The Effect of Refrigerated Storage on Anti-Diabetic and Antioxidant Potency of Probiotic Yogurt Treated with Some Medicinal Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Herbal Water Extract
2.2. Preparation of Starter Culture
2.3. Preparation of Yogurt
2.4. Preparation of Water-Soluble Extract from Herbal Yogurt
2.5. O-Phthaldialdehyde (OPA) Assay
2.6. Total Phenolic Content (TPC) Assay
2.7. Antioxidant Activity by 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical-Scavenging Activity Assay
2.8. Alpha-Amylase Inhibition Assay
2.9. Alpha-Glucosidase Inhibition Assay
2.10. Statistical Analysis
3. Results and Discussion
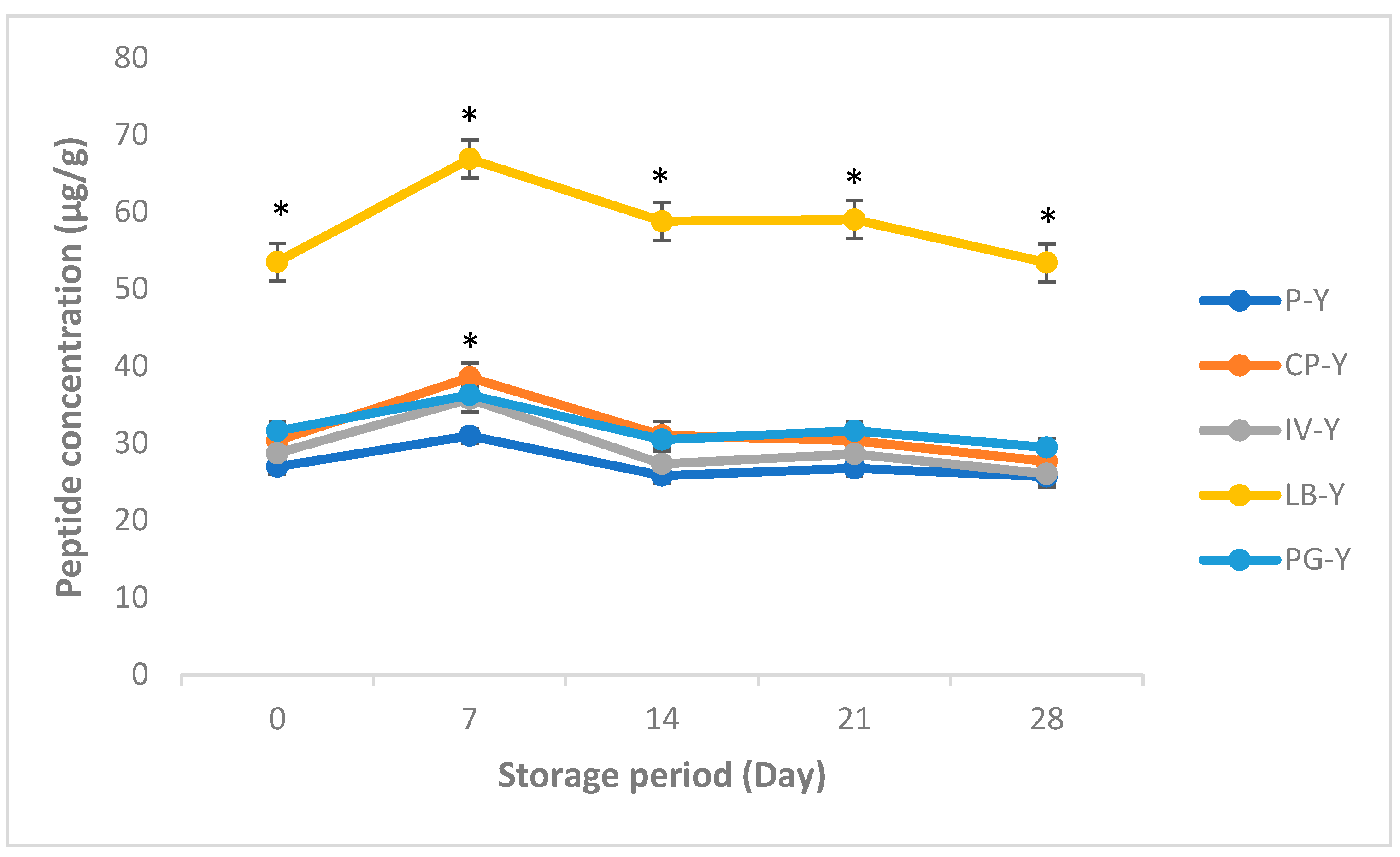
3.1. Peptides Concentration in Yogurt
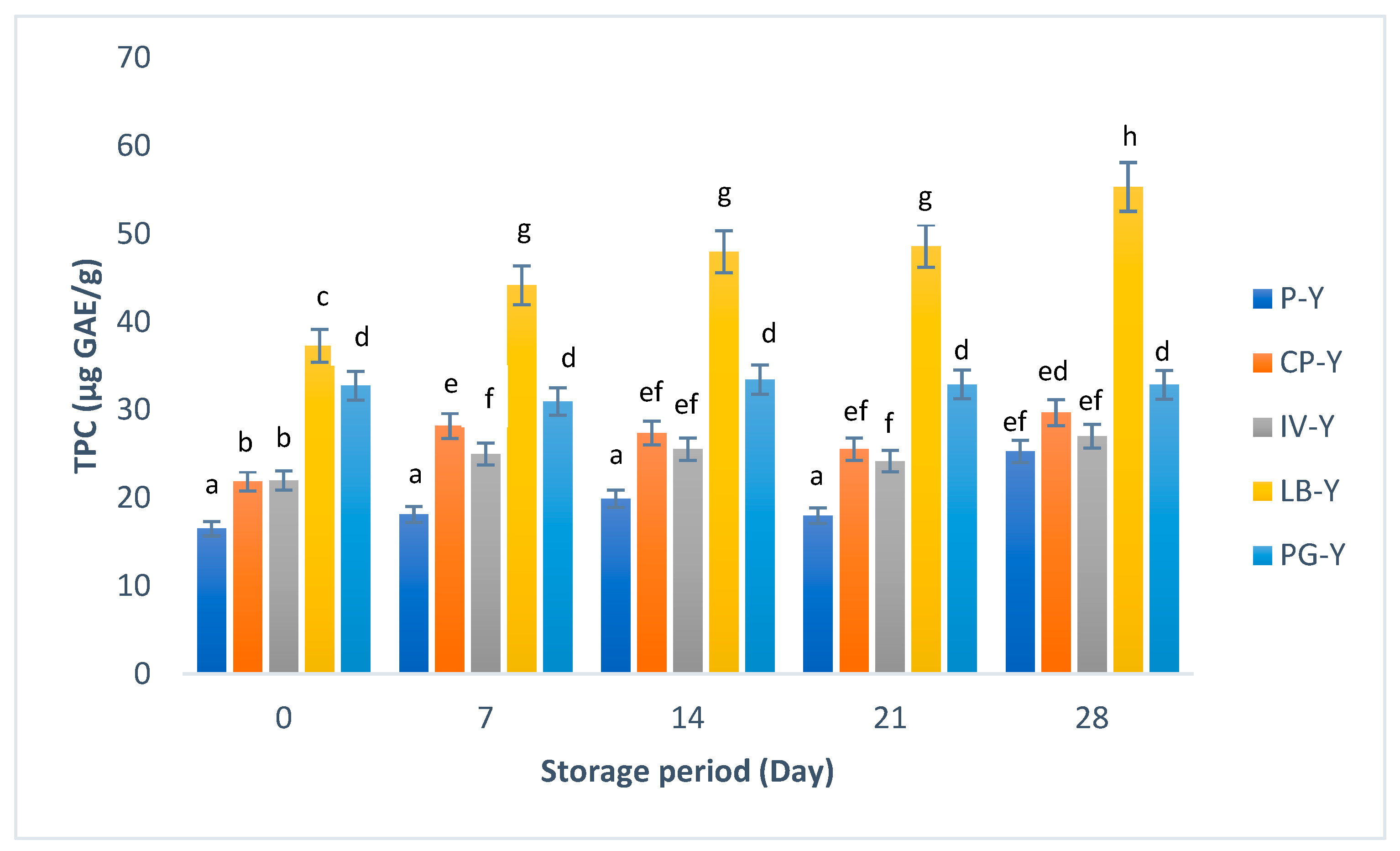
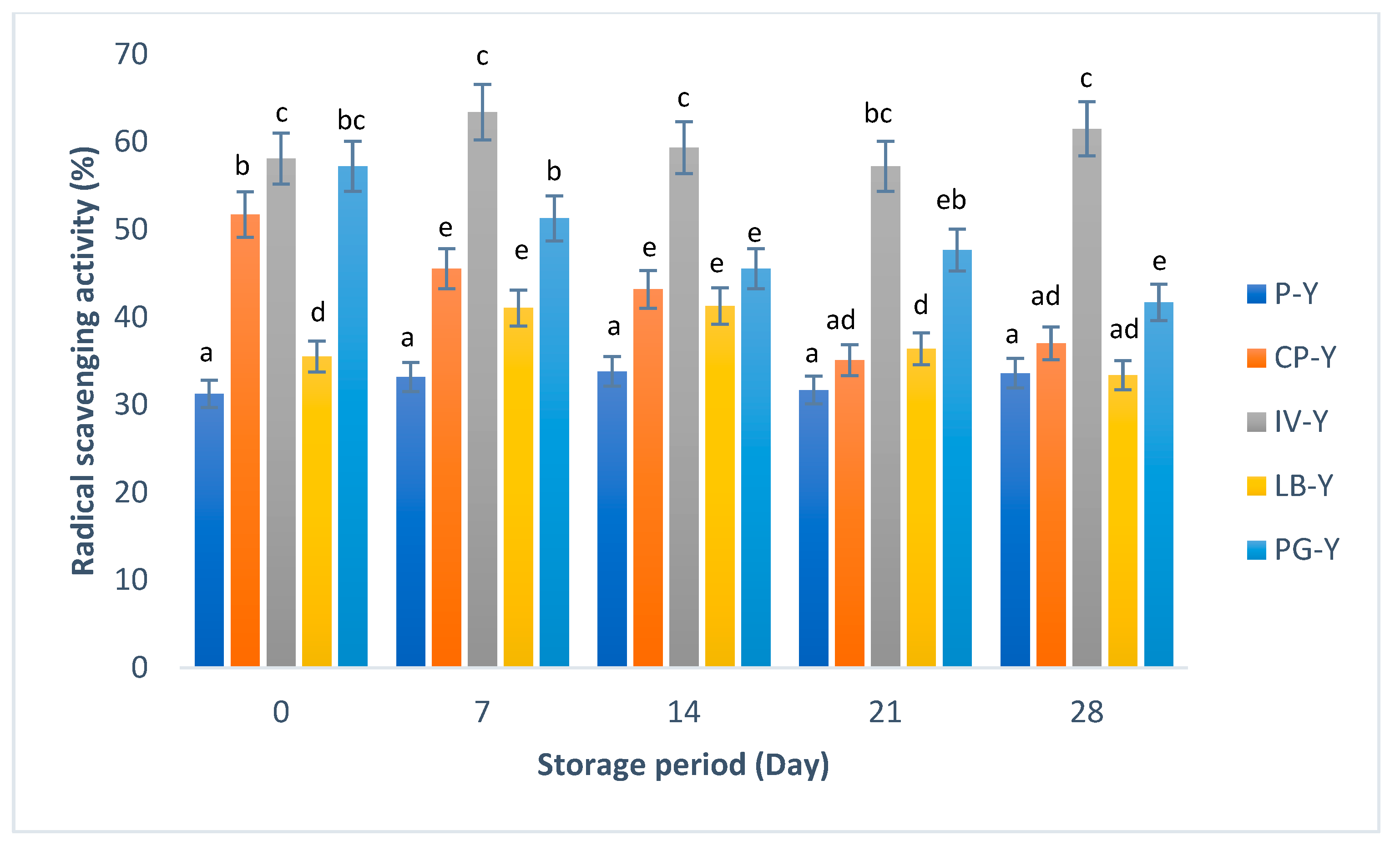
3.2. Total Phenolic Content (TPC) and Radical Scavenging Activity of Yogurt
3.3. α-Amylase and α-Glucosidase Inhibitory Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lotfy, M.; El-Wahab, A.; Hadad Hemeda, M.; Ezzat Abd El-Aziz Ali, A.; Metwally Bauomy, I.; Gamil Hammed Shola, M. Prevalence of pre diabetes and diabetes mellitus among Al-Azhar University male students Hostel in Cairo Egypt. Al-Azhar Med. J. 2020, 49, 931–938. [Google Scholar] [CrossRef]
- Moosaie, F.; Ghaemi, F.; Mechanick, J.I.; Shadnoush, M.; Firouzabadi, F.D.; Kermanchi, J.; Poopak, A.; Esteghamati, S.; Forouzanfar, R.; Abhari, S.M.F.; et al. Obesity and Diabetic Complications: A Study from the Nationwide Diabetes Report of the National Program for Prevention and Control of Diabetes (NPPCD-2021) Implications for Action on Multiple Scales. Prim. Care Diabetes 2022, 16, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B. Proteolytic activity, antioxidant, and α-Amylase inhibitory activity of yogurt enriched with coriander and cumin seeds. LWT 2020, 133, 109912. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-derived non-phenolic α-amylase and α-glucosidase inhibitors for controlling starch digestion rate and guiding diabetes-friendly recipes. LWT 2022, 153, 112455. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Antioxidant activity and inhibition of key enzymes linked to type-2diabetes and hypertension by Azadirachta indica-yogurt. J. Saudi Chem. Soc. 2013, 17, 295–301. [Google Scholar] [CrossRef]
- Shori, A.B. Screening of antidiabetic and antioxidant activities of medicinal plants. J. Integr. Med. 2015, 13, 297–305. [Google Scholar] [CrossRef]
- Bakr, A. Changes of hemoglobin content and glucose levels in the blood of Rattus norvegicus by water extracts of Azadirachta indica. Chin. J. Nat. Med. 2012, 10, 135–137. [Google Scholar]
- Lobo-Rojas, Á.; Quintero-Troconis, E.; Rondón-Mercado, R.; Pérez-Aguilar, M.C.; Concepción, J.L.; Cáceres, A.J. Consumption of Galactose by Trypanosoma cruzi Epimastigotes Generates Resistance against Oxidative Stress. Pathogens 2022, 11, 1174. [Google Scholar] [CrossRef]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag. Shelf Life 2016, 8, 1–6. [Google Scholar] [CrossRef]
- Shori, A.B. Camel milk and its fermented products as a source of potential probiotic strains and novel food cultures: A mini review. PharmaNutrition 2017, 5, 84–88. [Google Scholar] [CrossRef]
- Nyanzi, R.; Jooste, P.J.; Buys, E.M. Invited review: Probiotic yogurt quality criteria, regulatory framework, clinical evidence, and analytical aspects. J. Dairy Sci. 2021, 104, 1–19. [Google Scholar] [CrossRef]
- Ntuli, V.; Sibanda, T.; Elegbeleye, J.A.; Mugadza, D.T.; Seifu, E.; Buys, E.M. Dairy production: Microbial safety of raw milk and processed milk products. In Present Knowledge in Food Safety; Academic Press: Cambridge, MA, USA, 2023; pp. 439–454. [Google Scholar]
- Shori, A.B. Storage quality and antioxidant properties of yogurt fortified with polyphenol extract from nutmeg, black pepper, and white pepper. Electron. J. Biotechnol. 2022, 57, 24–30. [Google Scholar] [CrossRef]
- Shori, A.B.; Yong, Y.S.; Baba, A.S. Effects of medicinal plants extract enriched cheese with fish collagen on proteolysis and in vitro angiotensin-I converting enzyme inhibitory activity. LWT 2022, 159, 113218. [Google Scholar] [CrossRef]
- Shori, A.B.; Peng, C.W.; Bagheri, E.; Baba, A.S. Physicochemical analysis, proteolysis activity and exopolysaccharides production of herbal yogurt fortified with plant extracts. Int. J. Food Eng. 2021, 17, 227–236. [Google Scholar] [CrossRef]
- Shori, A.B. Inclusion of phenolic compounds from different medicinal plants to increase α-amylase inhibition activity and antioxidants in yogurt. J. Taibah Univ. Sci. 2020, 14, 1000–1008. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Dunshea, F.R.; Ajlouni, S. In vitro bioaccessibility of phenolic compounds and alpha-glucosidase inhibition activity in yoghurts enriched with mango peel powder. Food Biosci. 2022, 50, 102011. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Roux, E.; Perrin, C.; Miclo, L.; Dary-Mourot, A. Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Food Res. Int. 2014, 63, 71–80. [Google Scholar] [CrossRef]
- Shori, A.B. Application of Bifidobacterium spp in beverages and dairy food products: An overview of survival during refrigerated storage. Food Sci. Technol. 2022, 42, e41520. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; Kong, D.; Xu, P. Production and functionality of food-derived bioactive peptides: A review. Mini Rev. Med. Chem. 2018, 18, 1524–1535. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Fermented milk derives bioactive peptides with antihypertensive effects. Integr. Food Nutr. Metab. 2015, 2, 180–183. [Google Scholar]
- Shori, A.B.; Hong, Y.C.; Baba, A.S. Proteolytic profile, angiotensin-I converting enzyme inhibitory activity and sensory evaluation of Codonopsis pilosula and fish collagen cheese. Food Res. Int. 2021, 143, 110238. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.S.; Najarian, A.; Shori, A.B.; Lit, K.W.; Keng, G.A. In vitro inhibition of key enzymes related to diabetes and hypertension in Lycium barbarum yogurt. Arab. J. Sci. Eng. 2014, 39, 5355–5362. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Comparative antioxidant activity, proteolysis and in vitro α-amylase and α-glucosidase inhibition of Allium sativum-yogurts made from cow and camel milk. J. Saudi Chem. Soc. 2014, 18, 456–463. [Google Scholar] [CrossRef]
- Shori, A.B.; Baba, A.S. Cinnamomum verum improved the functional properties of bioyogurts made from camel and cow milks. J. Saudi Soc. Agric. Sci. 2011, 10, 101–107. [Google Scholar] [CrossRef]
- Shori, A.B.; Rashid, F.; Baba, A.S. Effect of the addition of phytomix-3+ mangosteen on antioxidant activity, viability of lactic acid bacteria, type 2 diabetes key-enzymes, and sensory evaluation of yogurt. LWT 2018, 94, 33–39. [Google Scholar] [CrossRef]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Shetty, P.H. Bacterial exopolysaccharides for improvement of technological, functional and rheological properties of yoghurt. Int. J. Biol. Macromol. 2021, 183, 1585–1595. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; pp. 517–532. [Google Scholar]
- Mustafa, R.A.; Hamid, A.A.; Mohamed, S.; Bakar, F.A. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J. Food Sci. 2010, 75, C28–C35. [Google Scholar] [CrossRef]
- Shori, A.B.; Albalawi, A.; Al Zahrani, A.J.; Al-sulbi, O.S.; Baba, A.S. Microbial analysis, antioxidant activity, and sensory properties of yoghurt with different starter cultures during storage. Int. Dairy J. 2022, 126, 105267. [Google Scholar] [CrossRef]
- Admassu, S.; Kebede, M. Application of antioxidants in food processing industry: Options to improve the extraction yields and market value of natural products. Adv. Food Technol. Nutr. Sci. 2019, 5, 38–49. [Google Scholar]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical properties, fatty-acid composition, and antioxidant activity of goji berry (Lycium barbarum L. and Lycium chinense Mill.) fruits. Antioxidants 2019, 8, 60. [Google Scholar] [CrossRef]
- Shori, A.B.; Ming, K.S.; Baba, A.S. The effects of Lycium barbarum water extract and fish collagen on milk proteolysis and in vitro angiotensin I-converting enzyme inhibitory activity of yogurt. Biotechnol. Appl. Biochem. 2021, 68, 221–229. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Panagiotakos, D.B.; Pitsavos, C.; Chrysochoou, C.; Detopoulou, P.; Skoumas, J.; Stefanadis, C. Dietary antioxidant capacity is inversely associated with diabetes biomarkers: The ATTICA study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 561–567. [Google Scholar] [CrossRef]
- Fialova, S.; Rendekova, K.; Mucaji, P.; Slobodnikova, L. Plant natural agents: Polyphenols, alkaloids and essential oils as perspective solution of microbial resistance. Curr. Org. Chem. 2017, 21, 1875–1884. [Google Scholar] [CrossRef]
- Padmashree, A.; Roopa, N.; Semwal, A.D.; Sharma, G.K.; Agathian, G.; Bawa, A.S. Star-anise (Illicium verum) and black caraway (Carum nigrum) as natural antioxidants. Food Chem. 2007, 104, 59–66. [Google Scholar] [CrossRef]
- Östman, E.M.; Liljeberg Elmståhl, H.G.; Björck, I.M. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am. J. Clin. Nutr. 2001, 74, 96–100. [Google Scholar] [CrossRef]
- Byambasuren, S.E.; Wang, J.; Gaudel, G. Medicinal value of wolfberry (Lycium barbarum L.). J. Med. Plants Stud. 2019, 7, 90–97. [Google Scholar]
- Liu, J.; Li, Y.; Pu, Q.; Qiu, H.; Di, D.; Cao, Y. A polysaccharide from Lycium barbarum L.: Structure and protective effects against oxidative stress and high-glucose-induced apoptosis in ARPE-19 cells. Int. J. Biol. Macromol. 2022, 201, 111–120. [Google Scholar] [CrossRef]
- Huizhen, C. Hyperglycemic Effect of Goji (Lycium barbarum) Polysaccharides. In Phytochemicals in Goji Berries; CRC Press: Boca Raton, FL, USA, 2020; pp. 79–92. [Google Scholar]
- Zhao, H.; Alexeev, A.; Chang, E.; Greenburg, G.; Bojanowski, K. Lycium barbarum glycoconjugates: Effect on human skin and cultured dermal fibroblasts. Phytomedicine 2005, 12, 131–137. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and anti-diabetic activities of polysaccharides from guava leaves. Molecules 2019, 24, 1343. [Google Scholar] [CrossRef]
- Shabbir, H.; Kausar, T.; Noreen, S.; Rehman, H.U.; Hussain, A.; Huang, Q.; Gani, A.; Su, S.; Nawaz, A. In vivo screening and antidiabetic potential of polyphenol extracts from guava pulp, seeds and leaves. Animals 2020, 10, 1714. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Lin, Y.C.; Yen, G.C.; Chen, H.Y. Preventive effects of guava (Psidium guajava L.) leaves and its active compounds against α-dicarbonyl compounds-induced blood coagulation. Food Chem. 2007, 103, 528–535. [Google Scholar] [CrossRef]
| Yogurt Samples | α-Amylase Inhibitory Activity (%) | |||||
|---|---|---|---|---|---|---|
| Storage Periods | 0 Day | 7 Day | 14 Day | 21 Day | 28 Day | |
| P-Y | 25.54 ± 1.28 | 46.53 ± 2.27 | 44.64 ± 2.96 | 24.92 ± 1.92 | 24.16 ± 1.74 | |
| CP-Y | 34.36 ± 2.20 * | 56.05 ± 2.19 * | 48.83 ± 2.18 | 46.58 ± 2.94 * | 26.4 ± 2.17 | |
| IV-Y | 31.55 ± 1.01 * | 51.21 ± 1.28 | 50.65 ± 1.32 * | 34.76 ± 1.13 * | 27.06 ± 1.58 | |
| LB-Y | 34.52 ± 2.19 * | 54.64 ± 2.52 * | 58.94 ± 2.30 * | 32.54 ± 1.85 * | 27.47 ± 0.92 * | |
| PG-Y | 35.28 ± 1.72 * | 55.88 ± 2.57 * | 58.09 ± 2.67 * | 35.84 ± 1.42 * | 29.87 ± 1.45 * | |
| IC50 (mg/g) | ||||||
| P-Y | 4.77 ± 1.98 | 1.46 ± 1.79 | 1.38 ± 1.84 | 4.04 ± 1.82 | 3.67 ± 1.28 | |
| CP-Y | 2.24 ± 1.72 * | 0.72 ± 2.61 * | 0.74 ± 1.24 * | 1.88 ± 1.77 * | 2.54 ± 2.08 * | |
| IV-Y | 1.95 ± 2.63 * | 0.72 ± 2.92 * | 0.86 ± 2.07 * | 1.74 ± 1.47 * | 2.39 ± 1.68 * | |
| LB-Y | 2.25 ± 0.51 * | 0.73 ± 1.72 * | 0.93 ± 2.27 * | 1.84 ± 1.45 * | 2.43 ± 1.57 * | |
| PG-Y | 2.19 ± 2.09 * | 0.79 ± 1.49 * | 0.75 ± 1.11 * | 1.69 ± 1.18 * | 2.54 ± 1.23 * | |
| Yogurt Samples | α-Glucosidase Inhibitory Activity (%) | |||||
|---|---|---|---|---|---|---|
| Storage Periods | 0 Day | 7 Day | 14 Day | 21 Day | 28 Day | |
| P-Y | 2.66 ± 0.23 | 6.27 ± 0.59 | 10.26 ± 1.11 | 3.62 ± 1.25 | 3.35 ± 1.74 | |
| CP-Y | 9.76 ± 1.40 * | 18.2 ± 2.61 * | 20.45 ± 1.94 * | 13.07 ± 1.95 * | 12.24 ± 1.66 * | |
| IV-Y | 7.73 ± 1.47 * | 11.99 ± 2.76 * | 15.09 ± 1.90 * | 7.79 ± 1.13 * | 6.71 ± 1.86 * | |
| LB-Y | 12.28 ± 0.43 * | 17.37 ± 2.02 * | 19.63 ± 1.24 * | 11.43 ± 2.42 * | 10.27 ± 0.93 * | |
| PG-Y | 9.71 ± 0.16 * | 14.81 ± 2.99 * | 16.98 ± 1.22 * | 10.45 ± 2.44 * | 9.44 ± 0.82 * | |
| IC50 (mg/g) | ||||||
| P-Y | 9.09 ± 2.67 | 3.57 ± 1.66 | 2.04 ± 1.28 | 6.39 ± 1.34 | 7.36 ± 1.61 | |
| CP-Y | 2.41 ± 0.58 * | 1.28 ± 1.58 * | 1.14 ± 1.90 * | 1.95 ± 0.54 * | 2.09 ± 1.15 * | |
| IV-Y | 3.47 ± 1.31 * | 2.19 ± 1.69 * | 1.48 ± 1.91 * | 3.17 ± 1.11 * | 3.67 ± 1.64 * | |
| LB-Y | 2.05 ± 1.67 * | 1.48 ± 1.04 * | 1.20 ± 2.27 * | 2.35 ± 2.31 * | 2.64 ± 1.02 * | |
| PG-Y | 2.39 ± 1.52 * | 1.76 ± 0.93 * | 1.37 ± 1.98 * | 2.22 ± 2.18 * | 2.81 ± 1.14 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shori, A.B.; Baba, A.S. The Effect of Refrigerated Storage on Anti-Diabetic and Antioxidant Potency of Probiotic Yogurt Treated with Some Medicinal Plants. Fermentation 2023, 9, 427. https://doi.org/10.3390/fermentation9050427
Shori AB, Baba AS. The Effect of Refrigerated Storage on Anti-Diabetic and Antioxidant Potency of Probiotic Yogurt Treated with Some Medicinal Plants. Fermentation. 2023; 9(5):427. https://doi.org/10.3390/fermentation9050427
Chicago/Turabian StyleShori, Amal Bakr, and Ahmad Salihin Baba. 2023. "The Effect of Refrigerated Storage on Anti-Diabetic and Antioxidant Potency of Probiotic Yogurt Treated with Some Medicinal Plants" Fermentation 9, no. 5: 427. https://doi.org/10.3390/fermentation9050427
APA StyleShori, A. B., & Baba, A. S. (2023). The Effect of Refrigerated Storage on Anti-Diabetic and Antioxidant Potency of Probiotic Yogurt Treated with Some Medicinal Plants. Fermentation, 9(5), 427. https://doi.org/10.3390/fermentation9050427






