Quality and Functional Characterization of Acetic Acid Bacteria Isolated from Farm-Produced Fruit Vinegars
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Sample
2.2. Isolation of Bacterial Strains
2.3. Spot Plate Assay
2.4. Bacterial Culture Condition and Preparation for Analysis
2.5. Identification of Bacterial Strains
2.6. Acetic Acid Production
2.7. Bacterial Growth and Titratable Acidity
2.8. Effect of Temperature on Growth
2.9. Antibacterial Activity
2.10. Antioxidant Activity
2.11. Angiotensin-Converting Enzyme (ACE) Inhibition
2.12. α-Glucosidase Inhibition
2.13. Statistical Analysis
3. Results and Discussion
3.1. Identification of Collected Vinegars
3.2. Identification of Isolates using 16S rRNA Sequencing
3.3. Effect of Temperature on Growth
3.4. Antibacterial Activity
3.5. Antioxidant Activity
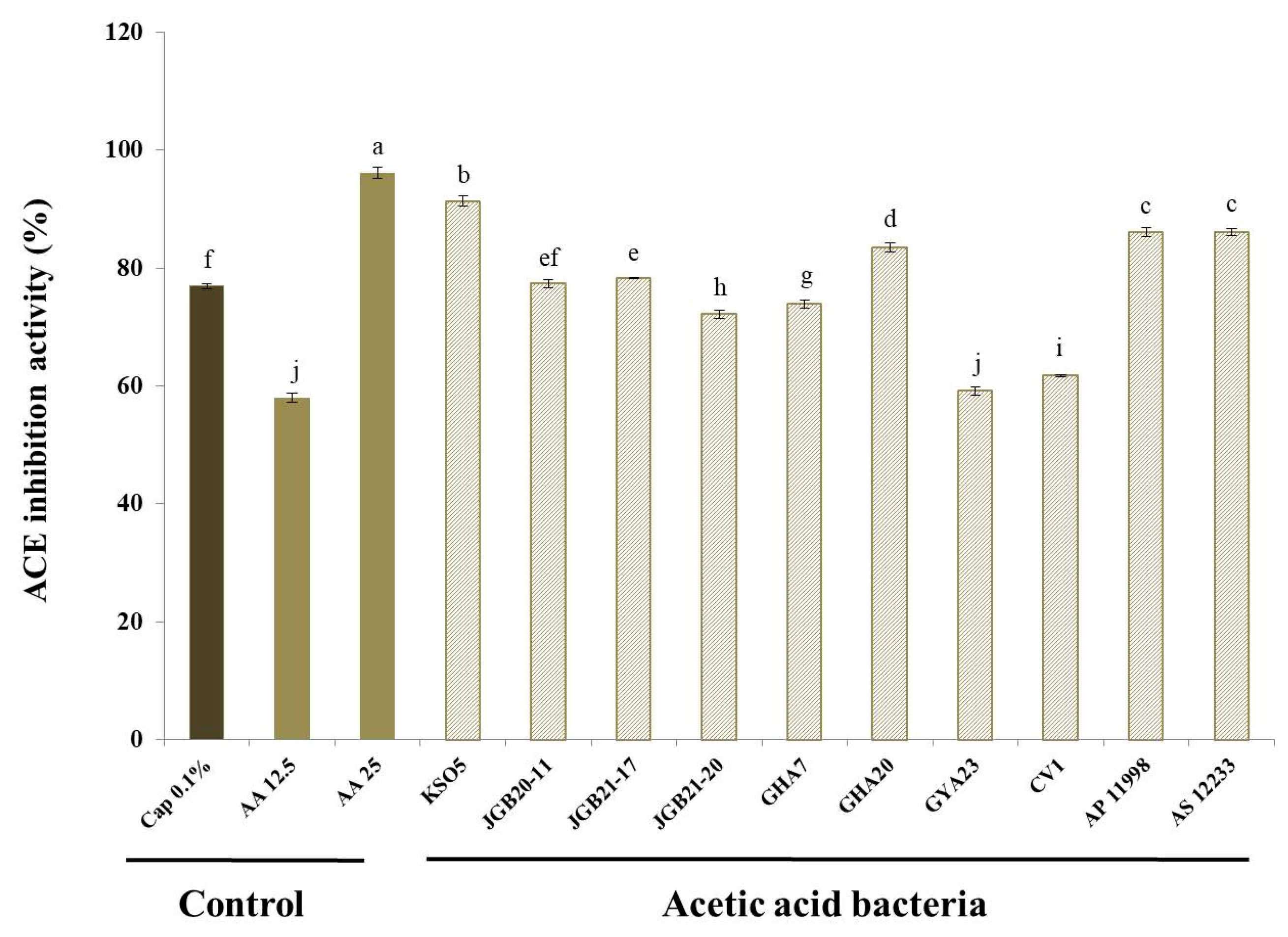
3.6. ACE Inhibition
3.7. α-Glucosidase Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.H.; Kim, J.Y.; Gwon, H.M.; Kim, S.Y.; Yeo, S.H. Determination of quality characteristics by the reproduction of grain vinegars reported in ancient literature. Korean J. Food Preserv. 2020, 27, 859–871. [Google Scholar] [CrossRef]
- Liu, L.; Yang, X. Hypolipidemic and antioxidant effects of freeze-dried powder of Shanxi mature vinegar in hyperlipidaemic mice. Food Sci. 2015, 36, 141–151. [Google Scholar]
- Seki, T.; Morimura, S.; Shigematsu, T.; Maeda, H.; Kida, K. Antitumor activity of rice-shochu post-distillation slurry and vinegar produced from the post-distillation slurry via oral administration in a mouse model. BioFactors 2004, 22, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, T.; Giudici, P.; Chen, F. Vinegar functions on health: Constituents, sources, and formation mechanisms. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1124–1138. [Google Scholar] [CrossRef]
- Lee, M.-K.; Choi, S.-R.; Lee, J.; Choi, Y.-H.; Lee, J.-H.; Park, K.-U.; Kwon, S.-H.; Seo, K.-I. Quality characteristics and anti-diabetic effect of yacon vinegar. J. Korean Soc. Food Sci. Nutr. 2012, 41, 79–86. [Google Scholar] [CrossRef]
- Chang, J.M.; Fang, T.J. Survival of Escherichia coli O157:H7 and Salmonella enterica serovars Typhimurium in iceberg lettuce and the antimicrobial effect of rice vinegar against E. coli O157. Food Microbiol. 2007, 24, 745–751. [Google Scholar] [CrossRef]
- Valduga, A.T.; Gonçalves, I.L.; Magri, E.; Delalibera-Finzer, J.R. Chemistry, pharmacology and new trends in traditional functional and medicinal beverages. Food Res. Int. 2019, 120, 478–503. [Google Scholar] [CrossRef]
- Sainz, F.; Navarro, D.; Mateo, E.; Torija, M.J.; Mas, A. Comparison of D-gluconic acid production in selected strains of acetic acid bacteria. Int. J. Food Microbiol. 2016, 222, 40–47. [Google Scholar] [CrossRef]
- Chouaia, B.; Gaiarsa, S.; Crotti, E.; Comandatore, F.; Degli Esposti, M.; Ricci, I.; Alma, A.; Favia, G.; Bandi, C.; Daffonchio, D. Acetic acid bacteria genomes reveal functional traits for adaptation to life in insect guts. Genome Biol. Evol. 2014, 6, 912–920. [Google Scholar] [CrossRef]
- Kersters, K.; Lisdiyanti, P.; Komagata, K.; Swings, J. The family Acetobacteraceae: The genera Acetobacter, Acidomonas, Asaia, Gluconacetobacter, Gluconobacter, and Kozakia. Prokaryotes 2006, 5, 163–200. [Google Scholar] [CrossRef]
- De Roos, J.; De Vuyst, L. Acetic acid bacteria in fermented foods and beverages. Curr. Opin. Biotechnol. 2018, 49, 115–119. [Google Scholar] [CrossRef]
- Saichana, N.; Matsushita, K.; Adachi, O.; Frébort, I.; Frebortova, J. Acetic acid bacteria: A group of bacteria with versatile biotechnological applications. Biotechnol. Adv. 2015, 33, 1260–1271. [Google Scholar] [CrossRef]
- Adachi, O.; Fujii, Y.; Ano, Y.; Moonmangmee, D.; Toyama, H.; Shinagawa, E.; Theeragool, G.; Lotong, N.; Matsushita, K. Membrane-bound sugar alcohol dehydrogenase in acetic acid bacteria catalyzes L-ribulose formation and NAD-dependent ribitol dehydrogenase independent of the oxidative fermentation. Biosci. Biotechnol. Biochem. 2001, 65, 115–125. [Google Scholar] [CrossRef]
- Gullo, M.; Verzelloni, E.; Canonico, M. Aerobic submerged fermentation by acetic acid bacteria for vinegar production: Process and biotechnological aspects. Process Biochem. 2014, 49, 1571–1579. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Wilkinson, S.; Daenen, L.; Arendt, E.K. Physiology of acetic acid bacteria and their role in vinegar and fermented beverages. Compr. Rev. Food Sci. Food Saf. 2019, 18, 587–625. [Google Scholar] [CrossRef]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Zhang, J.; Bao, J. Fermentative production of high titer gluconic and xylonic acids from corn stover feedstock by Gluconobacter oxydans and techno-economic analysis. Bioresour. Technol. 2016, 219, 123–131. [Google Scholar] [CrossRef]
- Zheng, Y.; Chang, Y.; Xie, S.; Song, J.; Wang, M. Impacts of bioprocess engineering on product formation by Acetobacter pasteurianus. Appl. Microbiol. Biotechnol. 2018, 102, 2535–2541. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Hong, H. Classification of acetic acid bacteria and their acid resistant mechanism. AMB Expr. 2021, 11, 29. [Google Scholar] [CrossRef]
- Kanchanarach, W.; Theeragool, G.; Inoue, T.; Yakushi, T.; Adachi, O.; Matsushita, K. Acetic acid fermentation of Acetobacter pasteurianus: Relationship between acetic acid resistance and pellicle polysaccharide formation. Biosci. Biotechnol. Biochem. 2010, 74, 1591–1597. [Google Scholar] [CrossRef]
- Wu, J.J.; Ma, Y.K.; Zhang, F.F.; Chen, F.S. Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of “Shanxi aged vinegar”, a traditional Chinese vinegar. Food Microbiol. 2012, 30, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Baek, C.H.; Park, E.H.; Baek, S.Y.; Jeong, S.T.; Kim, M.D.; Kwon, J.H.; Jeong, Y.J.; Yeo, S.H. Characterization of Acetobacter pomorum KJY8 isolated from Korean traditional vinegar. J. Microbiol. Biotechnol. 2014, 24, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, K.H.; Moon, J.Y.; Yeo, S.H.; Jeon, C.O. Acetobacter oryzoeni sp. Nov., isolated from Korean rice wine vinegar. Int. J. Syst. Evol. Microbiol. 2020, 70, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Cleenwerck, I.; Gonzalez, A.; Camu, N.; Engelbeen, K.; De Vos, P.; De Vuyst, L. Acetobacter fabarum sp. nov., an acetic acid bacterium from a Ghanaian cocoa bean heap fermentation. Int. J. Syst. Evol. Microbiol. 2008, 58, 2180–2185. [Google Scholar] [CrossRef]
- Iino, T.; Suzuki, R.; Kosako, Y.; Ohkuma, M.; Komagata, K.; Uchimura, T. Acetobacter okinawensis sp. nov, Acetobacter papayae sp. nov., and Acetobacter persicus sp. nov.; novel acetic acid bacteria isolated from stems of sugarcane, fruits, and a flower in Japan. J. Gen. Appl. Microbiol. 2012, 58, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Pitiwittayakul, N.; Theeragool, G.; Yukphan, P.; Chaipitakchonlatarn, W.; Malimas, T.; Muramatsu, Y.; Tanasupawat, S.; Nakagawa, Y.; Yamada, Y. Acetobacter suratthanensis sp. nov., an acetic acid bacterium isolated in Thailand. Ann. Microbiol. 2016, 66, 1157–1166. [Google Scholar] [CrossRef]
- Spitaels, F.; Li, L.; Wieme, A.; Balzarini, T.; Cleenwerck, I.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. Acetobacter lambici sp. nov., isolated from fermenting lambic beer. Int. J. Syst. Evol. Microbiol. 2014, 64, 1083–1089. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.Y.; Jeong, W.S.; Gwon, H.; Kim, S.Y.; Yeo, S.H. Culture and function-related characteristics of six acetic acid bacterial strains isolated from farm-made fermented vinegars. Korean J. Food Preserv. 2022, 29, 142–156. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Ei-El-Askri, T.; Yatim, M.; Sehli, Y.; Rahou, A.; Belhaj, A.; Castro, R.; Durán-Guerrero, E.; Hafidi, M.; Zouhair, R. Screening and characterization of new Acetobacter fabarum and Acetobacter pasteurianus strains with high ethanol-thermo tolerance and the optimization of acetic acid production. Microorganisms 2022, 10, 1741. [Google Scholar] [CrossRef]
- Chen, H.X.; Zhang, M.; Xie, B.J. Components and antioxidant activity of polysaccharide conjugate from green tea. Food Chem. 2005, 90, 17–21. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Luzón-Quintana, L.M.; Castro, R.; Durán-Guerrero, E. Biotechnological processes in fruit vinegar production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Sharafi, S.M.; Rasooli, I.; Beheshti-Maal, K. Isolation characterization and optimization of indigenous acetic acid bacteria and evaluation of their preservation methods. Iran. J. Microbiol. 2010, 2, 38–45. [Google Scholar]
- Adachi, O.; Moonmangmee, D.; Toyama, H.; Yamada, M.; Shinagawa, E.; Matsushita, K. New developments in oxidative fermentation. Appl. Microbiol. Biotechnol. 2003, 60, 643–653. [Google Scholar] [CrossRef]
- Moonmangmee, D.; Adachi, O.; Ano, Y.; Shinagawa, E.; Toyama, H.; Theeragool, G.; Lotong, N.; Matsushita, K. Isolation and characterization of thermotolerant Gluconobacter strains catalyzing oxidative fermentation at higher temperatures. Biosci. Biotechnol. Biochem. 2000, 64, 2306–2315. [Google Scholar] [CrossRef]
- Ge, J.; Kang, J.; Ping, W. Effect of acetic acid on bacteriocin production by Gram-positive bacteria. J. Microbiol. Biotechnol. 2019, 29, 1341–1348. [Google Scholar] [CrossRef]
- Axe, D.D.; Bailey, J.E. Transport of lactate and acetate through the energized cytoplasmic membrane of Escherichia coli. Biotechnol. Bioeng. 1995, 47, 8–19. [Google Scholar] [CrossRef]
- Ishii, S.; Kishi, M.; Yamagami, K.; Okada, S.; Abe, K.; Misaka, T. The use of mammalian cultured cells loaded with a fluorescent dye shows specific membrane penetration of undissociated acetic acid. Biosci. Biotechnol. Biochem. 2012, 76, 523–529. [Google Scholar] [CrossRef]
- Russell, J.B. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. Appl. Bacteriol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Surekha, M.; Robinson, R.K.; Batt, C.A.; Patel, C.; Reddy, S.M. Preservatives classification and properties. In Encyclopedia of Food Microbiology; Academic Press: New York, NY, USA, 2000; pp. 1710–1717. [Google Scholar] [CrossRef]
- Sakhare, P.Z.; Sachindra, N.M.; Yashoda, K.P.; Narasimha Rao, D.N. Efficacy of intermittent decontamination treatments during processing in reducing the microbial load on the broiler chicken carcass. Food Control 1999, 10, 189–194. [Google Scholar] [CrossRef]
- Xu, H.; Hong, J.H.; Kim, D.; Jin, Y.H.; Pawluk, A.M.; Mah, J.H. Evaluation of bioactive compounds and antioxidative activity of fermented green tea produced via one- and two-step fermentation. Antioxidants 2022, 11, 1425. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The evaluation of chemical antioxidant, antimicrobial and sensory properties of kombucha tea beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Harrison, C.; Acharya, K.R. ACE for all —A molecular perspective. J. Cell Commun. Signal. 2014, 8, 195–210. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Van Camp, J.; Verstraete, W. Optimisation and validation of an angiotensin-converting enzyme inhibition assay for the screening of bioactive peptides. J. Biochem. Biophys. Methods 2002, 51, 75–87. [Google Scholar] [CrossRef]
- Kondo, S.; Tayama, K.; Tsukamoto, Y.; Ikeda, K.; Yamori, Y. Antihypertensice effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2001, 65, 2690–2694. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, M.; Naz, A.; Siddiqui, H.; Ahmad, S.I.; Faizi, S. Hypotensive and toxicological study of citric acid and other constituents from Tagetes pathla roots. Arch. Pharm. Res. 2004, 27, 1037–1042. [Google Scholar] [CrossRef]
- Engler, M.M.; Engler, M.B.; Pierson, D.M.; Molteni, L.B.; Molteni, A. Effects of docosahexaenoic acid on vascular pathology and reactivity in hypertension. Exp. Biol. Med. 2003, 228, 299–307. [Google Scholar] [CrossRef]
- Kousar, M.; Salma, U.; Khan, T.; Shah, A.J. Antihypertensive potential of tartartic acid and exploration of underlying mechanistic pathways. Dose Response 2022, 20, 15593258221135728. [Google Scholar] [CrossRef]
- Caravaca, P.; Morán, L.; Dolores, M.; Delgado, J. The renin-angioensin-aldosterone system and COVID-19. Clin. Implic. 2020, 20, 27–32. [Google Scholar] [CrossRef]
- Elkhtab, E.; El-Alfy, M.; Shenana, M.; Mohamed, A.; Yousef, A.E. New potentially antihypertensive peptides liberated in milk during fermentation with selected acid bacteria and kombucha cultures. J. Dairy Sci. 2017, 100, 9508–9520. [Google Scholar] [CrossRef] [PubMed]
- Vitas, J.; Vukmanović, S.; Cakarevic, J.; Popovic, L.; Malbasa, R. Kombucha fermentation of six medicinal herbs: Chemical profile and biological activity. Chem. Ind. Chem. Eng. Q. 2020, 26, 157–170. [Google Scholar] [CrossRef]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. α-glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef]
- Shinde, J.; Taldone, T.; Barletta, M.; Kunaparaju, N.; Hu, B.; Kumar, S.; Placido, J.; Zito, S.W. α-glucosidase inhibitory activity of Syaygium cumini (Linn.) skeels seed kernel in vitro and in Goto-Kakizaki (GK) rats. Carbohydr. Res. 2008, 343, 1278–1281. [Google Scholar] [CrossRef]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martínez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- van de Laar, F.A. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef]
- Yim, E.J.; Jo, S.W.; Lee, E.S.; Park, H.S.; Ryu, M.S.; Uhm, T.B.; Kim, H.Y.; Cho, S.H. Fermentation characteristics of mulberry (Cudrania tricuspidata) fruit vinegar produced by acetic acid bacteria isolated from traditional fermented foods. Korean J. Food Preserv. 2015, 22, 108–118. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Wang, H.; Lo, C.Y.; Ho, C.T.; Li, S.M. Black tea in chemo-prevention of cancer and other human diseases. Food Sci. Hum. 2013, 2, 12–21. [Google Scholar] [CrossRef]




| Region | Origin (Vinegar) | Sample | PD 1 | CD 2 | Vessel |
|---|---|---|---|---|---|
| Gangwon-do | Apple | GHA | 11. 10. ‘20 | 03. 11. ‘21 | PET 3 |
| Jeollabuk-do | Korean blackberry (Rubus coreanus)_20 | JGB20 | 09. 25. ‘20 | 03. 20. ‘21 | Pottery |
| Korean blackberry (Rubus coreanus)_21 | JGB21 | 01. 25. ‘21 | 03. 20. ‘21 | Pottery | |
| Apple | JGA | 04. 25. ‘20 | 03. 20. ‘21 | Pottery | |
| Gyeonggi-do | Magnolia berry (Schisandra chinesis) | KSO | 01. 01. ‘21 | 03. 29. ‘21 | Glass |
| Pineapple | KSP | 01. 10. ‘21 | 03. 29. ‘21 | Glass | |
| Tomato | KST | 12. 18. ‘20 | 03. 29. ‘21 | Glass | |
| Gyeongsangbuk-do | Apple | GYA | 03. 16. ‘21 | 04. 26. ‘21 | Pottery |
| Alcohol Concentration | 3% | 5% | 7% | 9% | 10% | 12% | 15% |
|---|---|---|---|---|---|---|---|
| Strain | |||||||
| KSO 5 2 | 3.83 1 | 3.98 | 3.72 | 3.21 | 3.33 | 3.70 | 2.60 |
| KSO 6 | 3.88 | 3.93 | 3.94 | 3.29 | 3.48 | 3.62 | 2.23 |
| KSF 2 | 3.41 | 3.17 | 1.58 | 0.00 | 0.00 | 0.00 | 0.00 |
| KSF 6 | 2.78 | 3.14 | 1.55 | 0.00 | 0.00 | 0.00 | 0.00 |
| KSF 8 | 2.70 | 3.32 | 2.16 | 0.00 | 0.00 | 0.00 | 0.00 |
| JGB 20-11 | 3.69 | 4.00 | 5.11 | 2.70 | 3.56 | 2.58 | 0.00 |
| JGB 20-13 | 3.56 | 4.18 | 3.41 | 2.48 | 3.98 | 2.02 | 0.00 |
| JGB 21-17 | 3.13 | 3.56 | 3.15 | 1.75 | 2.60 | 2.52 | 0.00 |
| JGB 21-20 | 2.79 | 3.23 | 2.97 | 2.37 | 3.89 | 3.16 | 1.58 |
| JGB 21-24 | 3.17 | 3.59 | 3.12 | 1.97 | 1.70 | 1.32 | 0.00 |
| JGA 10 | 3.04 | 3.46 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| JGA 13 | 2.67 | 2.81 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| JGA 16 | 2.74 | 2.32 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| GHA 2 | 3.76 | 3.60 | 3.10 | 2.24 | 3.33 | 3.22 | 2.57 |
| GHA 7 | 3.65 | 3.58 | 3.22 | 2.24 | 4.24 | 3.08 | 3.53 |
| GHA 20 | 3.31 | 3.80 | 3.14 | 2.34 | 3.43 | 2.81 | 3.31 |
| GHA 112 | 2.94 | 3.55 | 3.26 | 2.07 | 3.30 | 2.70 | 2.38 |
| GYA 14 | 3.29 | 3.50 | 2.88 | 1.68 | 2.68 | 2.20 | 0.00 |
| GYA 17 | 3.39 | 3.30 | 2.84 | 1.92 | 3.04 | 2.28 | 2.24 |
| GYA 23 | 3.41 | 3.80 | 3.39 | 1.53 | 3.11 | 3.38 | 2.51 |
| GS CV1 3 | 2.84 | 3.68 | 2.51 | 2.06 | 2.24 | 2.41 | 1.84 |
| AP 11998 | 1.20 | 1.10 | 1.09 | 0.00 | 0.00 | 0.00 | 0.00 |
| AS 12233 | 4.18 | 4.74 | 3.06 | 2.56 | 2.38 | 0.00 | 0.00 |
| Strain 1 | Size (bp) | Closest Match | Identity (%) |
|---|---|---|---|
| KSO 5 | 1411 | A. cerevisiae | 99.85 |
| JGB 20-11 | 1408 | A. pasteurianus | 99.78 |
| JGB 21-17 | 1409 | A. pasteurianus | 99.70 |
| JGB 21-20 | 1406 | A. pasteurianus | 99.85 |
| GHA 7 | 1409 | A. pasteurianus | 99.85 |
| GHA 20 | 1410 | A. pasteurianus | 99.85 |
| GYA 23 | 1409 | A. pasteurianus | 99.85 |
| Sample Type | Name | Titratable Acidity (%) | Clear Zone (mm) | |||
|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | |||||
| B. cereus | S. aureus | E. coli | S. typhimurium | |||
| Strain | KSO 5 | 4.17 | 17.6 ± 0.1 g 2 | 22.0 ± 0.1 f | 19.8 ± 0.1 g | 20.2 ± 0.2 f |
| JGB 20-11 | 5.66 | 20.5 ± 0.1 e | 23.7 ± 0.2 e | 23.2 ± 0.1 f | 21.6 ± 0.1 d | |
| JGB 21-17 | 5.53 | 20.2 ± 0.2 d | 25.2 ± 0.1 c | 20.6 ± 0.1 e | 20.3 ± 0.2 f | |
| JGB 21-20 | 5.59 | 20.5 ± 0.1 f | 25.2 ± 0.2 c | 21.5 ± 0.1 f | 20.3 ± 0.2 f | |
| GHA 7 | 5.47 | 18.6 ± 0.2 c | 22.9 ± 0.1 d | 21.5 ± 0.2 c | 22.6 ± 0.1 f | |
| GHA 20 | 5.59 | 17.9 ± 0.2 c | 22.7 ± 0.1 d | 22.6 ± 0.2 c | 22.4 ± 0.1 f | |
| GYA 23 | 5.52 | 18.5 ± 0.1 b | 23.8 ± 0.2 e | 20.9 ± 0.1 d | 21.7 ± 0.1 b | |
| Positive control 3 | AA 50 | 27.3 ± 0.1 a | 31.1 ± 0.1 a | 30.1 ± 0.1 a | 30.1 ± 0.1 a | |
| AA 25 | 20.2 ± 0.2 f | 24.1 ± 0.1 b | 24.2 ± 0.2 b | 22.1 ± 0.1c | ||
| AA 12.5 | 16.2 ± 0.1 h | 16.1 ± 0.2 d | 17.1 ± 0.1 f | 17.2 ± 0.2 e | ||
| N.C. 1 | medium | 8.1 ± 0.1 i | 8.1 ± 0.1 g | 8.1 ± 0.1 h | 8.1 ± 0.1 g | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Jeong, W.-S.; Kim, S.-Y.; Yeo, S.-H. Quality and Functional Characterization of Acetic Acid Bacteria Isolated from Farm-Produced Fruit Vinegars. Fermentation 2023, 9, 447. https://doi.org/10.3390/fermentation9050447
Kim S-H, Jeong W-S, Kim S-Y, Yeo S-H. Quality and Functional Characterization of Acetic Acid Bacteria Isolated from Farm-Produced Fruit Vinegars. Fermentation. 2023; 9(5):447. https://doi.org/10.3390/fermentation9050447
Chicago/Turabian StyleKim, Sun-Hee, Woo-Soo Jeong, So-Young Kim, and Soo-Hwan Yeo. 2023. "Quality and Functional Characterization of Acetic Acid Bacteria Isolated from Farm-Produced Fruit Vinegars" Fermentation 9, no. 5: 447. https://doi.org/10.3390/fermentation9050447
APA StyleKim, S.-H., Jeong, W.-S., Kim, S.-Y., & Yeo, S.-H. (2023). Quality and Functional Characterization of Acetic Acid Bacteria Isolated from Farm-Produced Fruit Vinegars. Fermentation, 9(5), 447. https://doi.org/10.3390/fermentation9050447






