermented beverages are presented in Table 3.
Lactobacillus spp. are used as probiotics in the fermentation of different beverages, especially juices. For example, strains of Lactobacillus helveticus and L. acidophilus have already been used in the fermentation of goji berry juice; in addition to functional properties, the strains also contribute positively to the aroma and flavor of the beverage [107]. Lacticaseibacillus sp. and Lactiplantibacillus sp. resulted in increased levels of organic acids important for functional properties [120]. Strains of L. plantarum and L. fermentum isolated from fruits were used in the production of fermented blueberry juice, with a significant increase in the total bacterial population (>10 log CFU/mL), as well as in the lactic acid content (increase of 0 mg/ L for 2184.90 ± 335.80 mg/L). Furthermore, the total phenolic content in the fermented juice increased from 6.1 to 81.2%; this antioxidant activity, increased by 34.0% in the fermented beverage, was associated with an increase in rutin and gallic acid contents, of 136% and 38%, respectively, in relation to the control (non-fermented beverage) [105]. The contents of total phenolic compounds and flavonoids in a fermented beverage based on kiwi fruit were increased by the activity of probiotic strains of L. acidophilus, L. helveticus and L. plantarum, with their antioxidant capacity significantly increased. In general, this is associated to the formation of compounds such as protocatechuic acid and catechin, in addition to gallic acid and epicatechins naturally present in food matrices [85].
In apple juice, fermentation by LAB improves conversion of polyphenols into low molecular weight molecules with increased biological activity, thus enabling the development of beverages with functional properties related to preventing cardiovascular diseases and type II diabetes mellitus [121]. On the other hand, in mango juice, a natural source of phytochemicals, polyphenols and carbohydrates, LAB probiotic strains, such as P. pentosaceus and P. acidilactici, were effective in producing a nutraceutical fermented beverage due to the significant production of organic acids, as well as the increased levels of minerals such as Fe, Ca and Na [109]. Guedes et al. [122] developed a non-dairy probiotic beverage based on passion fruit and yam flour containing L. casei in populations greater than 6 Log CFU/mL after 28 days of refrigerated storage. The authors also demonstrated that the evaluated strain was relatively resistant to simulated in vitro gastrointestinal tract conditions, with populations greater than 4 Log CFU/mL. Angelov et al. [123] produced a functional beverage from oats fermented for 8 h by L. plantarum whose population in the final product was greater than 10 Log CFU/mL after 21 days of refrigeration. Ellendersen et al. [20] developed a probiotic beverage based on Fuji and Gala apple juice fermented by a mixed culture of L. casei and L. acidophilus; the final product showed a thick texture and an acidic flavor, with rates of acceptance by tasters greater than 96%.
For the Bifidobacterium genera, the fermentation of pineapple juice with probiotic strains of B. lactis, L. plantarum and L. acidophilus has also shown to be a promising strategy in the production of probiotic non-dairy beverages with good sensory acceptance [124]. Mixed cultures containing different species of LAB and B. animalis were able to ferment a fruit- and vegetable-based beverage with conserved probiotic activity. The pH reduction during the fermentation process generated an environment that is not suitable for the growth of undesirable microorganisms, which was related by the authors to the production of high levels of lactic acid, mainly by L. plantarum (1.74 g/L) [114].
Regarding yeasts, a single culture of S. cerevisiae promoted changes in the physicochemical composition of pomegranate juice, influencing its antioxidant properties which were reduced at the end of fermentation, as well as the levels of total phenolic compounds and flavonoids. This effect was attributed to oxidation reactions occurring during juice fermentation, indicating that methods for producing non-dairy probiotics should be continuously explored and the interactions between the used microorganisms should be better understood [116].
The co-cultivation between LAB and yeast has also been shown to be suitable to produce non-dairy probiotic beverages [38,119]. In addition to probiotic strains, yeast can positively influence the survival of probiotic bacteria. Increased survival and viability of LAB in coffees, such as L. rhamnosus, was observed when co-cultivated with S. boulardii. This interaction was able to guarantee bacterial growth for populations above 7 Log CFU/mL, as well as their survival in populations of around 6 Log CFU/mL after 14 weeks of storage under refrigeration at 4 °C [117]. Co-culture composed of L. plantarum and Pichia kluyveri, P. guilliermondii and Debaryomyces hansenii played an important role in the increase in growth and viability of probiotic populations during the fermentation of a beverage based on sunflower seed, oat, and almond. In addition, it prevented the loss of antioxidant activity during storage in comparison to a single culture of LAB. The beverage produced using D. hansenii and L. plantarum showed the highest antioxidant activity, while the one fermented by L. plantarum and P. kluyveri showed the highest concentration of lactic acid (5.81 g/L) [119].
One of most important production bottlenecks related to the use of yeast to produce non-dairy probiotic fermented beverages consists in the fermentation temperature. It is related to the fact that the growth and viability of many yeast species are impaired at temperatures around 37 °C, necessary for microbial adaptation to the host. In any case, the use of Pichia kudriavzevii, S. cerevisiae and Wickerhamomyces subpelliculosus has shown promise to produce functional beverages based on carnelian cherry capable of modulating the intestinal microbiota [118].
Despite the limited variety, there are currently some non-dairy probiotic beverages on the market. Proviva® was the first non-dairy probiotic beverage launched in Sweden by Skane Dairy in 1997. The base of the drink consists of an oat porridge fermented by L. plantarum with populations greater than 12 Log CFU/mL. The company Grainfields Australia® commercializes a beverage based on malted organic oats, corn, rice, alfalfa seeds and linseed, with a refreshing and effervescent character, which supplies lactic acid bacteria (L. acidophilus and L. delbreuckii), yeasts (S. boulardii and S. cerevisiae), vitamins, amino acids, and enzymes [125]. The company Vita Biosa® (Canada) uses a mixture of aromatic herbs and other plants fermented by a combination of LAB and yeasts [126].


Table 3.
Studies on the role of different species of bacteria and yeasts in the production of non-dairy fermented beverages.
Table 3.
Studies on the role of different species of bacteria and yeasts in the production of non-dairy fermented beverages.
| Microorganism | Fermentation | Study Proposal | Main Results | Beverage/Substrate | Reference |
|---|---|---|---|---|---|
| L. paracasei; L. plantarum and L. rhamnosus | 37 °C/ 48 h | Impact of different LAB strains on the taste, chemical profile and bioactivities of goji juice. | Increased organic acid levels, reduced sugar level, and improved sensory quality. | Goji berry juice | [120] |
| L. plantarum, L. acidophilus, L. helveticus, F. fructosus and W. cibaria | 30 °C/24 h | Characterization of flavor profiles, volatile compounds, non-volatile organic acids, reducing sugars, and sensory quality. | Decreased acetic acid levels and improved “goji berry” note in final products. | Goji berry juice | [107] |
| L. plantarum, P. kluyveri, P. guilliermondii and D. hansenii | 30 °C/24 h | Performance of potential probiotic yeasts and bacteria in co-cultivation for the elaboration of a non-dairy fermented beverage. | Fermentation with co-culture of LAB and yeasts showed a minor reduction in antioxidant activity. | Sunflower seeds, oats, and almonds | [119] |
| L. plantarum and L. fermentum | 37 °C/48 h | Kinetics and variations in the profile of organic acids, anthocyanins, and non-anthocyanin phenolic acid by isolated LAB. | Increased antioxidant capacity of fermented blueberry juice. | Blueberry juice | [105] |
| L. plantarum | 37 °C/24 h | Antioxidant functional characteristics of blueberry juice fermented by L. plantarum. | Increased phenolic compound levels, antioxidant activity, and inhibition of α-glucosidase and α-amylase. | Blueberry juice | [127] |
| P. pentosaceus and P. acidilactici | 37 °C/72 h | Determination of cell viability, antimicrobial potential, physicochemical, and sensory properties of a probiotic juice. | Increased production of organic acid and mineral (Fe, Ca, Na) levels. | Mango juice | [109] |
| L. acidophilus, L. plantarum and L. fermentum | 37 °C/24 h | Influence of the cultivar on the fermentative properties of the fermented beverage regarding the levels of sugars, organic acids, volatile compounds, and sensory quality. | Higher consumption of total sugars and improved sensory quality. | Apple juice | [21] |
| P. pentosaceus | 37 °C/18 h | Aroma and flavor of a juice fermented by LAB regarding the profile of non-volatile metabolites. | Improvement of sensory quality. | Broccoli juice | [110] |
| L. rhamnosus and S. cerevisiae | 30 °C/24 h | Growth and survival of probiotics in co-culture with yeast. | Co-cultivation with probiotic yeast increases LAB survival in coffee varieties. | Coffee | [117] |
| L. rhamnosus, L. paracasei, L. plantarum, L. acidophilus and B. animalis | 37 °C/24 h | Production of a probiotic juice fermented by LAB and Bifidobacterium. | The mixture provides a suitable medium for the growth and viability of LAB and Bifidobacterium in a bioreactor. | Mixture of fruits and vegetables | [114] |
| P. acidilactici | 37 °C/24 h | Orange juice-based probiotic drink with antimicrobial properties. | Probiotic and antimicrobial effect against L. monocytogenes. | Orange juice | [108] |
| P. kudriavzevii, S. cerevisiae and W. subpelliculosus | 37 °C/36 h | Fermentation protocol for a functional fermented beverage with profiles of bioactive compounds and effect on gut microbiota. | Higher content of alcohols and esters and lower levels of aldehydes and alkanes, with modulation of gut microbiota. | Cherry | [118] |
| L. brevis, L. plantarum, L. rhamnosus and F. tropaeoli | 30 °C/48 h | Ability of LAB to extend shelf life and improve biochemical and functional properties of fermented juice. | Preservation of antioxidant activity after long fermentation and shelf life. | Cherimoya juice | [128] |
| L. acidophilus, L. plantarum, L. rhamnosus and L.casei | 37 °C/72 h | Changes in cell viability, acidifying activities of LAB and production of volatile compounds and organic acids. | Production of flavor compounds such as acetaldehyde and ketones. | Apple juice | [106] |
| B. lactis, L. plantarum and L. acidophilus | 37 °C/24 h | Effects of prebiotics on probiotic viability and stability of BAL-fermented juice. | Supplementation with prebiotic fructooligosaccharides increased lactic acid production by bifidobacteria and improved stability of probiotics. | Pineapple juice | [124] |
| S. cerevisiae | 25 °C | Physicochemical, antioxidant and sensory characteristics of fermented pomegranate juice. | Decreased antioxidant activity, total phenolic compounds, flavonoids, and anthocyanins. | Pomegranate juice | [116] |
| B. animalis | - | Non-dairy probiotic product, incorporated with microencapsulated B. animalis by spray drying. | The presence of inulin increases the survival of bifidobacteria during spray drying. | Passion fruit juice | [112] |
| L. casei | 37 °C/48 h | Development of vegetal probiotic beverage of passion fruit, yam and Lacticaseibacillus casei. | The drinks were considered good sources of fiber and had good acceptance in terms of aroma, color, and appearance. | Passion fruit juice and yam flour | [122] |
| L. casei and L. acidophilus | 37 °C/10 h | Development and sensory profile of a probiotic beverage from apple fermented by Lactobacillus casei. | The fermented probiotic apple drink was characterized by a thick texture and sweet taste. The drink was tested by potential consumers, with an acceptance rate of 96%. | Apple juice | [20] |
| L. casei | 31 °C/24 h | Development of probiotic drink: process optimization and product stability. | The color was maintained throughout the shelf life. Sonicated pineapple juice proved to be a suitable substrate for the cultivation of L. casei. | Pineapple juice | [129] |
| L. plantarum | 37 °C/6–10 h | Development of a new oat-based probiotic drink. | The beta-glucan content in the beverage remained unchanged during beverage fermentation and storage. The shelf life of the oat drink was estimated to be 21 days under refrigeration. | Oat | [123] |
| L. acidophilus and L. plantarum | 30 °C/48 h | Development of a probiotic beverage using breadfruit flour as a substrate. | This study successfully demonstrated the development of a new breadfruit-based fermented beverage with acceptable sensory characteristics and cell viability. | Breadfruit flour | [130] |
| L. casei | 37 °C/72 h | Chemical and sensory properties of probiotic drink based on rice bran extract and honey. | Bacterial bioavailability decreased during refrigerator storage. The results of the sensorial evaluation showed that the sample with 10% of rice bran extract was more acceptable than the others. | Bran extract of rice and honey | [131] |
| L. acidophilus | 30 °C/8 h | Development of a non-dairy probiotic drink utilizing sprouted cereals, legume, and soymilk. | Acidity, pH and probiotic counts in samples of wheat, barley, millet and green grass-based probiotic drink were found to be dependent on the level of sprouted cereal flour and soy milk. | Cereal sprouts, legumes and soy milk | [22] |
| L. rhamnnosus, L. plantarum and L. delbrueckii | 37 °C/24 h | Development of a beetroot probiotic drink. | Total phenols, flavonoids and antioxidant activity were increased in the probiotic drink compared to the fresh juice sample. | Beet juice | [132] |
| L. acidophilus | 30 °C/8 h | Development of a non-dairy fermented probiotic drink based on germinated and ungerminated cereals and legume. | Fermentation improved the overall acceptability and functional properties of the drink. | Sprouted and non-sprouted cereals and legumes | [133] |
| L. bulgaricus and S. thermophilus | Room temperature/8 h | Nutritional composition of non-dairy yogurt from sprouted tigernut tubers. | Increased value of protein, ash, crude fiber and energy. It improved amino acid content and sensory attributes, but decreased fat and antinutritional content of yogurt samples. | Chufa “tigernut” | [134] |
| L. paracasei | 37 °C/24 h | Probiotic Gac fruit beverage fermented with Lactobacillus paracasei. | Fermentation increased β-carotene content, antioxidant activity, binding to bile acids and increased inhibition of cholesterol micellization. Furthermore, it altered the volatile compounds in Gac juice. | Melon | [135] |
| L. casei, L. fermentum and L. plantarum | 30 °C/72 h | Probiotic drink of mangosteen juice fermented with Lactobacillus strains. | The fermented juice showed good antioxidant activity compared to the control (without lactic acid bacteria). | Mangosteen juice | [136] |
| L. plantarum, L. acidophilus and L. delbrueckiiem | 37 °C/24 and 48 h | Evaluation of probiotics in vegetable juices: tomato, carrot, and beet juice. | Increase in the amount of vitamin C. | Tomato juice, carrot and beet | [137] |
6. Market Perspectives and Challenges in the Production of Non-Dairy Probiotic Beverages
In recent years, the notable increase in concern for health and well-being on the part of consumers has driven the market of probiotic beverages, currently valued at around 11 million dollars (2020), with estimates of around 23 million dollars in 2031 (Composite Annual Growth Rate—CAGR of 6.6% in the mentioned period). In this context, the COVID-19 pandemic has been stimulating the development of novel functional foods and beverages [138]. The Asia–Pacific region has dominated the global market for dairy and non-dairy probiotic beverages [138]. Currently, the global market for probiotic foods is more than ten times larger than that for probiotic supplements, suggesting a consumer preference for food and beverage consumption over nutraceuticals. Although dairy probiotic beverages and/or derivatives are available in a more diversified way on the market, non-dairy probiotic products have been standing out in another market niche related to the population with restriction on the consumption of dairy products and their derivatives, such as people who are lactose intolerant and those allergic to milk protein (casein), as well as people with restrictions on the consumption of animal foods (vegans) [111].
Despite the growing increase in the development of novel fermented plant-based probiotic beverages, the use of probiotic cultures in this type of raw material represents a major operational challenge. There are many gaps to be filled with the feasibility and development of adaptable technologies for non-dairy probiotic products, which are still obsolete compared to those of dairy products. Besides microbiological safety, technological features of the manufacturing process and marketing regulations are essential and equally challenging. Fruit juices, for example, correspond to the main matrices for the elaboration of new functional probiotic beverages; therefore, it is important that the probiotic strains can survive at low pH conditions, an intrinsic characteristic of most consumed juices [139,140]. Among other limitations, the inadequate amount of free amino acids, short peptides, and oligosaccharides necessary for probiotics to develop, as well as the presence of natural pigments subject to microbial oxidation negatively influencing the sensory characteristics of the product can be highlighted [141]. In this context, some promising strategies include the microencapsulation technology [142] and the concomitant use of prebiotics [143]. For instance, encapsulation of B. animalis using maltodextrin and spray drying processing showed promise in maintaining the viability of the probiotic [112]. Passion fruit juice fermented by this powdered bacterial culture resulted in a beverage with high viability of probiotic populations during 30 days of storage. This type of study demonstrates the possibility of using these technologies for the elaboration of probiotic cultures with a high maintenance capacity in unfavorable growth conditions, guaranteeing the stability of the beverage.
7. Safety of Probiotics Beverage and Relevant Concerns
Non-dairy probiotic beverages mainly based on fruits represent an interesting way of delivering probiotics [139]. In general, probiotic LAB are isolated from different types of food matrices, such as fish, meat, cereals, fruits, vegetables, and dairy products. Furthermore, many of these bacteria are widely found in the human intestinal tract, urogenital tract, and oral cavity. However, safety must be investigated based on the taxonomic identification of the microorganisms used, their origin and nature, pathogenicity, administered quantity, exposure level, and lack of ability to carry antibiotic-resistant genes. Thus, any novel probiotic strain needs to be evaluated for safety aspects before being used in the production of food and beverages for human consumption. Each country has its regulations regarding the production and marketing of probiotic beverages, which must be continuously updated based on new scientific studies, especially in human populations [144].
It is important to mention that everyone can respond differently to medications, dietary supplements, or foods (allergic reactions), which may be related to factors such as age, sex, and comorbidities [145]; in this context, probiotics are no exception [146]. In addition, children, the elderly, hospitalized patients, and immunodeficient people correspond to the most susceptible population groups and deserve a careful investigation of the risk–benefit ratio of probiotics before recommending consumption [147]. Thus, several approaches, including in vitro tests, animal models, and human populations, should be used to assess the safety of new probiotics on the market [146].
8. Conclusions
Cereal and fruit fermentations hold significant potential for the creation of novel probiotic beverages. Various substrates such as blueberry, mango, apple, orange, cherry, and pineapple, either individually or in combination, have been extensively explored in the literature. It is crucial to acknowledge that cereals and fruits possess numerous nutritional and health-promoting benefits. When synergistically combined with probiotics, they can further enhance a range of biological activities. Therefore, the emergence of non-dairy fermented beverages incorporating probiotics represents a growing trend in the functional food market. However, research in this field is relatively nascent, particularly when compared to its dairy-based counterparts. Consequently, comprehensive studies exploring probiotic strain adaptation to plant matrices are essential and need to be conducted extensively. By prospecting for novel microbial strains associated with fruits, it becomes possible to conduct more efficient fermentations and achieve desirable final functional products. Understanding this information is crucial for addressing challenges associated with the fermentation process. Although the production of non-dairy probiotic beverages remains technologically complex, encapsulation and spray drying have been proposed as promising approaches to enhance product shelf life.
Author Contributions
Conceptualization, G.V.d.M.P., J.G.P.M. and C.R.S.; formal analysis, A.d.S.V., B.L.M., G.B., C.R.S. and G.V.d.M.P.; writing—original draft preparation, A.d.S.V., B.L.M., G.B., B.C.V. and A.R.F.d.S.R.; writing—review and editing, G.V.d.M.P., J.G.P.M., C.R.S. and J.D.D.L.; project administration, G.V.d.M.P. and C.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed the National Council for Scientific and Technological Development (CNPq 440343/2022-4).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank CAPES and CNPq for the research scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to Select a Probiotic? A Review and Update of Methods and Criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Lee, B.H. New Perspectives on Probiotics in Health and Disease. Food Sci. Hum. Wellness 2015, 4, 56–65. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic Functional Foods: Survival of Probiotics during Processing and Storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mortazavian, A.M. Review Article: Technological Aspects of Prebiotics in Probiotic Fermented Milks. Food Rev. Int. 2011, 27, 192–212. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of Plant-Based Milk Alternatives for Improved Flavour and Nutritional Value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A.; Cosme, F.; Inês, A. Wine and Non-Dairy Fermented Beverages: A Novel Source of Pro-and Prebiotics. Fermentation 2020, 6, 113. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Khartad, A.; Chakraborty, S. The Potential of Non-Dairy Synbiotic Instant Beverage Powder: Review on a New Generation of Healthy Ready-to-Reconstitute Drinks. Food Biosci. 2021, 42, 101195. [Google Scholar] [CrossRef]
- Catanzaro, R.; Sciuto, M.; Marotta, F. Lactose Intolerance: An Update on Its Pathogenesis, Diagnosis, and Treatment. Nutr. Res. 2021, 89, 23–34. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Chakraborty, S. Review on Potential Non-dairy Synbiotic Beverages: A Preliminary Approach Using Legumes. Int. J. Food Sci. Technol. 2021, 56, 2068–2077. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of Food Matrix on the Viability of Probiotic Bacteria: A Review Based on Dairy and Non-Dairy Beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Roberts, D.; Reyes, V.; Bonilla, F.; Dzandu, B.; Liu, C.; Chouljenko, A.; Sathivel, S. Viability of Lactobacillus Plantarum NCIMB 8826 in Fermented Apple Juice under Simulated Gastric and Intestinal Conditions. LWT 2018, 97, 144–150. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical Analysis and Flavor Properties of Blended Orange, Carrot, Apple and Chinese Jujube Juice Fermented by Selenium-Enriched Probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and Non-Dairy Probiotic Beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Rasika, D.M.D.; Vidanarachchi, J.K.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ranadheera, C.S. Probiotic Delivery through Non-Dairy Plant-Based Food Matrices. Agriculture 2021, 11, 599. [Google Scholar] [CrossRef]
- Lima, T.T.M.; de Oliveira Hosken, B.; Venturim, B.C.; Lopes, I.L.; Martin, J.G.P. Traditional Brazilian Fermented Foods: Cultural and Technological Aspects. J. Ethn. Foods 2022, 9, 35. [Google Scholar] [CrossRef]
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and Disadvantages of Non-starter Lactic Acid Bacteria from Traditional Fermented Foods: Potential Use as Starters or Probiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1537–1567. [Google Scholar] [CrossRef]
- da Silva Vale, A.; de Melo Pereira, G.V.; Santana, L.M.; de Carvalho Neto, D.P.; Colonia, B.S.O.; Soccol, V.T.; Maske, B.L.; Soccol, C.R. Perspective on the Use of Synthetic Biology in Rudimentary Food Fermentations. Syst. Microbiol. Biomanuf. 2022, 3, 150–165. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional Foods and Nondairy Probiotic Food Development: Trends, Concepts, and Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- De Souza Neves Ellendersen, L.; Granato, D.; Bigetti Guergoletto, K.; Wosiacki, G. Development and Sensory Profile of a Probiotic Beverage from Apple Fermented with Lactobacillus Casei. Eng. Life Sci. 2012, 12, 475–485. [Google Scholar] [CrossRef]
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the Apple Cultivar on Cloudy Apple Juice Fermented by a Mixture of Lactobacillus Acidophilus, Lactobacillus Plantarum, and Lactobacillus Fermentum. Food Chem. 2021, 340, 127922. [Google Scholar] [CrossRef] [PubMed]
- Mridula, D.; Sharma, M. Development of Non-Dairy Probiotic Drink Utilizing Sprouted Cereals, Legume and Soymilk. LWT 2015, 62, 482–487. [Google Scholar] [CrossRef]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nuñez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.; et al. Fermented Beverages of Pre-and Proto-Historic China. Anthropology 2004, 51, 17593–17598. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.T.; Padam, B.S.; Chye, F.Y. Effect of Fermentation on the Antioxidant Properties and Phenolic Compounds of Bambangan (Mangifera pajang) Fruit. J. Food Sci. Technol. 2023, 60, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in Non-Dairy Probiotic Beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- da Silva Vale, A.; de Melo Pereira, G.V.; de Oliveira, A.C.; de Carvalho Neto, D.P.; Herrmann, L.W.; Karp, S.G.; Soccol, V.T.; Soccol, C.R. Production, Formulation, and Application of Postbiotics in the Treatment of Skin Conditions. Fermentation 2023, 9, 264. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Thakur, N.; Chand Bhalla, T. Characterization of Some Traditional Fermented Foods and Beverages of Himachal Pradesh. Indian J. Tradit. Knowl. 2004, 3, 325–335. [Google Scholar]
- Thakur, N.; Saris, P.E.J.; Bhalla, T.C. Microorganisms Associated with Amylolytic Starters and Traditional Fermented Alcoholic Beverages of North Western Himalayas in India. Food Biosci. 2015, 11, 92–96. [Google Scholar] [CrossRef]
- Kumari, A.; Swain, M.R.; Pandey, A.; Gupta, A.; Raj, A.; Sharma, A.; Kumar, A.; Chauhan, A.; Ann, A.; Neopany, B.; et al. Indigenous Alcoholic Beverages of South Asia. In Indigenous Fermented Foods of South Asia; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Targais, K.; Stobdan, T.; Mundra, S.; Ali, Z.; Yadav, A.; Korekar, G.; Singh, S.B. Chhang-A Barley Based Alcoholic Beverage of Ladakh, India. Indian J. Tradit. Knowl. 2012, 11, 190–193. [Google Scholar]
- Savitri, S.; Thakur, N.; Bhalla, T.C. Present Status and Future Prospects of Traditional Fermented Beverages of Himachal Pradesh, India. Int. J. Food Ferment. Technol. 2019, 9, 67–72. [Google Scholar] [CrossRef]
- Mayorga, G.A.C.; Arias Palma, G.B.; Sandoval-Cañas, G.J.; Ordoñez-Araque, R.H. Ancestral Fermented Indigenous Beverages from South America Made from Cassava (Manihot esculenta). Food Sci. Technol. 2021, 41, 360–367. [Google Scholar] [CrossRef]
- Ramos, C.L.; de Sousa, E.S.; Ribeiro, J.; Almeida, T.M.; Santos CC AD, A.; Abegg, M.A.; Schwan, R.F. Microbiological and Chemical Characteristics of Tarubá, an Indigenous Beverage Produced from Solid Cassava Fermentation. Food Microbiol. 2015, 49, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Faria-Oliveira, F.; Diniz, R.H.S.; Godoy-Santos, F.; Piló, F.B.; Mezadri, H.; Castro, I.M.; Brandão, R.L. The Role of Yeast and Lactic Acid Bacteria in the Production of Fermented Beverages in South America. In Food Production and Industry; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Guerra, L.S.; Cevallos-Cevallos, J.M.; Weckx, S.; Ruales, J. Traditional Fermented Foods from Ecuador: A Review with a Focus on Microbial Diversity. Foods 2022, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J.; Magalhães, F.; Aranda, A.; Matallana, E.; Gibson, B. Potential Application of Yeasts from Ecuadorian Chichas in Controlled Beer and Chicha Production. Food Microbiol. 2021, 98, 103644. [Google Scholar] [CrossRef]
- Freire, A.L.; Ramos, C.L.; Schwan, R.F. Microbiological and Chemical Parameters during Cassava Based-Substrate Fermentation Using Potential Starter Cultures of Lactic Acid Bacteria and Yeast. Food Res. Int. 2015, 76, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Piló, F.B.; Carvajal-Barriga, E.J.; Guamán-Burneo, M.C.; Portero-Barahona, P.; Dias, A.M.M.; Freitas, L.F.D.d.; Gomes, F.d.C.O.; Rosa, C.A. Saccharomyces Cerevisiae Populations and Other Yeasts Associated with Indigenous Beers (Chicha) of Ecuador. Br. J. Microbiol. 2018, 49, 808–815. [Google Scholar] [CrossRef]
- Colehour, A.M.; Meadow, J.F.; Liebert, M.A.; Cepon-Robins, T.J.; Gildner, T.E.; Urlacher, S.S.; Bohannan, B.J.M.; Snodgrass, J.J.; Sugiyama, L.S. Local Domestication of Lactic Acid Bacteria via Cassava Beer Fermentation. PeerJ 2014, 2014, e479. [Google Scholar] [CrossRef]
- Mendoza, L.M.; Neef, A.; Vignolo, G.; Belloch, C. Yeast Diversity during the Fermentation of Andean Chicha: A Comparison of High-Throughput Sequencing and Culture-Dependent Approaches. Food Microbiol. 2017, 67, 1–10. [Google Scholar] [CrossRef]
- Resende, L.V.; Pinheiro, L.K.; Miguel, M.G.C.P.; Ramos, C.L.; Vilela, D.M.; Schwan, R.F. Microbial Community and Physicochemical Dynamics during the Production of ‘Chicha’, A Traditional Beverage of Indigenous People of Brazil. World J. Microbiol. Biotechnol. 2018, 34, 34–46. [Google Scholar] [CrossRef]
- Bassi, D.; Orrù, L.; Vasquez, J.C.; Cocconcelli, P.S.; Fontana, C. Peruvian Chicha: A Focus on the Microbial Populations of This Ancient Maize-Based Fermented Beverage. Microorganisms 2020, 8, 93. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Impact of the Physicochemical Composition and Microbial Diversity in Apple Juice Fermentation Process: A Review. Molecules 2020, 25, 3698. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Du, J. A Comparative Study of the Effect of Bacteria and Yeasts Communities on Inoculated and Spontaneously Fermented Apple Cider. Food Microbiol. 2023, 111, 104195. [Google Scholar] [CrossRef] [PubMed]
- Valles, B.S.; Bedriñana, R.P.; Tascón, N.F.; Simón, A.Q.; Madrera, R.R. Yeast Species Associated with the Spontaneous Fermentation of Cider. Food Microbiol. 2007, 24, 25–31. [Google Scholar] [CrossRef]
- Schneedorf, J.M. Kefir D’Aqua and Its Probiotic Properties. In Probiotic in Animals; InTech: Rijeka, Croatia, 2012; pp. 53–75. [Google Scholar]
- Magalhães, K.T.; de Pereira, G.V.M.; Dias, D.R.; Schwan, R.F. Microbial Communities and Chemical Changes during Fermentation of Sugary Brazilian Kefir. World J. Microbiol. Biotechnol. 2010, 26, 1241–1250. [Google Scholar] [CrossRef]
- da CP Miguel, M.G.; Cardoso, P.G.; Magalhães, K.T.; Schwan, R.F. Profile of Microbial Communities Present in Tibico (Sugary Kefir) Grains from Different Brazilian States. World J. Microbiol. Biotechnol. 2011, 27, 1875–1884. [Google Scholar] [CrossRef]
- Laureys, D.; De Vuyst, L. The Water Kefir Grain Inoculum Determines the Characteristics of the Resulting Water Kefir Fermentation Process. J. Appl. Microbiol. 2016, 122, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Laureys, D.; De Vuyst, L. Microbial Species Diversity, Community Dynamics, and Metabolite Kinetics of Water Kefir Fermentation. Appl. Environ. Microbiol. 2014, 80, 2564–2572. [Google Scholar] [CrossRef]
- Miranda, J.F.; Ruiz, L.F.; Silva, C.B.; Uekane, T.M.; Silva, K.A.; Gonzalez, A.G.M.; Fernandes, F.F.; Lima, A.R. Kombucha: A Review of Substrates, Regulations, Composition, and Biological Properties. J. Food Sci. 2022, 87, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.M.D.; de Almeida, A.L.; do Amaral, R.Q.G.; da Mota, R.N.; de Sousa, P.H.M. Kombucha: Review. Int. J. Gastron. Food Sci. 2020, 22, 100272. [Google Scholar] [CrossRef]
- Yang, J.; Lagishetty, V.; Kurnia, P.; Henning, S.M.; Ahdoot, A.I.; Jacobs, J.P. Microbial and Chemical Profiles of Commercial Kombucha Products. Nutrients 2022, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha Tea Fermentation: Microbial and Biochemical Dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Health, Wellness, and Safety Aspects of the Consumption of Kombucha. J. Chem. 2015, 2015, 591869. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Sathishkumar, M. Kombucha. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid Contents and the Effect of Fermentation Condition of Kombucha Tea Beverages on Physicochemical, Microbiological and Sensory Properties. CyTA-J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Gholami, A.; Lai, C.W.; Chiang, W.H.; Omidifar, N.; Bahrani, S.; Mazraedoost, S. Recent Progress in Chemical Composition, Production, and Pharmaceutical Effects of Kombucha Beverage: A Complementary and Alternative Medicine. Evid.-Based Complement. Altern. Med. 2020, 2020, 4397543. [Google Scholar] [CrossRef]
- Kapp, J.M.; Sumner, W. Kombucha: A Systematic Review of the Empirical Evidence of Human Health Benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Maske, B.L.; Pereira, G.V.d.M.; Carvalho Neto, D.P.d.; Lindner, J.d.D.; Letti, L.A.J.; Pagnoncelli, M.G.; Ssccol, C.R. Presence and Persistence of Pseudomonas Sp. during Caspian Sea-Style Spontaneous Milk Fermentation Highlights the Importance of Safety and Regulatory Concerns for Traditional and Ethnic Foods. Food Sci. Technol. 2021, 41, 273–283. [Google Scholar] [CrossRef]
- Patz, S.; Witzel, K.; Scherwinski, A.-C.; Ruppel, S. Culture Dependent and Independent Analysis of Potential Probiotic Bacterial Genera and Species Present in the Phyllosphere of Raw Eaten Produce. Int. J. Mol. Sci. 2019, 20, 3661. [Google Scholar] [CrossRef]
- Merabti, R.; Madec, M.N.; Chuat, V.; Becila, F.Z.; Boussekine, R.; Bekhouche, F.; Valence, F. First Insight into the Technological Features of Lactic Acid Bacteria Isolated from Algerian Fermented Wheat Lemzeiet. Curr. Microbiol. 2019, 76, 1095–1104. [Google Scholar] [CrossRef]
- Aguilar, G.; Morlon-Guyot, J.; Trejo-Aguilar, B.; Guyot, J.P. Purification and Characterization of an Extracellular α-Amylase Produced by Lactobacillus Manihotivorans LMG 18010T, an Amylolytic Lactic Acid Bacterium. Enzyme Microb. Technol. 2000, 27, 406–413. [Google Scholar] [CrossRef]
- Maske, B.L.; de Melo Pereira, G.V.; da S. Vale, A.; de Carvalho Neto, D.P.; Karp, S.G.; Viesser, J.A.; De Dea Lindner, J.; Pagnoncelli, M.G.; Soccol, V.T.; Soccol, C.R. A Review on Enzyme-Producing Lactobacilli Associated with the Human Digestive Process: From Metabolism to Application. Enzyme Microb. Technol. 2021, 149, 109836. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic Potential and Amylolytic Properties of Lactic Acid Bacteria Isolated from Chinese Fermented Cereal Foods. Food Control. 2020, 111, 107057. [Google Scholar] [CrossRef]
- Petrova, P.; Emanuilova, M.; Petrov, K. Amylolytic Lactobacillus Strains from Bulgarian Fermented Beverage Boza. Z. Nat. C 2010, 65, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bolaños-Núñez, S.; Santiago-Urbina, J.A.; Guyot, J.-P.; Díaz-Ruiz, G.; Wacher, C. Microbial Interactions between Amylolytic and Non-Amylolytic Lactic Acid Bacteria Strains Isolated during the Fermentation of Pozol. Foods 2021, 10, 2607. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic Acid Bacteria as Sensory Biomodulators for Fermented Cereal-Based Beverages. Trends Food Sci. Technol. 2016, 54, 17–25. [Google Scholar] [CrossRef]
- Gomes, R.J.; de Fatima Borges, M.; de Freitas Rosa, M.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139. [Google Scholar] [CrossRef] [PubMed]
- WoldemariamYohannes, K.; Wan, Z.; Yu, Q.; Li, H.; Wei, X.; Liu, Y.; Wang, J.; Sun, B. Prebiotic, Probiotic, Antimicrobial, and Functional Food Applications of Bacillus Amyloliquefaciens. J. Agric. Food Chem. 2020, 68, 14709–14727. [Google Scholar] [CrossRef]
- Freire, A.L.; Zapata, S.; Mosquera, J.; Mejia, M.L.; Trueba, G. Bacteria Associated with Human Saliva Are Major Microbial Components of Ecuadorian Indigenous Beers (Chicha). PeerJ 2016, 4, e1962. [Google Scholar] [CrossRef]
- Yerlikaya, O.; Akan, E.; Kinik, Ö. The Metagenomic Composition of Water Kefir Microbiota. Int. J. Gastron. Food Sci. 2022, 30, 100621. [Google Scholar] [CrossRef]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.; Stewart, G. Saccharomyces Cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; Maske, B.L.; de Carvalho Neto, D.P.; Karp, S.G.; De Dea Lindner, J.; Martin, J.G.P.; de Oliveira Hosken, B.; Soccol, C.R. What Is Candida Doing in My Food? A Review and Safety Alert on Its Use as Starter Cultures in Fermented Foods. Microorganisms 2022, 10, 1855. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Im, J.H.; Chun, H.S. Mycotoxins in Soybean-based Foods Fermented with Filamentous Fungi: Occurrence and Preventive Strategies. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5131–5152. [Google Scholar] [CrossRef]
- Zajc, J.; Gunde-Cimerman, N. The Genus Wallemia—From Contamination of Food to Health Threat. Microorganisms 2018, 6, 46. [Google Scholar] [CrossRef]
- Grijalva-Vallejos, N.; Aranda, A.; Matallana, E. Evaluation of Yeasts from Ecuadorian Chicha by Their Performance as Starters for Alcoholic Fermentations in the Food Industry. Int. J. Food Microbiol. 2020, 317, 108462. [Google Scholar] [CrossRef]
- Dellacassa, E.; Trenchs, O.; Fariña, L.; Debernardis, F.; Perez, G.; Boido, E.; Carrau, F. Pineapple (Ananas comosus L. Merr.) Wine Production in Angola: Characterisation of Volatile Aroma Compounds and Yeast Native Flora. Int. J. Food Microbiol. 2017, 241, 161–167. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Immune Boosting Functional Foods and Their Mechanisms: A Critical Evaluation of Probiotics and Prebiotics. Biomed. Pharmacother. 2020, 130, 110625. [Google Scholar] [CrossRef]
- Rerksuppaphol, S. A Randomized Double-Blind Controlled Trial of Lactobacillus Acidophilus Plus Bifidobacterium Bifidum versus Placebo in Patients with Hypercholesterolemia. J. Clin. Diagn. Res. 2015, 9, KC01. [Google Scholar] [CrossRef]
- Junnarkar, M.; Gaikwad, S.C.; Pawar, S.; Nawani, N. Probiotic Potential of Lactic Acid Bacteria from Fresh Vegetables: Application in Food Preservation. Indian J. Exp. Biol. 2019, 57, 825–838. [Google Scholar]
- Wang, Z.; Feng, Y.; Yang, N.; Jiang, T.; Xu, H.; Lei, H. Fermentation of Kiwifruit Juice from Two Cultivars by Probiotic Bacteria: Bioactive Phenolics, Antioxidant Activities and Flavor Volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef]
- Pinto, A.; Barbosa, J.; Albano, H.; Isidro, J.; Teixeira, P. Screening of Bacteriocinogenic Lactic Acid Bacteria and Their Characterization as Potential Probiotics. Microorganisms 2020, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Kim, S.H. Anti-Inflammatory and Anti-Osteoporotic Potential of Lactobacillus Plantarum A41 and L. Fermentum SRK414 as Probiotics. Probiotics Antimicrob. Proteins 2020, 12, 623–634. [Google Scholar] [CrossRef]
- Shah, N.J.; Swami, O.C. Role of Probiotics in Diabetes: A Review of Their Rationale and Efficacy. EMJ Diabetes 2017, 5, 104–110. [Google Scholar] [CrossRef]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health—A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Syiemlieh, I.; Morya, S. Dairy and Non-Dairy Based Probiotics: A Review. Pharma Innov. 2022, 11, 2956–2964. [Google Scholar] [CrossRef]
- Abatenh, E.; Gizaw, B.; Tsegay, Z.; Tefera, G.; Aynalem, E. Health Benefits of Probiotics; 2018. [Google Scholar]
- Markowiak, P.; Śliżewska, K. The Role of Probiotics, Prebiotics and Synbiotics in Animal Nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Kahouli, I.; Malhotra, M.; Westfall, S.; Alaoui-Jamali, M.A.; Prakash, S. Design and Validation of an Orally Administrated Active L. Fermentum-L. Acidophilus Probiotic Formulation Using Colorectal Cancer Apc Min/+ Mouse Model. Appl. Microbiol. Biotechnol. 2017, 101, 1999–2019. [Google Scholar] [CrossRef]
- Bedada, T.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for Cancer Alternative Prevention and Treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar] [CrossRef] [PubMed]
- Zaharuddin, L.; Mokhtar, N.M.; Nawawi, K.N.M.; Ali, R.A.R. A Randomized Double-Blind Placebo-Controlled Trial of Probiotics in Post-Surgical Colorectal Cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, B.P.; Yuan, C.Y.; Wood, D.R.; Nicolas, J.D.; Grothaus, J.S.; Hunter, C.J. Probiotic Lactobacillus Species Strengthen Intestinal Barrier Function and Tight Junction Integrity in Experimental Necrotizing Enterocolitis. J. Probiotics Health 2017, 5, 159. [Google Scholar] [CrossRef]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. A Critical Review of Antibiotic Resistance in Probiotic Bacteria. Food Res. Int. 2020, 136, 109571. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Frauwallner, A.; Langerholc, T.; Krebs, B.; ter Haar (née Younes), J.A.; Heschl, A.; Turk, D.M.; Rogelj, I. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. Biomed. Res. Int. 2019, 2019, 7585486. [Google Scholar] [CrossRef] [PubMed]
- Szutowska, J.; Gwiazdowska, D. Probiotic Potential of Lactic Acid Bacteria Obtained from Fermented Curly Kale Juice. Arch. Microbiol. 2021, 203, 975–988. [Google Scholar] [CrossRef]
- Guinane, C.M.; Lawton, E.M.; O’Connor, P.M.; O’Sullivan, Ó.; Hill, C.; Ross, R.P.; Cotter, P.D. The Bacteriocin Bactofencin A Subtly Modulates Gut Microbial Populations. Anaerobe 2016, 40, 41–49. [Google Scholar] [CrossRef]
- Jia, B.; Zou, Y.; Han, X.; Bae, J.-W.; Jeon, C.O. Gut Microbiome-Mediated Mechanisms for Reducing Cholesterol Levels: Implications for Ameliorating Cardiovascular Disease. Trends Microbiol. 2023, 31, 76–91. [Google Scholar] [CrossRef]
- Iqbal, Z.; Ahmed, S.; Tabassum, N.; Bhattacharya, R.; Bose, D. Role of Probiotics in Prevention and Treatment of Enteric Infections: A Comprehensive Review. 3 Biotech 2021, 11, 242. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Li, D.; Wen, G.; Zhou, J.; Manickam, S.; Han, Y.; Chai, W.S. Fermentation of Blueberry Juices Using Autochthonous Lactic Acid Bacteria Isolated from Fruit Environment: Fermentation Characteristics and Evolution of Phenolic Profiles. Chemosphere 2021, 276, 130090. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 Lactic Acid Bacteria on the Flavor Profile of Fermented Apple Juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, P.; Laaksonen, O.; Wei, B.; Zhu, Y.; Zhang, B.; Zhu, B.; Li, H. Lactic Acid Bacteria Incubation and Aging Drives Flavor Enhancement of Goji Berry Juice. J. Food Compos. Anal. 2022, 105, 104202. [Google Scholar] [CrossRef]
- De Oliveira Vieira, K.C.; Ferreira, C.D.S.; Bueno, E.B.T.; De Moraes, Y.A.; Toledo, A.C.C.G.; Nakagaki, W.R.; Pereira, V.C.; Winkelstroter, L.K. Development and Viability of Probiotic Orange Juice Supplemented by Pediococcus Acidilactici CE51. LWT 2020, 130, 109637. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.C.; Olomitutu, F.O.; Adebami, G.E. Production and Evaluation of Probioticated Mango Juice Using Pediococcus Pentosaceus and Pediococcus Acidilactici during Storage at Different Temperature. J. Agric. Food Res. 2021, 6, 100202. [Google Scholar] [CrossRef]
- Xu, X.; Bi, S.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Comprehensive Investigation on Volatile and Non-Volatile Metabolites in Broccoli Juices Fermented by Animal- and Plant-Derived Pediococcus Pentosaceus. Food Chem. 2021, 341, 128118. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in Dairy and Non-Dairy Probiotic Products—A Review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef]
- Dias, C.O.; dos Santos Opuski de Almeida, J.; Pinto, S.S.; de Oliveira Santana, F.C.; Verruck, S.; Müller, C.M.O.; Prudêncio, E.S.; de Mello Castanho Amboni, R.D. Development and Physico-Chemical Characterization of Microencapsulated Bifidobacteria in Passion Fruit Juice: A Functional Non-Dairy Product for Probiotic Delivery. Food Biosci. 2018, 24, 26–36. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Güney, D.; Güngörmüşler, M. Development and Comparative Evaluation of a Novel Fermented Juice Mixture with Probiotic Strains of Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2021, 13, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, M.; Zendo, T.; Nakayama, J. Diversity and Dynamics of Sourdough Lactic Acid Bacteriota Created by a Slow Food Fermentation System. J. Biosci. Bioeng. 2021, 131, 333–340. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; Guerrero-Beltrán, J.Á. Antioxidant and Physicochemical Characteristics of Unfermented and Fermented Pomegranate (Punica granatum L.) Beverages. J. Food Sci. Technol. 2019, 56, 132–139. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Toh, M.; Liu, S.-Q. Growth, Survival, and Metabolic Activities of Probiotics Lactobacillus Rhamnosus GG and Saccharomyces Cerevisiae Var. Boulardii CNCM-I745 in Fermented Coffee Brews. Int. J. Food Microbiol. 2021, 350, 109229. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Polo, A.; Celano, G.; Martinovic, A.; Cavoski, I.; Gobbetti, M. Design of Potential Probiotic Yeast Starters Tailored for Making a Cornelian Cherry (Cornus mas L.) Functional Beverage. Int. J. Food Microbiol. 2020, 323, 108591. [Google Scholar] [CrossRef]
- Ferreira, I.; de Sousa Melo, D.; Menezes, A.G.T.; Fonseca, H.C.; de Assis, B.B.T.; Ramos, C.L.; Magnani, M.; Dias, D.R.; Schwan, R.F. Evaluation of Potentially Probiotic Yeasts and Lactiplantibacillus Plantarum in Co-Culture for the Elaboration of a Functional Plant-Based Fermented Beverage. Food Res. Int. 2022, 160, 111697. [Google Scholar] [CrossRef]
- Duan, W.; Guan, Q.; Zhang, H.-L.; Wang, F.-Z.; Lu, R.; Li, D.-M.; Geng, Y.; Xu, Z.-H. Improving Flavor, Bioactivity, and Changing Metabolic Profiles of Goji Juice by Selected Lactic Acid Bacteria Fermentation. Food Chem. 2023, 408, 135155. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, C.; Guo, Y.; Wang, X.; Meng, Y. Polyphenols in Fermented Apple Juice: Beneficial Effects on Human Health. J. Funct. Foods 2021, 76, 104294. [Google Scholar] [CrossRef]
- Do Monte Guedes, C.K.R.; do Monte Guedes, A.F.L.; da Silva, J.R.; da Silva, E.B.B.; dos Santos, E.C.M.; Stamford, T.C.M.; Stamford, T.L.M. Development of Vegetal Probiotic Beverage of Passion Fruit (Passiflora edulis Sims), Yam (Dioscorea cayenensis) and Lacticaseibacillus casei. Food Sci. Technol. 2021, 41, 619–626. [Google Scholar] [CrossRef]
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a New Oat-Based Probiotic Drink. Int. J. Food Microbiol. 2006, 112, 75–80. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Bujna, E.; Fekete, N.; Tran, A.T.M.; Rezessy-Szabo, J.M.; Prasad, R.; Nguyen, Q.D. Probiotic Beverage from Pineapple Juice Fermented With Lactobacillus and Bifidobacterium Strains. Front. Nutr. 2019, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Grainfields Australia Fermented Foods. Available online: https://agmfoods.com/ (accessed on 5 January 2023).
- Vita Biosa. Available online: https://vitabiosa.com/ (accessed on 5 January 2023).
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of Functional Characteristics of Blueberry Juice Fermented by Lactobacillus Plantarum. LWT 2021, 139, 110590. [Google Scholar] [CrossRef]
- Isas, A.S.; Celis, M.S.M.; Correa, J.R.P.; Fuentes, E.; Rodríguez, L.; Palomo, I.; Mozzi, F.; Nieuwenhove, C. Van Functional Fermented Cherimoya (Annona cherimola Mill.) Juice Using Autochthonous Lactic Acid Bacteria. Food Res. Int. 2020, 138, 109729. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Rodrigues, S. Sonicated Pineapple Juice as Substrate for L. Casei Cultivation for Probiotic Beverage Development: Process Optimisation and Product Stability. Food Chem. 2013, 139, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hamid, N.; Gutierrez-Maddox, N.; Kantono, K.; Kitundu, E. Development of a Probiotic Beverage Using Breadfruit Flour as a Substrate. Foods 2019, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Hatami, S.; Tajabadi, N.; Massoud, R.; Sharifan, A. Chemical and Sensorial Properties of Probiotic Beverage Based on Rice Bran Extract and Honey. Biomass Convers. Biorefin 2021, 13, 5151–5156. [Google Scholar] [CrossRef]
- Panghal, A.; Virkar, K.; Kumar, V.; Dhull, S.B.; Gat, Y.; Chhikara, N. Development of Probiotic Beetroot Drink. Curr. Res. Nutr. Food Sci. J. 2017, 5, 257–262. [Google Scholar] [CrossRef]
- Chavan, M.; Gat, Y.; Harmalkar, M.; Waghmare, R. Development of Non-Dairy Fermented Probiotic Drink Based on Germinated and Ungerminated Cereals and Legume. LWT 2018, 91, 339–344. [Google Scholar] [CrossRef]
- Ogundipe, O.O.; Fasogbon, B.M.; Ogundipe, F.O.; Oredope, O.; Amaezenanbu, R.U. Nutritional Composition of Non-dairy Yogurt from Sprouted Tigernut Tubers. J. Food Process. Preserv. 2021, 45, e15884. [Google Scholar] [CrossRef]
- Marnpae, M.; Chusak, C.; Balmori, V.; Kamonsuwan, K.; Dahlan, W.; Nhujak, T.; Hamid, N.; Adisakwattana, S. Probiotic Gac Fruit Beverage Fermented with Lactobacillus Paracasei: Physiochemical Properties, Phytochemicals, Antioxidant Activities, Functional Properties, and Volatile Flavor Compounds. LWT 2022, 169, 113986. [Google Scholar] [CrossRef]
- Mongkontanawat, N.; Boonna, S.; Wasikadilok, N. Probiotic Beverage from Mangosteen Juice Fermented with Lactobacillus Strains. Trends Sci. 2022, 19, 6305. [Google Scholar] [CrossRef]
- Goderska, K.; Dombhare, K.; Radziejewska-Kubzdela, E. Evaluation of Probiotics in Vegetable Juices: Tomato (Solanum lycopersicum), Carrot (Daucus carota Subsp. Sativus) and Beetroot Juice (Beta vulgaris). Arch. Microbiol. 2022, 204, 300. [Google Scholar] [CrossRef]
- Lumina intelligence. Reserarch and Markets Probiotic Drink Market: Insights on the Probiotic Drinks Global Market to 2031—Asia-Pacific Has Been Dominating the Industry. Available online: https://www.lumina-intelligence.com/ (accessed on 10 April 2023).
- Pimentel, T.C.; Klososki, S.J.; Rosset, M.; Barão, C.E.; Marcolino, V.A. Fruit Juices as Probiotic Foods. Sport. Energy Drink. 2019, 10, 483–513. [Google Scholar]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. In Vitro Bioaccessibility of Health-Related Compounds as Affected by the Formulation of Fruit Juice- and Milk-Based Beverages. Food Res. Int. 2014, 62, 771–778. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Laursen, R.R.; Ouwehand, A. The Production and Delivery of Probiotics: A Review of a Practical Approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M. Challenges for the Production of Probiotic Fruit Juices. Beverages 2015, 1, 95–103. [Google Scholar] [CrossRef]
- Rakin, M.; Vukasinovic, M.; Siler-Marinkovic, S.; Maksimovic, M. Contribution of Lactic Acid Fermentation to Improved Nutritive Quality Vegetable Juices Enriched with Brewer’s Yeast Autolysate. Food Chem. 2007, 100, 599–602. [Google Scholar] [CrossRef]
- Žuntar, I.; Petric, Z.; Kovačević, D.B.; Putnik, P. Safety of Probiotics: Functional Fruit Beverages and Nutraceuticals. Foods 2020, 9, 947. [Google Scholar] [CrossRef]
- Nielsen, D.E.; El-Sohemy, A. Applying Genomics to Nutrition and Lifestyle Modification. Per Med. 2012, 9, 739–749. [Google Scholar] [CrossRef]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive Approaches for Assessing the Safety of Probiotic Bacteria. Food Control. 2020, 108, 106872. [Google Scholar] [CrossRef]
- Sotoudegan, F.; Daniali, M.; Hassani, S.; Nikfar, S.; Abdollahi, M. Reappraisal of Probiotics’ Safety in Human. Food Chem. Toxicol. 2019, 129, 22–29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).