1. Introduction
Livestock production, the main economic engine of the Azores, is currently facing multiple challenges at a global level, including the need to respond to human population growth and food security, but also environmental pollution and accelerating climate change [
1].
Considering the context of the limited agricultural area of the archipelago of the Azores, obtaining a sustainable productive increase, capable of responding to an increasingly demanding and globalized market, is a challenge. In this context, the answer must necessarily come from the optimization of available resources and the valorization of the specific potential of the territory [
2].
As nutrition is one of the factors that has the greatest impact on animal production and can represent over 50% of production costs, meeting the growing competitive challenges must necessarily involve improving the quality, quantity, and availability of the used fodder [
3].
In the Azores, ruminant production depends heavily on pastures as a food source. During periods of greater food scarcity, forage is preserved as silage, prepared from pasture surplus produced in the spring [
4]. It is, thus, a system highly dependent on climate and very susceptible to climate change [
5]. Consequently, the need to research non-conventional animal feed sources has been increasing, not only in the Azores but also in the rest of the world [
6]. Taking advantage of invasive plants in this context is particularly beneficial, as it can not only result in a competitive advantage from an economic perspective, which is an increasingly important aspect in a globalized market, but also make livestock production more eco-sustainable [
7].
Invasive plants are indeed a problem that, to a greater or lesser extent, affects the entire region of the Azores, constituting a threat to the endemic flora and fauna of the region. From a resource optimization perspective, making use of them as a non-conventional animal feed source makes particular sense, as it not only constitutes a complementary means of combating their spread, as suggested by [
4], but also provides an alternative coarse feed, given that they are rich in fiber, and because they typically present higher levels of protein and minerals than the straw fed to ruminants [
8]. Furthermore, their use can contribute to meeting climate change targets [
9].
This study aims to assess the viability from a nutritional standpoint of several invasive plants as non-conventional animal feed sources: Pennisetum setaceum, Ricinus communis, Arundo donax, Acacia melanoxylon, Opuntia ficus-indica, Agave americana, Pittosporum undulatum, and Hedychium gardnerianum.
3. Results
Table 1 presents the results obtained for the chemical composition and the dry and organic matter digestibility (DMD and OMD). The lowest dry matter (DM) value was observed for
Opuntia ficus-indica (6.65%), while the highest was found for
Acacia melanoxylon (39.43%).
Ricinus communis was observed to have the highest crude protein (CP) value (24.51% DM), while
Opuntia ficus-indica had the lowest recorded value (4.94% DM). The high level of ash present in
Opuntia ficus-indica (17.66% DM) was also noteworthy, considering the chemical parameters tested in this study.
Regarding the study of dry matter digestibility, it was noted that the results varied between 27.32% for Acacia melanoxylon and 84.41% for Opuntia ficus-indica. The same was observed in the determination of organic matter digestibility (OMD), with values varying between 23.50% for Acacia melanoxylon and 77.14 for Ricinus communis.
Most plants showed digestibility values greater than 50%; however, Acacia melanoxylon, Pittosporum undulatum, and Hedychium gardnerianum obtained lower percentages.
As summarized in
Table 2, the maximum value found for NDF was for
Pennisetum setaceum (80.39% DM), and it is also this plant that contains the maximum value found in this study for HEM (39.03% DM) and the maximum value of CEL (36.81% DM). Conversely, the minimum value found for NDF was for
Opuntia ficus-indica (21.23% DM), which was also the plant to contain the minimum value found in this study for ADF (14.31% DM), while the maximum value for ADF was observed with
Acacia melanoxylon (64.01% DM).
The ADL values found ranged from 3.76% DM for Arundo donax to 17.42 for Pittosporum undulatum, this plant being the one that also contained the minimum value of HEM (3.60% DM) found in this study.
The values for estimated dry matter intake (DMI), digestible dry matter (DDM), and relative feed value (RFV) are shown in
Table 3, with the highest DMI value (5.75%) found for
Opuntia ficus-indica being very similar to that found for
Ricinus communis (4.82%) and
Agave americana (4.24%). The minimum value was 1.49 for
Pennisetum setaceum, which is comparable to
Hedychium gardnerianum,
Arundo donax, and
Acacia melanoxylon.
The maximum estimated for DDM was 77.75% for Opuntia ficus-indica, similar to the value estimated for Ricinus communis (73.27%) and Agave americana L. (70.30%). In contrast, the minimum estimated value was 48.22 for Acacia melanoxylon. The highest RFV value found in this study was 346.79 for Opuntia ficus-indica, followed by Ricinus communis (273.86) and Agave americana L. (231.33).
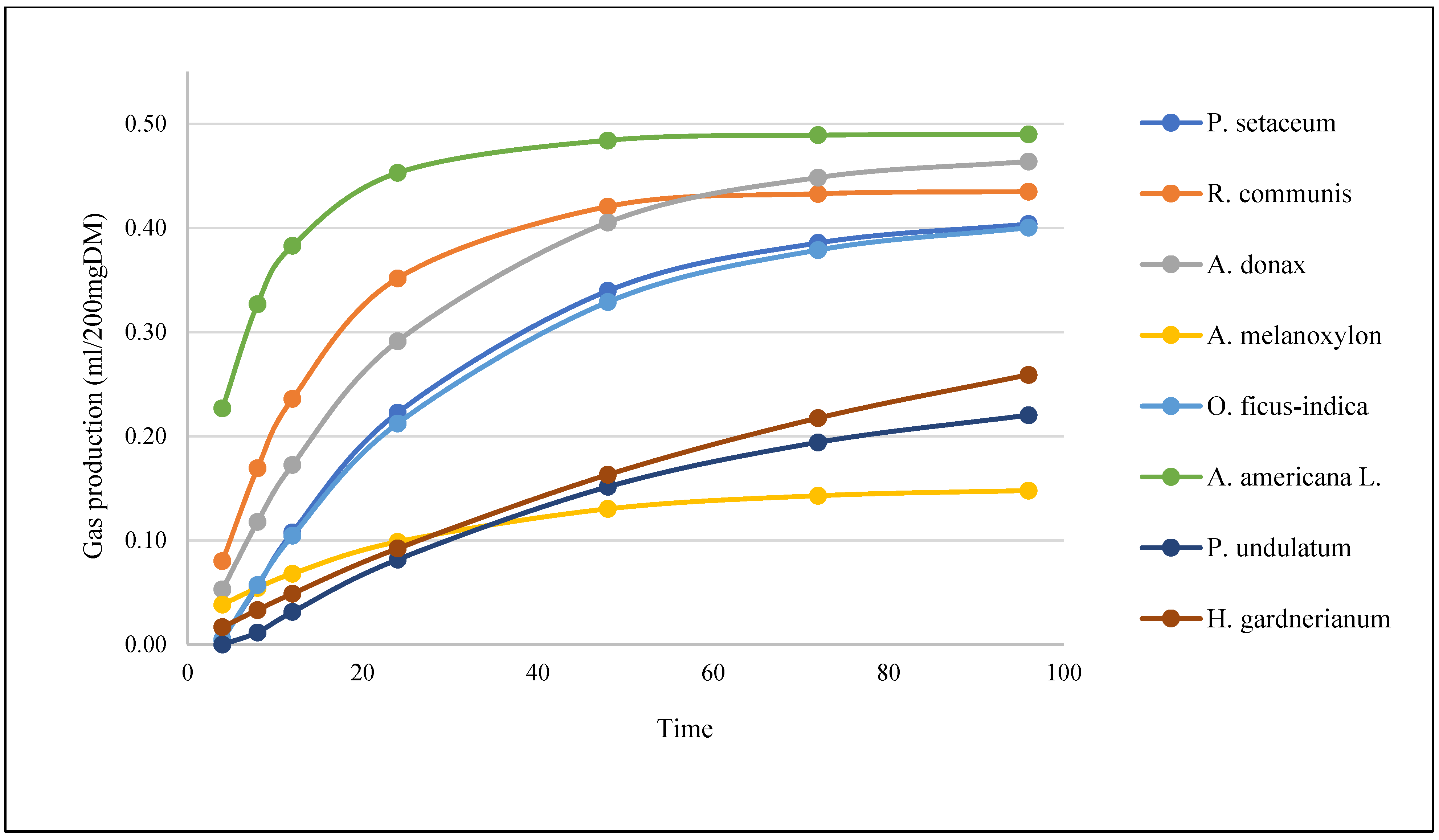
The in vitro gas production recorded over the tested incubation hours (4, 8, 12, 24, 48, 72, and 96 h) varied considerably (
p < 0.05). As shown in
Table 4,
Agave americana L. produced the highest amount of gas from the start of incubation to the end (22.68 to 48.99 mL/200 mgDM), contrasting with
Acacia melanoxylon, which produced the least gas (3.83 to 14.78 mL/200 mgDM).
Figure 2 shows the cumulative patterns of in vitro gas production of the different plants over the hours of incubation.
The in vitro gas production kinetics parameters are shown in
Table 5. All of
Acacia melanoxylon,
Agave americana L., and
Hedychium gardnerianum showed lag times of 0 h. This means that the fermentation started right as the element was incubated. The
a constant of the reaction kinetics varied between a value of −7.56 mL/200 mgDM pa for
Pennisetum setaceum and 1.96 mL/200 mgDM for
Acacia melanoxylon. Arundo donax produced the highest insoluble fraction,
b (49.65 mL), at the degradation rate
c (0.0389 mL/h), which is similar to the value obtained for
Penisetum setaceum, which produced
b (49.10 mL) at a rate of 0.0419 mL/h. In contrast,
Acacia melanoxylon generated
b (13.16 mL) at a degradation rate
c of 0.0382 mL/h.
Table 6 shows the values of the metabolizable energy and net energy lactation of the different plants. The highest estimates of ME (8.72 MJ/kgDM) and NEL (5.06 MJ/kgDM) were obtained for
Agave Americana, while
Ricinus communis recorded the second highest estimates of ME (8.40 MJ/kgDM), and NEL (5.08 MJ/kgDM). On the other hand,
Pittosporum undulatum and
Hedychium gardnerianum had similar ME (3.81 vs. 3.99 MJ/kgDM) and NEL (1.60 vs. 1.57 MJ/kgDM) values, which were the lowest estimated values.
It is possible to observe in
Table 7 the existing correlations between the chemical compositions and the feed quality indices of the studied plants. In general, the results showed strong negative correlations between ADF and DMD, OMD, and DMI (r > 0.86,
p < 0.01). There was also a very strong negative relationship between ADF and RFV (r = 0.91,
p < 0.01), and a perfect negative correlation between ADF and DDM (r = 1.00,
p < 0.01). We can also highlight a very strong relationship between NDF, DMI, and RFV, showing a value of r > 0.92 (
p < 0.01).
Table 8 shows us the existing association between chemical composition and gas production. We observe that there are not very strong correlations in this association; the strongest positive links found were between digestibility (dry matter and organic matter) and gas production at 24, 48, 72, and 96 h, with the r ranging between 0.791 and 0.899 with a
p < 0.01.
Table 9 shows the relationships between chemical compositions and in vitro degradation kinetics (b, c, ME, and NEL). Only the ADL content is strongly negatively correlated with
c (r = 0.93,
p = 0.01), with all other correlations being moderate, weak, or very weak.
4. Discussion
Agriculture is an activity that is highly dependent on climatic factors such as temperature, rainfall, soil moisture, and solar radiation. Climate change can affect agricultural production due to the increase in temperature and the reduction of precipitation, resulting in longer periods of drought. The demand for using plants found in the natural flora as unconventional fodder for animal production has increased, not only in areas of the globe where the drought is increasingly severe and where fodder is scarce but also in developed countries where this type of food can make the activity more economically and environmentally sustainable [
21]. Investigating how to best use the plants of a region is an important step towards finding a balance between the highest nutritional value and the lowest emission of polluting agents [
22]. There have been several studies carried out with the aim of deepening the understanding of non-conventional forages [
4], namely their interference with reproduction [
23], their nutritional value, and their potential in combating the emission of greenhouse gases [
19,
24].
As animal feed is one of the aspects that most influence production [
3], it is important to understand and know the specific characteristics of forages regarding their nutrients and digestibility [
21,
25], which allow supporting the design of a diet that meets the nutritional needs of animals [
26,
27].
Forages can have their chemical composition influenced by several factors, which include their genotype, maturity stage, and harvest time [
28].
A routine system of chemical characterization of forages should include the determination of fiber, crude protein, and crude ash, among others. For this to be possible, the accurate determination of dry matter, which represents the percentage of plant tissue that remains after drying, is fundamental [
5].
Opuntia ficus-indica (6.65%) and
Agave americana (11.15%) showed the lowest dry matter values among the plants being studied. This can be justified by the fact that these are the only CAM plants (crassulacean acid metabolism) considered in this study. These species usually have a high-water retention capacity and, compared to other species, can produce up to 5 times more DM per millimeter of rainfall [
29], making them a viable complementary source of water for ruminants.
In addition,
Hedychium gardnerianum obtained a low value of dry matter (11.89%), similar to that found by [
5], and is also considered a good source of water, especially during the dry season.
The highest value of dry matter found was 39.43% (
Acacia melanoxylon), similar to that referenced by [
5]. According to [
30], Acacia is distributed in most parts of the world, occupying vast areas of land due to its phenotypic plasticity, which allows it to adapt and establish itself successfully in changing environments.
The lowest crude protein content found in these plants was for
Opuntia ficus-indica (4.94% DM) and
Agave americana (5.69% DM), which is below the threshold of 7%, typically seen as the required minimum value for normal ruminal microorganism function [
31,
32]. Regarding
Opuntia, it should be noted that [
33] reports variable amounts of CP across several plant varieties and that some clones from Brazil could exceed 11% DM of CP. In the case of
Agave americana L. [
7], crude protein values were also similar to those found in this study, ranging between 5.16% DM and 6.30% DM.
Moreover, for
Pittosporum undulatum and
Hedychium gardnerianum, low crude protein values were found, 7.84% DM and 8.78% DM respectively, which are similar to the values found in a study by [
19], although in this study the crude protein of
Pittosporum undulatum did not exceed 6.11% DM. On the other hand, [
5] references crude protein values for
Pittosporum undulatum of 7.96% DM and for
Hedychium gardnerianum of 12.03% DM.
Crude protein values above 15% DM, required for optimum growth and lactation of dairy cattle [
34], were found for the remaining plants in the study.
For
Pennisetum setaceum, Acacia melanoxylon, and
Arundo donax, the crude protein values were not very different (16.61% DM, 16.99% DM, and 15.69% DM, respectively). [
35] found crude protein values between 13.0% DM and 16.9% DM for different types of Acacia, while [
5] reported crude protein values of 16.86% DM for the same Acacia species in our study.
In addition, [
5] reported slightly higher crude protein values for
Arundo donax (16.86% DM), while [
36] reported protein values of 7.61% DM lower than those found in our study.
The highest crude protein value found in this study, 24.51% DM, for
Ricinus communis, was very similar to that reported by [
37]. However, the plant contains ricin, a highly toxic compound in the seeds that has a potent cytotoxic effect that promotes severe gastroenteritis [
38]. Ricin is a toxic alkaloid present in the leaves, stems, and pericarps of
Ricinus communis seeds that can induce severe neurological disorders [
38,
39]. Although there are now studies that suggest ways for the plant’s toxicity to be overcome [
40], in particular, a number of chemical and physical methods have been tested. The chemical methods consisted of the use of formaldehyde, ammonia, lime, tannic acid, sodium chloride, and sodium hydroxide at different concentrations. The physical methods were boiling, immersion, steaming, autoclaving, and heating. The conclusion was that, from the means studied, the calcium hydroxide treatment was the only effective method for eliminating the ricin [
41] determined that the thermal detoxification process is able to degrade toxic molecules present in ricin, making it a practical and efficient method to be used on a large scale.
According to [
8], in vitro dry matter digestibility is one of the main criteria for assessing the usefulness of feeds used in livestock production since it reflects the amount of plant material that can be digested by the ruminants. In this study,
Acacia melanoxylon (27.32%),
Pittosporum undulatum (39.54%), and
Hedychium gardnerianum (31.37%) showed DMD values below 50%, which, according to [
42], is the minimum DMD value for the maintenance needs of animals [
5] also found similar DMD values but admits, citing [
43], that the digestibility of shrubs and trees is underestimated, possibly due to secondary metabolites, such as tannins or saponins, being present. These can have a negative impact on in vitro digestibility values.
Of all the plants studied,
Opuntia ficus-indica was the one that obtained the highest DMD of 84.41%, which is consistent with the value reported by [
44]. According to [
45],
Opuntia ficus-indica is highly digestible. It was also found that
Ricinus communis has a high digestibility value (79.19%), similar to that observed by other authors [
46,
47].
Table 2 shows the analytical detergent fiber (ADF) of the different plants. Fiber is made up of complex polysaccharides and represents the plant fraction that is partially digestible in the gastrointestinal tract of herbivorous animals. By determining the NDF, ADF, and ADL, information about different cell wall fractions can be obtained, such as cellulose, hemicellulose, and lignin. Non-conventional plants are characterized by having a higher cell wall-to-cell content ratio than other forage types. Plant quality is not solely influenced by the species, however, as the state of maturity, handling, and soil quality all play a role as they impact the leaf area and photosynthetic capacity of the plants [
48]. Dry seasons usually lead to an increase in cellulose, hemicellulose, and lignin. This, in turn, increases the forage resistance due to the existence of more cellulose and lignin connections, which results in a harder process of ingestion, rumination, and fermentation by microorganisms [
28].
According to [
49], when a diet’s NDF content is above 55%, the feed intake is lower. This was corroborated in this trial, where it was found that there was a negative correlation between plant NDF values and dry matter intake. There were several plants that obtained values higher than 55%, such as
Pennisetum setaceum, which presents the highest NDF value in this study (80.39% DM) and also has high contents of HEM and CEL (39.03% DM and 38.81% DM, respectively), comparable to the results found by [
37].
Hedychium gardnerianun also obtained a high value of NDF (72.39% DM), which is a very different result from that found by [
5] (38.02% DM). These differences in data between previous studies and the present one for the same plant can be attributed to variations in soil characteristics and different leaf maturation stages when these studies were conducted [
50].
Ricinus communis,
Opuntia ficus-indica,
Agave Americana L., and
Pittosporum undulatum had NDF values below 50% DM.
The in vitro evaluation of these plants (
Table 4,
Table 5 and
Table 6) provides further insights into the nutritional value and energy contents of these plants, leveraging on the relation between the amount of gas produced in in vitro fermentation, the extent of fermentation and digestibility of a forage [
51], and the rate at which the substrate is degraded [
8].
The highest gas production and energy estimates recorded were for Ricinus communis, Arundo donax, and Agave americana L., which were also the ones that obtained the highest DMD values.
As shown in
Table 5,
Acacia melanoxylin,
Agave americana, and
Hedychium gardnerianum have a lag time of 0, which means the process of fermentation initiates just as the element is incubated. This plant has a high saponin content, as noted by some authors [
52]. It would, therefore, be of interest to determine anti-nutritive substances in future works. These plants were also the only ones for which a positive value for
a was obtained. This indicates the component started to degrade quickly. Conversely, a negative value would have meant that there was an initial period during which no cell wall degradation occurred, called the lag phase [
53].
According to the Quality Rating Standard assigned by the Hay Marketing Working Group of the American Forage and Grassland Council, Pennisetum setaceum, Acacia melanoxylon, and Hedychium gardnerianum were considered of no interest for animal feed. Arundo donax is also considered of poor quality. Ricinus communis, Opuntia ficus-indica, and Agave americana L. were classified as premium. Pittosporum undulatum was rated good.
5. Conclusions
With the results obtained in this work and based on the chemical composition, gas production, and other estimates of nutritional importance evaluated, we can conclude that all these unconventional invasive plants show promising nutritional qualities that would make them a viable alternative feed for ruminants when there is a shortage of forage.
Among the species that were studied, Opuntia ficus-indica, Agave americana L., and Hedychium gardnerianum were the ones that presented the lowest dry matter values, meaning they are rich in water and can be used to complement diets during dry periods. Pittosporum undulatum, Acacia melanoxylon, and Arundo donax showed high fiber values and can therefore be used as roughage during the winter.
Pennisetum setaceum, Ricinus communis, and Opuntia ficus-indica were the plants with the highest digestibility, whereas Pittosporum undulatum showed low digestibility values despite having a low protein content. Pittosporum also achieved a good RFV fodder grade, which means it can be used as an alternative source of fiber fodder for ruminants.
Overall, Ricinus communis gave the best results, although further studies are needed to suggest ways for the plant’s toxicity to be overcome.
Regarding gas production, Agave americana obtained the highest values from the beginning to the end of the incubation, while Acacia melanoxylon produced the least amount of gas.
However, further research is needed to identify the presence of secondary metabolites as well as in vivo animal response tests to determine their acceptability and impact on animal health and performance.