Abstract
Biological control agents are a promising substitute for chemical pesticides in agricultural pest management. In this study, Penicillium sp. with high pathogenicity to the agricultural pests oriental leafworm moth (Spodoptera litura) and diamondback moth (Plutella xylostella) were isolated from naturally infected insects and grown on different agricultural residues as an inexpensive substrate for their sporulation. Ten strains of Penicillium (P.01~P.10) were identified as P. citrinum based on morphological features and molecular studies, with sequence analysis using an internal transcribed spacer region. Different fungal isolates exhibited a varying degree of pathogenicity against S. litura and Pl. xylostella, and strains P.04 and P.09 showed the highest pathogenicity to S. litura, with a mortality rate of 92.13% after 7 days of treatments, while strain P.06 resulted in the highest mortality of Pl. xylostella (100%) after 6 days of treatment. Moreover, among ten isolates infected with both S. litura and P. xylostella, P.06 showed potential virulence against S. litura and Pl. xylostella, with lethal time for 50% mortality (LT50) values of 4.5 days and 3.0 days, respectively. The ten isolates showed higher virulence to Pl. xylostella than to S. litura. The agro-industrial-based medium showed efficiency for the cultivation of isolates for sporulation on an industrial scale, suggesting that the newly isolated P. citrinum is a potential biological control agent for controlling insect pests and could be further developed for microbial pesticide production.
1. Introduction
Modern agricultural techniques result in the growth of pests due to disruption of the natural ecosystem. In addition, the widespread application of synthetic pesticides kills natural enemies that help control pest populations [1]. As an alternative, entomopathogenic fungi (EPF) provide a distinctive and sustainable solution for pest control [2,3]. These spore-forming fungi exhibit a variety of nutritional modes, ranging from biotrophy to necrotrophy, and have the capability to penetrate insect defenses [4]. They are suitable for development as an ecofriendly biopesticide. Isolation and identification of ubiquitous EPF across naturally infected insects and soils play a key role in understanding the ecological requirements of these organisms and can aid in the selection of appropriate fungal candidates for pest management [5]. In recent years, the use of fungi-based insecticides for biological plant protection has attracted considerable attention since this method minimizes the use of chemical insecticides and efficiently protects the ecosystem and environment [6,7]. Therefore, using insect-pathogenic microorganisms of EPF agents for biological control of agricultural pests to protect crops is important for integrated pest management program approaches to increase agricultural output [8,9].
Various species of EPF, including Beauveria, Metarhizium, Aspergillus, Fusarium, Penicillium, and Paecilomyces, are naturally present in agricultural soils; however, their spore levels in nature are insufficient to effectively control pest outbreaks [10]. Therefore, the application of EPF for pest management requires a product with sufficient spore numbers. In addition to isolation and identification of new strains of EPF, the development of specific fungi as bio-pesticides poses challenges, as their ability to produce spores is dependent on the species and the ingredients used in the media [3]. Several factors have been reported to influence the production of mycopesticides, such as the availability of fungi for mass production and commercialization and cost-effective fermentation processes with high concentrations of persistent and virulent spores [3]. Solid-state fermentation is a widely used system for the production of EPF [11,12]. This process consumes less water and uses agricultural and industrial by-product residues as a cheap and eco-friendly solid substrate (e.g., corn bran, wheat bran, rice bran, brown rice, rice husks, coffee husks, sugarcane bagasse, and palm kernel cake) [13,14]. Moreover, the use of these residues for EPF production enables a biorefinery approach for their valorization, reduces waste generation, and facilitates sustainable production of valuable bioproducts [15,16].
EPF have been documented to exhibit virulence against piercing and sucking insect pests, such as aphids and thrips [17]. The oriental leafworm moth (Spodoptera litura) and diamondback moth (Plutella xylostella) are major lepidopteran pests of broccoli, canola, cabbage, and other vegetables. These insects are known for their high migratory ability and wide distribution in both temperate and tropical regions [18]. Currently, the management of these insects mainly uses chemical pesticides, but the extensive use of such chemicals has caused serious problems of environmental pollution and pest resistance. Therefore, there is significant interest in screening and identifying strains with promising pathogenicity to both S. litura and Pl. xylostella. Previously, we isolated five strains of Paecilomyces javanicus and one strain of P. lilacinus that exhibited pathogenicity to both S. litura and Pl. xylostella [19]. Penicillium citrinum strains have been reported to be associated with mosquito larvae [20,21]. However, no data has been reported on the efficacy of different fungal P. citrinum isolates against S. litura and Pl. xylostella. Investigation of this EPF not only improves microbiota biodiversity knowledge but also provides new EPF for biocontrol potential. Hence, this work aimed to isolate and identify Penicillium sp. as a biocontrol agent with high pathogenicity against S. litura and Pl. xylostella and to analyze different residues as inexpensive substrates for the cultivation of isolates for sporulation.
2. Materials and Methods
2.1. Collection, Isolation, Identification, and Phylogenetic Analysis of Penicillium sp.
Naturally infected insects and soils were obtained from cultivated fields in Lam Dong, Dak Nong, Long An, and Ho Chi Minh City (Vietnam). The methods from our previous work were used to isolate fungal strains from the soil and infected insect samples [19]. Briefly, external conidia from infected insects were transferred onto potato dextrose agar (PDA) medium supplemented with 3% (w/w) sodium chloride and 0.01% (w/w) chloramphenicol plate (PDA+) and incubated at 30 °C for 5 days. For soil samples, 2 g of soil was suspended in 20 mL of sterilized water. A serial dilution (10−1, 10−2, and 10−3 g/mL) of the soil suspension was then prepared, spread onto PDA+ plates, and placed in an incubator at 30 °C for 5 days. The fungal colony’s hyphal tip was then transferred to PDA plates to obtain pure culture isolation. The macro- and microscopic features of the isolates were examined using the methods of Samson [22] and Houbraken et al. [23] to preliminarily identify the isolated fungi.
Ribosomal RNA (rRNA)-internal transcribed spacer (ITS) sequencing was used to further identify Penicillium isolates. A pair of primers for 18S-ITS1-5.8S-ITS2-28S rRNA was designed and provided by Nam Khoa BioTek Co., Ltd. (Ho Chi Minh City, Vietnam) and used for the polymerase chain reaction (PCR). The PCR products were then subjected to agarose gel electrophoresis, and the DNA sequences were subsequently compared to those deposited in the National Center of Biotechnology Information (NCBI) server (http://www.ncbi.nlm.nih.gov) using Entrez and BLAST (accessed on 20 March 2021).
The nucleotide sequences of the ITS-rRNA gene of the isolates and related strains were aligned using Clustal X version 2.1 (http://www.clustal.org/clustal2/; accessed on 7 April 2023). The phylogenetic tree was then constructed using bootstrap analysis with a default setting of 1000 trials and a seed value of 111.
2.2. Spore Suspension Preparation
The isolates were grown on sterilized substrates at 37 °C for 10 days. After the solid-state fermentation was completed, the substrate (10 g) was collected and mixed with 20 mL of 0.05% Tween 80 in a sterile test tube by vortexing to dislodge and suspend the spores. The spores were then collected by filtering through a double-layered sterile cheesecloth and used for the determination of spore concentration using a Marienfeld hemocytometer (Germany). The spore suspension was subsequently standardized to 108 spores/mL for further experiments.
2.3. Pathogenicity of P. citrinum to Diamondback Moth and Oriental Leafworm Moth
The method of Altre et al. [24] was used to examine the pathogenicity tests. Briefly, third instar larvae of S. litura and Pl. xylostella were obtained from a laboratory colony, reared on a wheat germ diet, and maintained in laboratory conditions of 15:9 h light:dark and 30 °C until used. Plastic containers were prepared by placing filter paper at the bottom, lightly moistening them with sterile distilled water, and placing greenhouse-grown cabbage (Brassica chinensis L var. gracious) in them as food. Third instar S. litura and Pl. xylostella larvae were exposed to a spore suspension of each isolate (108 spores/mL) by dipping them in the solution for 5 seconds and then placed in a container. For the control, the larvae were treated with sterile aqueous 0.05% Tween-80 solutions. The mortality was then monitored daily for 7 days. Corrected mortality was determined using Abbott’s formula (equation 1) [25]. The experiment was conducted in triplicates.
2.4. Influence of Different Substrates on Spore Production of P. citrinum
Different agricultural residues (sugarcane bagasse, wheat bran, rice bran, corn bran, rice husks, coffee husks, and broken rice) were used as substrates for the solid-state fermentation of P. citrinum for spore production. The composition of each tested medium is shown in Table S1 (Supplementary Material). The media were prepared by mixing each substrate (25 g) with 20 mL of distilled water and sterilizing it in an autoclave for 20 min at 121 °C. The prepared substrates were then inoculated with 108 spores of each isolate and placed in an incubator at 37 °C for 10 days. After the fermentation was completed, the spore suspension was collected and used for the determination of spore concentration.
2.5. Statistical Analysis
Data obtained from the pathogenicity test (Table 1 and Table 2) were analyzed by non-parametric analysis (Kruskal-Wallis test at p ≤ 0.05) using PC SAS 8.2 (SAS Institute, Cary, NC, USA). The lethal time required to cause 50% mortality (LT50) was determined using the Kaplan-Meier analysis method. An analysis of variance (ANOVA) with the least significant difference (LSD) test at p ≤ 0.05 was conducted to analyze the statistical significance of data obtained from the solid-state fermentation by using the same SAS software.

Table 1.
Corrected mortality of third instar larvae of Spodoptera litura exposed in fungal suspension of ten isolates with different infection times (day).

Table 2.
Corrected mortality of third instar larvae of Plutella xylostella exposed in fungal suspension of ten isolates with different infection times (day).
3. Results
3.1. Isolation and Identification of P. citrinum
Ten strains of Penicillium isolates (namely P.01~P.10) were isolated from the infected insects (Table S2, Supplementary Material). These isolates grew well on PDA, with diameters of colonies ranging 3.5~4.3 cm within 10 days of incubation at 37 °C. The Penicillium colonies on the PDA medium formed a basal felt with floccose aerial mycelium giving rise to conidiophores. Colonies were short, floccose, flat, round, and olive green at the top and white at the periphery (Figure 1A,B). Conidiophores are born singly, and the wall was smooth and thin, typically bearing irregular penicillus that contained conidia and phialide. Conidia are produced in distorted chains, spherical to subspherical (Figure 1C). The morphology of isolates was similar to the characterization and identification of P. citrinum reported by Kumar et al. [26] and Mukherjee et al. [27]. Studies have reported the analysis of rRNA gene sequences for the polyphylogeny of the genus Penicillium [28], and the ITS region was useful for the identification and classification of Penicillium. In this study, the ITS1 region was amplified and sequenced to identify the isolated strains. Sequence analysis of P.01~P.10 clones demonstrated 100% similarity to the sequence of P. citrinum isolate C2C in the GenBank database (accession no. MH990629.1). The nucleotide sequence of these isolates was deposited in the GenBank, and the accession number is shown in Table S2 (Supplementary Material). A phylogenetic tree was constructed using the ITS1 sequences of the isolates (ca 840 nucleotides) and the Penicillium genus (Figure 2). Phylogeny consisted of 20 trains and indicated that the isolated strains were attached with P. citrinum strain SUMS0392 (KU128405.1) with 100% bootstrap support (Figure 2). These results confirmed that the P.01~P.10 isolates were the species P. citrinum.
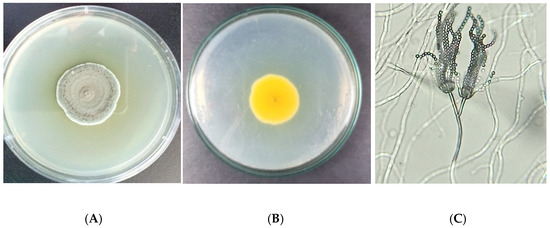
Figure 1.
Obverse (A) and reverse (B) photograph of isolated Penicillium citrinum colony on PDA medium at 7 day. The morphology of fungal conidiophores cells was observed under a compound microscope (×40 magnification) view (C).
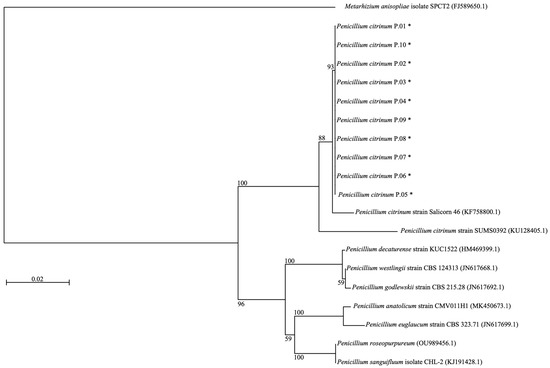
Figure 2.
Phylogenetic tree of the isolated P. citrinum (indicated by asterisk) and other Penicillium strains. The strains are identified by their EMBL/GenBank/DDBJ accession numbers after species names. Bootstrap values, indicated at the nodes, were obtained from 1000 bootstrap replicates and shown as percentages. Bar indicates 2% sequence divergence. The sequence of Metarhizium anisopliae isolate SPCT2 (FJ589650.1) was used as an outgroup.
3.2. Pathogenicity of P. citrinum to S. litura and Pl. xylostella
This study investigated the pathogenicity of ten isolates to S. litura and Pl. xylostella. It was observed that all the isolated P. citrinum strongly infected S. litura and Pl. xylostella larvae. The mortality of S. litura ranged from 76.39% (P.05) to 92.13% (P.04 and P.09) after 7 days of treatment (Table 1). More than 95.83% of Pl. xylostella was infected by all isolates after 7 days of treatment, except the P.06 isolate strongly infected Pl. xylostella with a mortality of 100% after 6 days of treatment (Table 2). All isolates exhibited higher pathogenicity to Pl. xylostella than S. litura after day 1 of infection. The larval mortality progressively increased with increasing time after infection.
To further evaluate the pathogenicity of the isolates, Kaplan–Meier survival curves (Figure S1, Supplementary Material) were established to estimate the LT50 values. All fungal isolates were pathogenic to S. litura and Pl. xylostella, but their virulence against the two tested insects differed (Table 3). The LT50 values for S. litura ranged from 4.5 days to 5.0 days, whereas a range of 2.5–3.0 days was observed for Pl. xylostella. The P.06 and P.01 isolates showed the highest ability to infect and kill S. litura (LT50 value of 2.5 days) and Pl. xylostella (LT50 value of 2.5 days), respectively. There were statistical differences in all isolates of P. citrinum for the LT50 values in the third instar larvae of S. litura and Pl. xylostella, and the infection rate of those ten isolated P. citrinum to Pl. xylostella was significantly higher than to S. litura.

Table 3.
Lethal time (days) for 50% mortality (LT50) of S. litura and Pl. xylostell third instar larvae exposed to ten isolated P. citrinum.
3.3. Influence of Different Substrates on Sporulation of P. citrinum
This study compared the spore production of ten isolates on different substrates. It was found that the number of spores varied among those ten isolates and tested media (Table 4). The composition of all media (M1~M10), including broken rice, wheat bran, rice bran, corn bran, rice husks, coffee husks, sugarcane bagasse, and water, is shown in Table S1. The P.01 isolate showed a significantly higher number of spores (4.87 × 108~4.96 × 108 spores/g) from M4, M5, and M7 media compared to other media. The highest and lowest number of spores was observed when P.02 isolate was grown in M4 (7.07 × 108 spores/g) medium and M8 (1.49 × 108 spores/g) medium, respectively. M7 was the most efficient medium for spore production of P.03 isolate, with a spore number of 5.48 × 108 spores/g. M4 and M5 media were suitable for cultivating P.04 to produce spores of 4.28 × 108 spores/g and 4.04 × 108 spores/g, respectively. A significantly higher number of spores (3.15 × 108~3.66 × 108 spores/g) from the M6, M9, and M10 media were observed in the P05 isolate than those from other media. The highest number of spores was observed when P.06, P.07, and P.10 isolates were grown in M4 (3.16 × 108 spores/g), M6 (3.10 × 108 spores/g), and M4 (4.33 × 108 spores/g) media, respectively. Both M3 and M7 were suitable for sporulation of the P.08 isolate, with the number of spores being 3.17 × 108 and 3.44 × 108 spores/g, respectively, while M4, M5, and M9 were suitable for sporulation of P.09, with the number of spores being 3.40 × 108~3.95 × 108 spores/g.

Table 4.
The number of spores of ten Penicillium isolates on different media.
4. Discussion
This work describes the isolation and identification of ten strains of P. citrinum from infected insects based on morphological and rRNA-ITS studies [29,30], whereas no strains of Penicillium were isolated from the soil samples, suggesting that the soil environment impacts EPF distribution. This impact may be due to more human activities in fallow lands and the use of chemical pesticides [31]. Moreover, it could be due to the presence of these EPF in the soil samples being extremely low. However, further investigations are needed to clarify if different environmental protections, cropping systems, and other factors affect the EPF biodiversity in the sample collection regions.
Pathogenicity tests were used to evaluate the potential use of EPF as a biocontrol agent against the target pests. The infection of ten isolates to S. litura and Pl. xylosterlla is shown in Figure S2 (Supplementary Material). This study showed that strains P02 and P03 strongly infected both S. litura (mortality of 83.80% and 84.26%, respectively) and Pl. xylosterlla (mortality of 100% and 95.83%, respectively) after 7 days of treatment. However, strains P04 and P09 showed the highest ability to infect S. litura, with a mortality of 92.13% after 7 days of treatments, while strain P.06 resulted in the highest mortality of Pl. xylosterlla (100%) after 6 days of treatment, indicating that larval mortality varied with EPF species. Effects were clearly exposure time related. One of the major drawbacks of EPF is their slow speed to kill insects, thus requiring a longer exposure time to cause insect death compared to chemical insecticides. However, EPF minimize crop damage by inducing infection of host pests, leading to a decrease in oviposition, development, feeding, mating, and other physiological traits of insects [32,33]. Altre et al. [24] observed P. fumosoroseus-infected Pl. xylostella with 79% mortality. In addition, 100% mortality of S. litura eggs was detected due to infection with Aspergillus spp. [34], while P. citrinum resulted in 2.5% egg mortality in Pteroptyx bearni fireflies [35]. Maketon et al. [21] stated that P. citrinum strains (1 × 106 conidia/mL) caused 100% mortality to the third instar mosquito larvae within 2 h. Furthermore, Herlinda et al. [36] reported that P. citrinum caused insignificant mortality to S. frugiperda larvae, but it resulted in 98.67% mortality to the second instar cotton leafworm larvae. Soliman et al. [37] illustrated that Metarhizium anisopliae, Beauveria bassiana, and Paecilomyces lilacinus caused 15~60%, 15~57.50%, and 10~17.5% mortality in the third instar larvae of Ceratitis capitata at 106~1010 conidia/mL, respectively. Ramanujam et al. [38] described that M. anisopliae isolate caused 67.8% mortality after 86.04 h of exposure at 108 spores/mL, followed by B. bassiana isolate with 64.3% mortality at 108 spores/mL after 88.30 h against second instar larvae of the maize fall armyworm S. frugiperda. Idrees et al. [39] declared that P. citrinum isolate significantly caused egg mortality and a reduction in the feeding of the third instar of S. frugiperda larvae. Baksh and Khan [40] revealed that Paecilomyces tenuipes (spore concentration of 108 spores/mL) infected third instar larvae of Pl. xylostella with an LT50 of 2.33 days. Mukherjee et al. [27] showed that P. verrucosum had significant LT50 of 37.5 h with 105 spores/mL on papaya aphid 3rd~4th instar. In addition, A. oryzae and B. bassiana were used to manage S. litura infesting corn in Indonesia [18].
In our study, although infections with EFP varied for the ten P. citrinum strains, all isolates showed a strong pathogenicity against both S. litura and Pl. xylostella with the LT50 values of 4.5–5.0 days and 2.5–3.0 days, respectively, suggesting that these isolates are a promising alternative to synthetic insecticides for insect management. Variation of the isolated EPF in pathogenicity to Pl. xylostella and S. litura larvae indicates that the isolates and species examined were adapted differently to particular insects (or particular taxonomic groups of insects) or that there is a strong intraspecies difference among these isolates, and further research is needed for these. The pathogenicity of the ten isolated P. citrinum to S. litura was much lower than to Pl. xylostella, suggesting that it was more difficult to infect S. litura than Pl. xylostella by these isolates penetrating the insect through the cuticle [41]. During the infection process, EPF synthesize and release extracellular enzymes (i.e., chitinase, lipases, and proteases) to hydrolyze the cuticle constituents (lipid, chitin, and protein) and subsequently allow penetration of EPF through the cuticle after their conidia attach to and germinate on the insect host cuticle and sporulate inside host bodies, resulting in insect death [42,43,44]. The external surface of conidia has a fundamental protein with a hydrophobic rodlet layer that connects to the insect epicuticle [45], and these unique fungal hydrophobins are important in the growth, development, sporulation, thermotolerance, and pathogenesis of EPF [46,47]. Environmental conditions also affect the virulence of EPF. Rizal et al. [48] demonstrated that temperature had an idiosyncratic effect across the virulence of Metarhizium and B. bassiana in terms of sporulation rates, mortality, and time to death. In our study, the isolated P. citrinum strongly infected oriental leafworm moth and diamondback moth under laboratory conditions. Therefore, these isolates are potential microbial biocides and can be integrated into pest management strategies and used for crop protection against S. litura and Pl. xylostella. However, further study needs to be conducted to validate the results under field conditions, as well as determining their influence on non-target organisms including natural enemies, their stability, and their tolerance to environmental pressure.
Selection of EPF for industrial application as biological control agents requires their high spore production and efficient growth on a low-cost medium. To reduce production costs and facilitate a biorefinery approach for sustainable production, various agro-industrial waste residues have been utilized as inexpensive substrates for solid-state fermentation of EPF for spore production. Soybean meals have been used as a substrate for lipase production from Penicillium and spore production of Bacillus thuringiensis [49,50]. In addition, cottonseed flour and soybean meal (1:1) have been reported as inexpensive substrates for the solid-state fermentation of EPF Cordyceps javanica [51]. The produced conidia showed potential pathogenicity to the Asian citrus psyllid, Diaphorinacitri (LT50 of 3.58 days), with a mortality of 100% after an exposure period of 7 days [50]. Furthermore, sorghum resulted in the greatest amount of Metarhizium flavoviride spores (102.80 × 109 spores/g substrate), whereas the highest number of spores for B. bassiana (141.0 × 109 spores/g) was achieved by growing this EPF on white rice for 60 days [52].
In the current work, a mixture of different substrates (broken rice, wheat bran, rice bran, corn bran, rice husks, coffee husks, and sugarcane bagasse) were formulated and used as media to grow the isolated P. citrinum for sporulation. As shown in Table 4, the sporulation of ten isolates was significantly influenced by the medium composition. Different substrates caused variation in the spore numbers of all isolates. Among the examined media, M4 (a mixture of sugarcane bagasse, wheat bran, and broken rice) was more efficient for the spore production of six isolated strains, P.01, P.02, P.04, P.06, P.09, and P.10 than that of other isolates. In particular, M4 was identified as the most efficient medium for spore production of the P.02 isolate, with a spore number of 7.07 × 108 spores/g, while M8 (a mixture of rice husks, corn bran, and broken rice) was unsuitable for cultivating this isolate to produce spores of 1.49 × 108 spores/g. The maximal spore production of P.03 was found in the M7 medium (containing sugarcane bagasse, rice bran, and broken rice). The best medium for spore production of P.05 with the highest number of spores was M6, M9, or M10, while the most suitable medium for P.08 was M3 or M7, suggesting that a specific media may need to be customized for each individual isolate. Notably, when all isolates were grown in all media, a lower number of spores produced was observed from M1 (broken rice only) and M2 (containing rice husks and broken rice) compared to other media. The isolates preferred the use of wheat bran or rice bran mixed with sugarcane bagasse for their sporulation. Mishra and Thawani [53] reported that the growth and sporulation of EPF depend on the amount of moistening, the utilization of starch in grains by the action of the enzyme amylase, and other agents for the growth of fungus. High levels of moistening agents could cause clumping of the substrate particles to hinder the optimal utilization of substrate, but less moistening agents cause dried and hard conditions of the grains, thus lowering the growth and sporulation of fungus. A nutrient-poor medium is insufficient for extensive mycelial growth, whereas a nutrient-rich medium may not stimulate spore production.
Sufficient spore production and conidial germination of EPF are crucial components for the successful management of target pests, as they affect the virulence, proliferation, and the shelf-life of EPF [54]. In this study, the use of tested substrates for spore production by P. citrinum was shown to have the potential to facilitate an efficient and low-cost process for industrial application. The abilities of P. citrinum isolates P.03, P.04, P.06, and P.09 to cause high mortality and to sporulate at high rates across all media supports a potential for the development of a virulent mycopesticide for the management of S. litura and Pl. xylosterlla. Long-term control using EPF is one of the most promising strategies to prevent further pest attacks and outbreaks.
5. Conclusions
This study reports the isolation and identification of ten strains of P. citrinum. The isolated P. citrinum were evaluated for their pathogenicity to S. litura and Pl. xylostella. The infection of insects was different for each of the ten P. citrinum strains, and all isolates showed higher infectivity and stronger virulence against Pl. xylostella than S. litura. Suitable agro-industrial-based media were determined for the solid-state fermentation of isolates for sporulation, among which broken rice mixed with wheat bran and sugarcane bagasse were efficient for fermentation with high yields of spores, particularly for sporulation of P.01, P.02, P.04, P.06, P.09, and P.10 isolates. The newly isolated P. citrinum strains exhibit properties that make them potentially useful as biocontrol agents for controlling S. litura and Pl. xylostella.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/2311-5637/9/5/438/s1, Table S1: Composition of medium; Table S2: Location of sample collection and GenBank accession number of isolated Penicillium citrinum; Figure S1: Kaplan-Meier survival curve showing the percent survival of Spodoptera litura (A) and Plutella xylostell (B) exposed to ten isolates; Figure S2: Dead Spodoptera litura (A) Plutella xylostella (B) infected by isolated Penicillium citrinum in the pathogenicity test.
Author Contributions
Conceptualization, H.C.N., K.-H.L. and C.J.B.; methodology, H.C.N., K.-H.L. and C.J.B.; validation, T.P.N., H.S.L. and K.N.N.; formal analysis, H.C.N., T.P.N., D.C.P., T.N.T. and K.-H.L.; investigation, H.C.N., T.P.N., H.S.L. and K.N.N.; resources, H.C.N., C.J.B., K.-H.L. and C.-H.S.; writing—original draft preparation, H.C.N. and K.-H.L.; writing—review and editing, C.J.B.; visualization, H.C.N. and K.-H.L.; supervision, H.C.N. and C.J.B.; project administration, H.C.N.; funding acquisition, H.C.N. and C.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Renuka, S.; Vani, H.C.; Alex, E. Entomopathogenic fungi as a potential management tool for the control of urban malaria vector, Anopheles stephensi (Diptera: Culicidae). J. Fungi 2023, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Jaronski, S.T. Mass production of entomopathogenic fungi—State of the art. In Mass Production of Beneficial Organisms, 2nd ed.; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 317–357. [Google Scholar]
- Shah, P.; Pell, J. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Assadi, B.H.; Chouikhi, S.; Ettaib, R.; M’hamdi, N.B.; Belkadhi, M.S. Effect of the native strain of the predator Nesidiocoris tenuis Reuter and the entomopathogenic fungi Beauveria bassiana and Lecanicillium muscarium against Bemisia tabaci (Genn.) under greenhouse conditions in Tunisia. Egypt. J. Biol. Pest Control. 2021, 31, 47. [Google Scholar] [CrossRef]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research progress on phytopathogenic fungi and their role as biocontrol agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef]
- Deans, C.; Krischik, V. The current state and future potential of microbial control of scarab pests. Appl. Sci. 2023, 13, 766. [Google Scholar] [CrossRef]
- Lian, T.; Qin, C.; Jie, Y.; Xu, J.; Zhao, D.; Qiu, H.; Hua, Y.; Lai, G. Biological characteristics of six strains of entomophytic fungi and their pathogenicity against Curculio chinensis (Coleoptera: Curculionidae). J. Environ. Entomol. 2019, 41, 642–649. [Google Scholar]
- Hoarau, C.; Campbell, H.; Prince, G.; Chandler, D.; Pope, T. Biological control agents against the cabbage stem flea beetle in oilseed rape crops. Biol. Control. 2022, 167, 104844. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Lopes, R.B.; Delalibera, Í., Jr.; Fernandes, É.K.K.; Luz, C.; Faria, M. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebr. Pathol. 2019, 165, 46–53. [Google Scholar] [CrossRef]
- Muñiz-Paredes, F.; Miranda-Hernández, F.; Loera, O. Production of conidia by entomopathogenic fungi: From inoculants to final quality tests. World J. Microbiol. Biotechnol. 2017, 33, 57. [Google Scholar] [CrossRef]
- Sala, A.; Barrena, R.; Artola, A.; Sánchez, A. Current developments in the production of fungal biological control agents by solid-state fermentation using organic solid waste. Crit. Rev. Environ. Sci. Technol. 2019, 49, 655–694. [Google Scholar] [CrossRef]
- Pham, T.A.; Kim, J.J.; Kim, K. Optimization of solid-state fermentation for improved conidia production of Beauveria bassiana as a mycoinsecticide. Mycobiology 2010, 38, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento Silva, J.; Mascarin, G.M.; dos Santos Gomes, I.C.; Tinôco, R.S.; Quintela, E.D.; dos Reis Castilho, L.; Maria Guimaraes Freire, D. New cost-effective bioconversion process of palm kernel cake into bioinsecticides based on Beauveria bassiana and Isaria javanica. Appl. Microbiol. Biotechnol. 2018, 102, 2595–2606. [Google Scholar] [CrossRef]
- Ginni, G.; Kavitha, S.; Kannah, Y.; Bhatia, S.K.; Kumar, A.; Rajkumar, M.; Gopalakrishnan, K.; Arivalagan, P.; Nguyen, T.L.C.; Rajesh, B. Valorization of agricultural residues: Different biorefinery routes. J. Environ. Chem. Eng. 2021, 9, 105435. [Google Scholar]
- Chatterjee, S.; Mohan, S.V. Fungal biorefinery for sustainable resource recovery from waste. Bioresour. Technol. 2022, 345, 126443. [Google Scholar] [CrossRef]
- Shipp, J.; Zhang, Y.; Hunt, D.; Ferguson, G. Influence of humidity and greenhouse microclimate on the efficacy of Beauveria bassiana (Balsamo) for control of greenhouse arthropod pests. Environ. Entomol. 2003, 32, 1154–1163. [Google Scholar] [CrossRef]
- Fitriana, Y.; Suharjo, R.; Swibawa, I.G.; Semenguk, B.; Pasaribu, L.T.; Hartaman, M.; Rwandini, R.A.; Indriyati, I.; Purnomo, P.; Solikhin, S. Aspergillus oryzae and Beauveria bassiana as entomopathogenic fungi of Spodoptera litura Fabricius (Lepidoptera: Noctuidae) infesting corn in Lampung, Indonesia. Egypt. J. Biol. Pest Control 2021, 31, 127. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Tran, T.V.A.; Nguyen, Q.L.; Nguyen, N.N.; Nguyen, M.K.; Nguyen, N.T.T.; Su, C.H.; Lin, K.H. Newly isolated Paecilomyces lilacinus and Paecilomyces javanicus as novel biocontrol agents for Plutella xylostella and Spodoptera litura. Not. Bot. Horti Agrobo. 2017, 45, 280–286. [Google Scholar] [CrossRef]
- Pereira, E.d.S.; Sarquis, M.I.d.M.; Ferreira-Keppler, R.L.; Hamada, N.; Alencar, Y.B. Filamentous fungi associated with mosquito larvae (Diptera: Culicidae) in municipalities of the Brazilian Amazon. Neotrop. Entomol. 2009, 38, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Maketon, M.; Amnuaykanjanasin, A.; Kaysorngup, A. A rapid knockdown effect of Penicillium citrinum for control of the mosquito Culex quinquefasciatus in Thailand. World J. Microbiol. Biotechnol. 2014, 30, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; Gams, W. The taxonomic situation in the hyphomycete genera Penicillium, Aspergillus and Fusarium. Antonie Leeuwenhoek 1984, 50, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; de Vries, R.P.; Samson, R.A. Modern taxonomy of biotechnologically important Aspergillus and Penicillium sp. Adv. Appl. Microbiol. 2014, 86, 199–249. [Google Scholar] [PubMed]
- Altre, J.; Vandenberg, J.; Cantone, F. Pathogenicity of Paecilomyces fumosoroseus isolates to diamondback moth, Plutella xylostella: Correlation with spore size, germination speed, and attachment to cuticle. J. Invertebr. Pathol. 1999, 73, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, H.; Vishal, V.; Lal, S. Studies on the morphology, phylogeny, and bioremediation potential of Penicillium citrinum and Paecilomyces variotii (Eurotiales) from oil-contaminated areas. Arch. Microbiol. 2023, 205, 50. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Debnath, P.; Ghosh, S.K.; Medda, P.K. Biological control of papaya aphid (Aphis gossypii Glover) using entomopathogenic fungi. Vegetos 2020, 33, 1–10. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Hu, Q.; Weng, Q. Entomopathogenic fungi in the soils of China and their bioactivity against striped flea beetles Phyllotreta striolata. Diversity 2022, 14, 464. [Google Scholar] [CrossRef]
- Driver, F.; Milner, R.J.; Trueman, J.W. A taxonomic revision of Metarhizium based on a phylogenetic analysis of rDNA sequence data. Mycol. Res. 2000, 104, 134–150. [Google Scholar] [CrossRef]
- Schoch, C.L.; Robbertse, B.; Robert, V.; Vu, D.; Cardinali, G.; Irinyi, L.; Meyer, W.; Nilsson, R.H.; Hughes, K.; Miller, A.N.; et al. Finding needles in haystacks: Linking scientific names, reference specimens and molecular data for Fungi. Database 2014, 2014, bau061. [Google Scholar] [CrossRef]
- Flandroy, L.; Poutahidis, T.; Berg, G.; Clarke, G.; Dao, M.C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 2018, 627, 1018–1038. [Google Scholar] [CrossRef]
- Thomas, M.B.; Blanford, S.; Lomer, C.J. Reduction of feeding by the variegated grasshopper, Zonocerus variegatus, following infection by the fungal pathogen. Metarhizium flavoviride. Biocontrol Sci. Technol. 1997, 7, 327–334. [Google Scholar] [CrossRef]
- Opisa, S.; Du Plessis, H.; Akutse, K.; Fiaboe, K.; Ekesi, S. Effects of entomopathogenic fungi and Bacillus thuringiensis-based biopesticides on Spoladea recurvalis (Lepidoptera: Crambidae). J. Appl. Entomol. 2018, 142, 617–626. [Google Scholar] [CrossRef]
- Anand, R.; Tiwary, B.N. Pathogenicity of entomopathogenic fungi to eggs and larvae of Spodoptera litura, the common cutworm. Biocontrol Sci. Technol. 2009, 19, 919–929. [Google Scholar] [CrossRef]
- Foo, K.; Sathiya Seelan, J.S.; Dawood, M.M. Microfungi associated with Pteroptyx bearni (Coleoptera: Lampyridae) eggs and larvae from Kawang River, Sabah (northern Borneo). Insects 2017, 8, 66. [Google Scholar] [CrossRef]
- Herlinda, S.; Efendi, R.A.; Suharjo, R.; Hasbi, H.; Setiawan, A.; Elfita, E.; Verawaty, M. New emerging entomopathogenic fungi isolated from soil in South Sumatra (Indonesia) and their filtrate and conidial insecticidal activity againts Spodoptera litura. Biodivers. J. Biol. Divers. 2020, 21, 5102–5113. [Google Scholar]
- Soliman, N.; Al-amin, S.M.; Mesbah, A.E.; Ibrahim, A.M.; Mahmoud, A.M. Pathogenicity of three entomopathogenic fungi against the Mediterranean fruit fly, Ceratitis capitata (Wiedemann)(Diptera: Tephritidae). Egypt. J. Biol. Pest Control 2020, 30, 49. [Google Scholar] [CrossRef]
- Ramanujam, B.; Poornesha, B.; Shylesha, A.N. Effect of entomopathogenic fungi against invasive pest Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) in maize. Egypt. J. Biol. Pest Control 2020, 30, 100. [Google Scholar]
- Idrees, A.; Qadir, Z.A.; Akutse, K.S.; Afzal, A.; Hussain, M.; Islam, W.; Waqas, M.S.; Bamisile, B.S.; Li, J. Effectiveness of entomopathogenic fungi on immature stages and feeding performance of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. Insects 2021, 12, 1044. [Google Scholar] [CrossRef] [PubMed]
- Baksh, A.; Khan, A. Pathogenicity of Paecilomyces tenuipes to diamond back moth, Plutella xylostella at three temperatures in Trinidad. Int. J. Agric. Biol. 2012, 14, 261–265. [Google Scholar]
- Han, J.H.; Jin, B.R.; Kim, J.J.; Lee, S.Y. Virulence of entomopathogenic fungi Metarhizium anisopliae and Paecilomyces fumosoroseus for the microbial control of Spodoptera exigua. Mycobiology 2014, 42, 385–390. [Google Scholar] [CrossRef]
- Dhawan, M.; Joshi, N. Enzymatic comparison and mortality of Beauveria bassiana against cabbage caterpillar Pieris brassicae LINN. Brazilian J. Microbiol. 2017, 48, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Mohamed, H.; El-Naggar, S.; Swelim, M.; Elkhawaga, O. Isolation and selection of entomopathogenic fungi as biocontrol agent against the greater wax moth, Galleria mellonella L.(Lepidoptera: Pyralidae). Egypt. J. Biol. Pest Control. 2016, 26, 249. [Google Scholar]
- Mannino, M.C.; Huarte-Bonnet, C.; Davyt-Colo, B.; Pedrini, N. Is the insect cuticle the only entry gate for fungal infection? Insights into alternative modes of action of entomopathogenic fungi. J. Fungi 2019, 5, 33. [Google Scholar] [CrossRef]
- Pedrini, N. Molecular interactions between entomopathogenic fungi (Hypocreales) and their insect host: Perspectives from stressful cuticle and hemolymph battlefields and the potential of dual RNA sequencing for future studies. Fungal Biol. 2018, 122, 538–545. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Ligoxygakis, P.; Xia, Y.X. HYD3, a conidial hydrophobin of the fungal entomopathogen Metarhizium acridum induces the immunity of its specialist host locust. Int. J. Biol. Macromol. 2020, 165, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Zibaee, A.; Khodaparast, S.A.; Fazeli-Dinan, M. Screening and virulence of the entomopathogenic fungi associated with Chilo suppressalis walker. J. Fungi 2021, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Rizal, L.M.; Furlong, M.J.; Walter, G.H. Responses of diamondback moth to diverse entomopathogenic fungi collected from non-agricultural habitats–Effects of dose, temperature and starvation. Fungal Biol. 2022, 126, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Wolski, E.; Rigo, E.; Di Luccio, M.; Oliveira, J.; De Oliveira, D.; Treichel, H. Production and partial characterization of lipases from a newly isolated Penicillium sp. using experimental design. Lett. Appl. Microbiol. 2009, 49, 60–66. [Google Scholar] [CrossRef]
- Ballardo, C.; Abraham, J.; Barrena, R.; Artola, A.; Gea, T.; Sánchez, A. Valorization of soy waste through SSF for the production of compost enriched with Bacillus thuringiensis with biopesticide properties. J. Environ. Manag. 2016, 169, 126–131. [Google Scholar] [CrossRef]
- Awan, U.A.; Meng, L.; Xia, S.; Raza, M.F.; Zhang, Z.; Zhang, H. Isolation, fermentation, and formulation of entomopathogenic fungi virulent against adults of Diaphorina citri. Pest Manag. Sci. 2021, 77, 4040–4053. [Google Scholar] [CrossRef]
- Mar, T.T.; Suwannarach, N.; Lumyong, S. Isolation of entomopathogenic fungi from Northern Thailand and their production in cereal grains. World J. Microbiol. Biotechnol. 2012, 28, 3281–3291. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.P.; Thawani, V. Mass Production of Paecilomyces Fumosoroseus from agricultural products and waste material. Paripex Indian J. Res. 2016, 5, 271–273. [Google Scholar]
- Sain, S.K.; Monga, D.; Hiremani, N.S.; Nagrale, D.T.; Kranthi, S.; Kumar, R.; Kranthi, K.R.; Tuteja, O.P.; Waghmare, V.N. Evaluation of bioefficacy potential of entomopathogenic fungi against the whitefly (Bemisia tabaci Genn.) on cotton under polyhouse and field conditions. J. Invertebr. Pathol. 2021, 183, 107618. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).