Effect of Pretreatments on the Production of Biogas from Castor Waste by Anaerobic Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Pretreatments
2.3. Scanning Electron Microscopy
2.4. Physicochemical Analysis
2.5. Anaerobic Digestion Process
2.6. Gas Chromatograph
3. Results
3.1. Impact of Pretreatments
3.2. Physicochemical Characterization
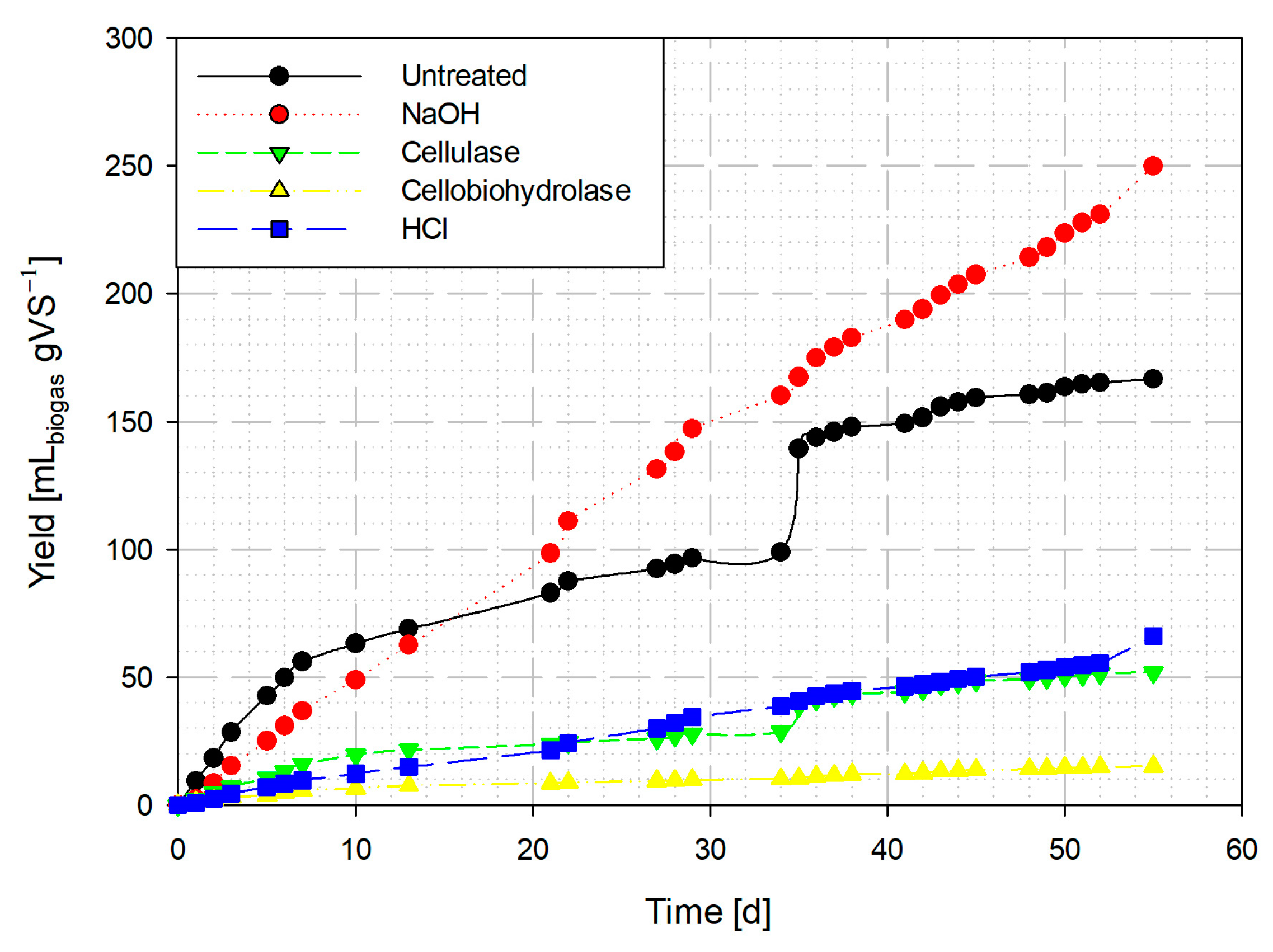
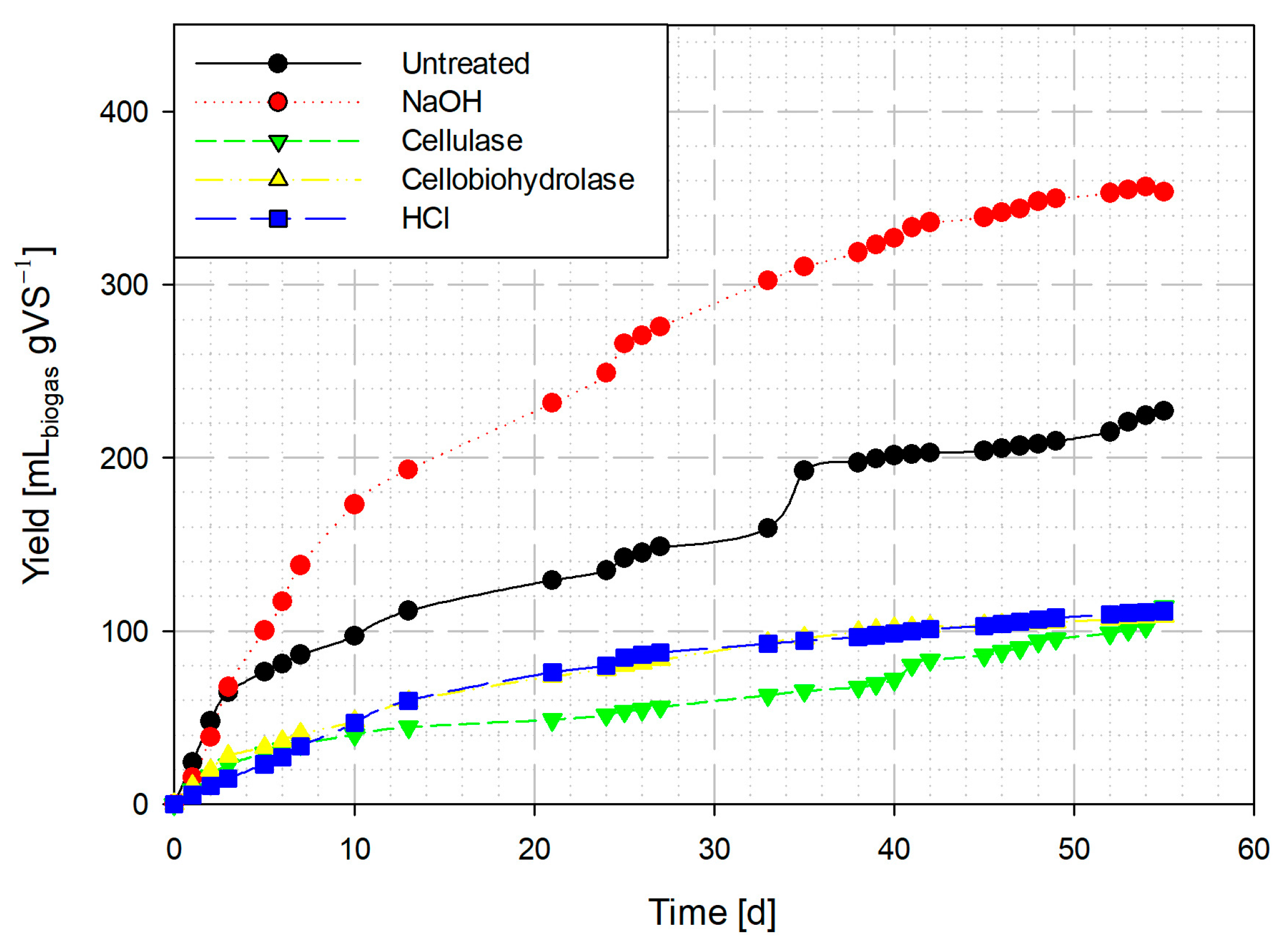
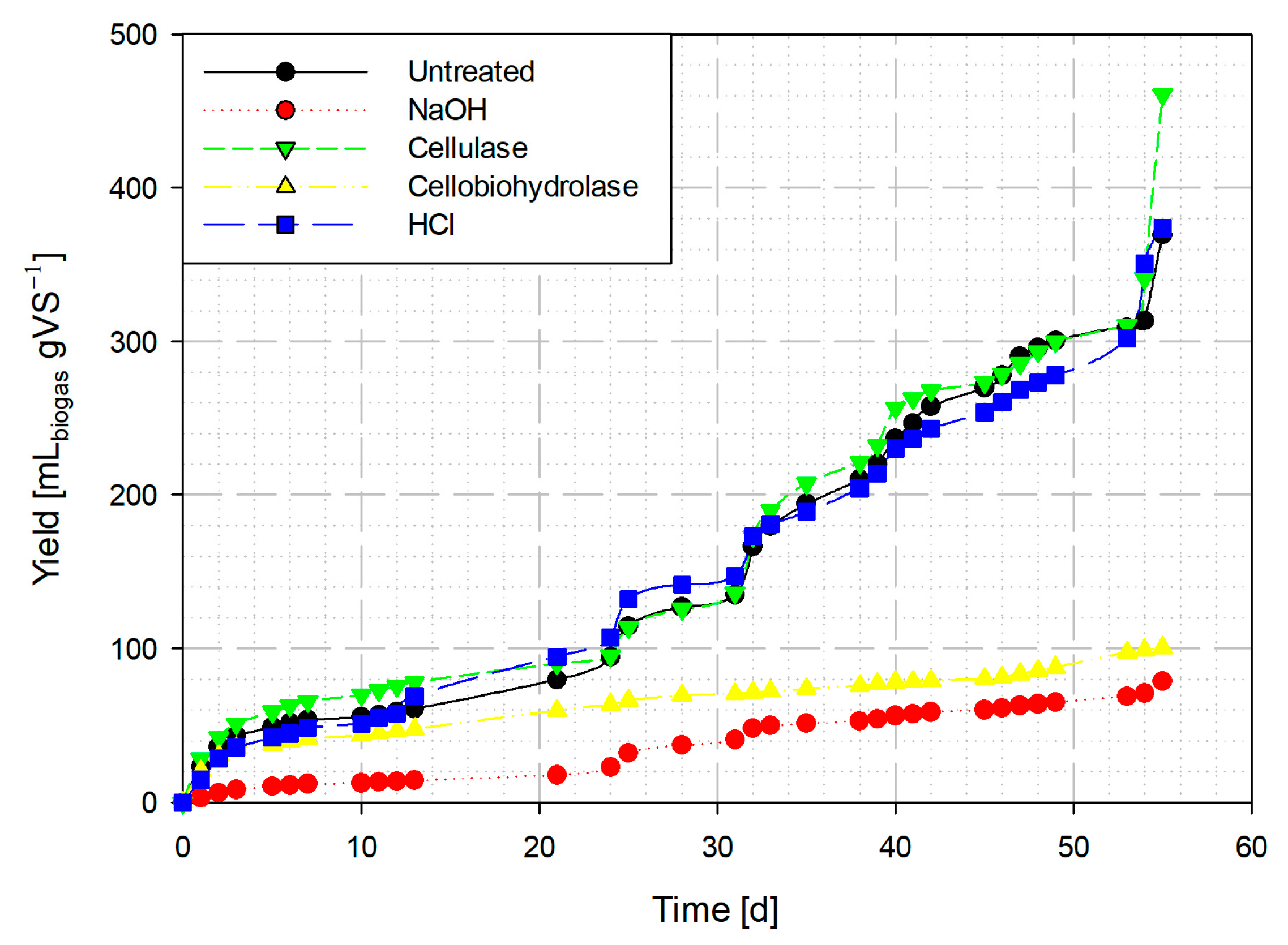
3.3. Effect of Pretreatments on the Generation of Biogas
3.3.1. Biogas from Aerial Parts of Ricinus communis
3.3.2. Biogas from Bagasse Seed of Ricinus communis
3.4. Methane Content
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| APRc | Aerial parts of Ricinus communis |
| BSRc | Bagasse seed of Ricinus communis |
| C/N | Carbon/Nitrogen ratio |
| COD | Chemical oxygen demand |
| FS | Fixed solid |
| GHGs | Greenhouse gases |
| HTR | Hydraulic retention time |
| SEM | Scanning electron microscopy |
| TS | Total solid |
| VS | Volatile solid |
References
- Shurpali, N.; Agarwal, A.K.; Srivastava, V.K. Introduction to Greenhouse Gas Emissions. In Greenhouse Gas Emissions: Challenges, Technologies and Solutions; Shurpali, N., Agarwal, A.K., Srivastava, V., Eds.; Springer: Singapore, 2019; pp. 1–5. ISBN 978-981-13-3272-2. [Google Scholar]
- Sonwani, S.; Saxena, P. Introduction to Greenhouse Gases: Sources, Sinks and Mitigation. In Greenhouse Gases: Sources, Sinks and Mitigation; Sonwani, S., Saxena, P., Eds.; Springer Nature: Singapore, 2022; pp. 1–7. ISBN 978-981-16-4482-5. [Google Scholar]
- Maji, S.; Ahmed, S.; Ghosh, S. Source Apportionment of Greenhouse Gases in the Atmosphere. In Greenhouse Gases: Sources, Sinks and Mitigation; Sonwani, S., Saxena, P., Eds.; Springer Nature: Singapore, 2022; pp. 9–37. ISBN 978-981-16-4482-5. [Google Scholar]
- Uprety, D.C.; Saxena, P. (Eds.) Methane. In Technologies for Green House Gas Assessment in Crop Studies; Springer: Singapore, 2021; pp. 59–76. ISBN 978-981-16-0204-7. [Google Scholar]
- McKendry, P. Energy Production from Biomass (Part 1): Overview of Biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Fazzino, F.; Pedullà, A.; Calabrò, P.S. Boosting the Circularity of Waste Management: Pretreated Mature Landfill Leachate Enhances the Anaerobic Digestion of Market Waste. Biofuel Res. J. 2023, 10, 1764–1773. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Pyrolysis Kinetics and Thermodynamic Parameters of Castor (Ricinus communis) Residue Using Thermogravimetric Analysis. Bioresour. Technol. 2018, 250, 422–428. [Google Scholar] [CrossRef]
- Kuete, V. Physical, Hematological, and Histopathological Signs of Toxicity Induced by African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Cameroon, Africa, 2014; pp. 635–657. ISBN 9780128000182. [Google Scholar]
- Fatimah, I.; Sagadevan, S.; Murugan, B.; Muraza, O. Castor Oil (Ricinus communis). In Biorefinery of Oil Producing Plants for Value-Added Products; John Wiley & Sons Ltd.: New York, NY, USA, 2022; pp. 51–78. ISBN 9783527830756. [Google Scholar]
- Ogunniyi, D.S. Castor Oil: A Vital Industrial Raw Material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, H.; Meier, M.A.R. Castor Oil as a Renewable Resource for the Chemical Industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Worbs, S.; Köhler, K.; Pauly, D.; Avondet, M.-A.; Schaer, M.; Dorner, M.B.; Dorner, B.G. Ricinus communis Intoxications in Human and Veterinary Medicine-A Summary of Real Cases. Toxins 2011, 3, 1332–1372. [Google Scholar] [CrossRef]
- Monlau, F.; Latrille, E.; Da Costa, A.C.; Steyer, J.-P.; Carrère, H. Enhancement of Methane Production from Sunflower Oil Cakes by Dilute Acid Pretreatment. Appl. Energy 2013, 102, 1105–1113. [Google Scholar] [CrossRef]
- Adl, M.; Sheng, K.; Gharibi, A. Technical Assessment of Bioenergy Recovery from Cotton Stalks through Anaerobic Digestion Process and the Effects of Inexpensive Pre-Treatments. Appl. Energy 2012, 93, 251–260. [Google Scholar] [CrossRef]
- Shafiei, M.; Zilouei, H.; Zamani, A.; Taherzadeh, M.J.; Karimi, K. Enhancement of Ethanol Production from Spruce Wood Chips by Ionic Liquid Pretreatment. Appl. Energy 2013, 102, 163–169. [Google Scholar] [CrossRef]
- Bochmann, G.; Montgomery, L.F.R. Storage and Pre-Treatment of Substrates for Biogas Production. In The Biogas Handbook; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing: Vienna, Austria, 2013; pp. 85–103. ISBN 9780857094988. [Google Scholar]
- Salehian, P.; Karimi, K. Alkali Pretreatment for Improvement of Biogas and Ethanol Production from Different Waste Parts of Pine Tree. Ind. Eng. Chem. Res. 2013, 52, 972–978. [Google Scholar] [CrossRef]
- Gomez-Tovar, F.; Celis, L.B.; Razo-Flores, E.; Alatriste-Mondragón, F. Chemical and Enzymatic Sequential Pretreatment of Oat Straw for Methane Production. Bioresour. Technol. 2012, 116, 372–378. [Google Scholar] [CrossRef]
- Badshah, M.; Lam, D.M.; Liu, J.; Mattiasson, B. Use of an Automatic Methane Potential Test System for Evaluating the Biomethane Potential of Sugarcane Bagasse after Different Treatments. Bioresour. Technol. 2012, 114, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Ziemiński, K.; Romanowska, I.; Kowalska, M. Enzymatic Pretreatment of Lignocellulosic Wastes to Improve Biogas Production. Waste Manag. 2012, 32, 1131–1137. [Google Scholar] [CrossRef]
- Murphy, J.D.; Thamsiriroj, T. Fundamental Science and Engineering of the Anaerobic Digestion Process for Biogas Production. In The Biogas Handbook; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing: Limerick, Ireland, 2013; pp. 104–130. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Valijanian, E.; Aghbashlo, M.; Ghanavati, H.; Sulaiman, A.; Wakisaka, M. Prominent Parameters in Biogas Production Systems. In Biogas: Fundamentals, Process, and Operation; Tabatabaei, M., Ghanavati, H., Eds.; Springer International Publishing: Cham, Switzerland; Karaj, Iran, 2018; pp. 135–161. [Google Scholar] [CrossRef]
- Anuario Estadístico de la Producción Agrícola. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 29 March 2023).
- Bateni, H.; Karimi, K.; Zamani, A.; Benakashani, F. Castor Plant for Biodiesel, Biogas, and Ethanol Production with a Biorefinery Processing Perspective. Appl. Energy 2014, 136, 14–22. [Google Scholar] [CrossRef]
- Lingaiah, V.; Rajasekaran, P. Biodigestion of Cowdung and Organic Wastes Mixed with Oil Cake in Relation to Energy. Agric. Wastes 1986, 17, 161–173. [Google Scholar] [CrossRef]
- Gollakota, K.G.; Meher, K.K. Effect of Particle Size, Temperature, Loading Rate and Stirring on Biogas Production from Castor Cake (Oil Expelled). Biol. Wastes 1988, 24, 243–249. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Song, W.; Ding, L.; Xie, B.; Zhou, J.; Cen, K. Characterisation of Water Hyacinth with Microwave-Heated Alkali Pretreatment for Enhanced Enzymatic Digestibility and Hydrogen/Methane Fermentation. Bioresour. Technol. 2015, 182, 1–7. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, C.; Li, Y. Enhanced Solid-State Anaerobic Digestion of Corn Stover by Alkaline Pretreatment. Bioresour. Technol. 2010, 101, 7523–7528. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of Lignocellulosic Biomass for Enhanced Biogas Production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Telliard, W.A. Method 1684: Total, Fixed, and Volatile Solids in Water, Solids, and Biosolids; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2001.
- Kar, S.; Milstein, D. Oxidation of Organic Compounds Using Water as the Oxidant with H2 Liberation Catalyzed by Molecular Metal Complexes. Acc. Chem. Res. 2022, 55, 2304–2315. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mirahmadi, K.; Kabir, M.M.; Jeihanipour, A.; Karimi, K.; Taherzadeh, M.J. Alkaline pretreatment of spruce and birch to improve bioethanol and biogas production. Bioresources 2010, 5, 928–938. [Google Scholar] [CrossRef]
- Passos, F.; Hom-Diaz, A.; Blanquez, P.; Vicent, T.; Ferrer, I. Improving Biogas Production from Microalgae by Enzymatic Pretreatment. Bioresour. Technol. 2016, 199, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Bateni, H.; Karimi, K. Biorefining of Eruca sativa Plant for Efficient Biofuel Production. RSC Adv. 2016, 6, 34492–34500. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Sárvári Horváth, I. Sustainable and Efficient Sugar Production from Wheat Straw by Pretreatment with Biogas Digestate. RSC Adv. 2019, 9, 27692–27701. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, H.; Zou, H.; Sun, T.; Li, M.; Zhai, J.; He, Q.; Gu, L.; Tang, W.Z. Effects of Acid/Alkali Pretreatments on Lignocellulosic Biomass Mono-Digestion and Its Co-Digestion with Waste Activated Sludge. J. Clean. Prod. 2020, 277, 123998. [Google Scholar] [CrossRef]
- Sánchez-Cantú, M.; Ortiz-Moreno, L.; Ramos-Cassellis, M.E.; Marín-Castro, M.; De la Cerna-Hernández, C. Solid-State Treatment of Castor Cake Employing the Enzymatic Cocktail Produced from Pleurotus djamor Fungi. Appl. Biochem. Biotechnol. 2018, 185, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Bateni, H.; Bateni, F.; Karimi, K. Effects of Oil Extraction on Ethanol and Biogas Production from Eruca sativa Seed Cake. Waste Biomass Valorization 2017, 8, 1897–1905. [Google Scholar] [CrossRef]
- Bateni, H.; Karimi, K. Biodiesel Production from Castor Plant Integrating Ethanol Production via a Biorefinery Approach. Chem. Eng. Res. Des. 2016, 107, 4–12. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.D.; Michailof, C.M.; Lappas, A.A. Castor Bean Cake Residues Upgrading towards High Added Value Products via Fast Catalytic Pyrolysis. Biomass Bioenergy 2016, 95, 405–415. [Google Scholar] [CrossRef]
- Hilioti, Z.; Michailof, C.M.; Valasiadis, D.; Iliopoulou, E.F.; Koidou, V.; Lappas, A.A. Characterization of Castor Plant-Derived Biochars and Their Effects as Soil Amendments on Seedlings. Biomass Bioenergy 2017, 105, 96–106. [Google Scholar] [CrossRef]
- Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Khura, T.K. Production of Methane from Anaerobic Digestion of Jatropha and Pongamia Oil Cakes. Appl. Energy 2012, 93, 148–159. [Google Scholar] [CrossRef]
- Sinbuathong, N.; Munakata-Marr, J.; Sillapacharoenkul, B.; Chulalaksananukul, S. Effect of the Solid Content on Biogas Production from Jatropha curcas Seed Cake. Int. J. Glob. Warm. 2011, 3, 403–416. [Google Scholar] [CrossRef]
- Raheman, H.; Mondal, S. Biogas Production Potential of Jatropha Seed Cake. Biomass Bioenergy 2012, 37, 25–30. [Google Scholar] [CrossRef]
- Şenol, H.; Açıkel, Ü.; Oda, V. Anaerobic Digestion of Sugar Beet Pulp after Acid Thermal and Alkali Thermal Pretreatments. Biomass Convers. Biorefinery 2021, 11, 895–905. [Google Scholar] [CrossRef]
- Wang, D.; Ai, P.; Yu, L.; Tan, Z.; Zhang, Y. Comparing the Hydrolysis and Biogas Production Performance of Alkali and Acid Pretreatments of Rice Straw Using Two-Stage Anaerobic Fermentation. Biosyst. Eng. 2015, 132, 47–55. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for Transformation of Biogas to Biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Uçkun Kiran, E.; Liu, Y. Bioethanol Production from Mixed Food Waste by an Effective Enzymatic Pretreatment. Fuel 2015, 159, 463–469. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, L.; Wan, Y.; Ye, M.; Ye, J.; Li, Y.-Y.; Liu, J. Using an Expended Granular Sludge Bed Reactor for Advanced Anaerobic Digestion of Food Waste Pretreated with Enzyme: The Feasibility and Its Performance. Bioresour. Technol. 2020, 311, 123504. [Google Scholar] [CrossRef]
- Venkateshkumar, R.; Shanmugam, S.; Veerappan, A.R. Evaluation of Biogas Through Chemically Treated Cottonseed Hull in Anaerobic Digestion With/Without Cow Dung: An Experimental Study. BioEnergy Res. 2023, 16, 660–672. [Google Scholar] [CrossRef]
- Kevrekidis, I.G.; Shvartsman, S.Y. Chemical Reactor Analysis and Design Fundamentals: Jim Rawlings and John Ekerdt; Nob Hill Publishing, Madison, WI, 2002. ISBN 0-615-11884-4. Chem. Eng. Sci. 2004, 59, 2123–2124. [Google Scholar] [CrossRef]
- Petersson, A.; Thomsen, M.H.; Hauggaard-Nielsen, H.; Thomsen, A.-B. Potential Bioethanol and Biogas Production Using Lignocellulosic Biomass from Winter Rye, Oilseed Rape and Faba Bean. Biomass Bioenergy 2007, 31, 812–819. [Google Scholar] [CrossRef]
- Almeida, A.; Raadam, M.H.; Lopez-Villanueva, A.; Lira, I.O.H.D.; Khakimov, B. In-Depth Investigation on Triterpenoid Production from the Desert Plants Aloe vera (L.) Burm.f. and Opuntia robusta J.C. Wendl. Prompted by Their Low Specific Methane Production. BioEnergy Res. 2022, 15, 1–14. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Nguyen, D.; Surendra, K.C.; Shrestha, S.; Rajendran, K.; Oechsner, H.; Xie, L.; Khanal, S.K. Anaerobic Biorefinery: Current Status, Challenges, and Opportunities. Bioresour. Technol. 2016, 215, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, Y.; Zhou, X. Effect of Ca(OH)2 Pretreatment on Extruded Rice Straw Anaerobic Digestion. Bioresour. Technol. 2015, 196, 116–122. [Google Scholar] [CrossRef]
- Rasaeian, N.; Mirmohamadsadeghi, S.; Denayer, J.F.M.; Karimi, K. Improving Biogas Production from Different Parts of Spruce Tree Using Leading Pretreatments. Fuel 2022, 329, 125539. [Google Scholar] [CrossRef]
- Linyi, C.; Yujie, Q.; Buqing, C.; Chenglong, W.; Shaohong, Z.; Renglu, C.; Shaohua, Y.; Lan, Y.; Zhiju, L. Enhancing Degradation and Biogas Production during Anaerobic Digestion of Food Waste Using Alkali Pretreatment. Environ. Res. 2020, 188, 109743. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, Q.; Gu, Y.; Yu, H.; Zhang, Y.; Zhou, X. Improving Biodegradability and Biogas Production of Miscanthus Using a Combination of Hydrothermal and Alkaline Pretreatment. Ind. Crops Prod. 2020, 144, 111985. [Google Scholar] [CrossRef]
- Hashemi, S.; Joseph, P.; Mialon, A.; Moe, S.; Lamb, J.J.; Lien, K.M. Enzymatic Pretreatment of Steam-Exploded Birch Wood for Increased Biogas Production and Lignin Degradation. Bioresour. Technol. Rep. 2021, 16, 100874. [Google Scholar] [CrossRef]



| Pretreatment | Time [h] | Temperature [°C] |
|---|---|---|
| Untreated (control) | 0 | Room temperature |
| Cellobiohydrolase enzyme extracted from Hypocrea jecorina (0.1% w/v) | 18 | 60 |
| Cellulase enzyme extracted from Trichoderma longibrachiaum (0.5% w/w) | 18 | 60 |
| NaOH (4% w/w) | 24 | Room temperature |
| HCl (4% w/v) | 2 | 80 |
| Sample | Moisture [%] | TS [%] | VS [%] | FS [%] |
|---|---|---|---|---|
| APRc | 9.9 | 90.2 | 74.4 | 15.9 |
| BSRc | 4.4 | 95.6 | 71.1 | 24.5 |
| Sample | TS [g L−1] | VS [g L−1] | Total Hexoses [mg L−1] | COD [mg L−1] |
|---|---|---|---|---|
| APRc Untreated | 31 | 26 | 514.4 | 749.4 |
| APRc NaOH | 44 | 37 | 451.1 | 1607.3 |
| APRc Cellulase | 92 | 87 | 815.6 | 1661.7 |
| APRc Cellobiohydrolase | 280 | 270 | 246.8 | 4355.3 |
| APRc HCl | 138 | 101 | 898.5 | 4816 |
| BSRc Untreated | 32 | 31 | 238.2 | 918.6 |
| BSRc NaOH | 166 | 163 | 106.3 | 1801 |
| BSRc Cellulase | 24 | 22 | 528.3 | 2800.2 |
| BSRc Cellobiohydrolase | 48 | 46 | 379.2 | 1376.7 |
| BSRc HCl | 68 | 36 | 618.2 | 1720.4 |
| Sample at Room Temperature | CH4 [%] | CO2 * [%] | Sample at 37 °C | CH4 [%] | CO2 * [%] |
|---|---|---|---|---|---|
| APRc Untreated | 15.5 ± 2.9 D | 84.5 ± 2.9 A | APRc Untreated | 20.7 ± 12.6 D | 79.3 ± 12.6 A |
| APRc NaOH | 25.7 ± 6.1 CD | 74.3 ± 6.1 AB | APRc NaOH | 75.1 ± 14.7 AB | 24.9 ± 14.7 CD |
| APRc Cellulase | 21.3 ± 7.1 D | 78.7 ± 7.1 A | APRc Cellulase | 60.2 ± 12 ABCD | 39.8 ± 12 ABCD |
| APRc Cellobiohydrolase | 16.8 ± 2.3 D | 83.2 ± 2.3 A | APRc Cellobiohydrolase | 60.1 ± 7.6 ABCD | 39.9 ± 7.6 ABCD |
| APRc HCl | 54.5 ± 7.7 BC | 45.5 ± 7.7 BC | APRc HCl | 31.2 ± 16.8 CD | 68.8 ± 16.8 AB |
| BSRc Untreated | 60.3 ± 15.8 AB | 39.7 ± 15.8 CD | BSRc Untreated | 50.9 ± 8.5 BCD | 49.1 ± 8.5 ABC |
| BSRc NaOH | 66.5 ± 6.7 AB | 33.5 ± 6.7 CD | BSRc NaOH | 85.1 ± 17.2 AB | 14.9 ± 17.2 CD |
| BSRc Cellulase | 70.4 ± 15.9 AB | 29.6 ± 15.9 CD | BSRc Cellulase | 65.1 ± 11.4 ABC | 34.9 ± 11.4 BCD |
| BSRc Cellobiohydrolase | 89.6 ± 13.5 AB | 10.4 ± 13.5 D | BSRc Cellobiohydrolase | 92.2 ± 12.9 A | 7.8 ± 12.9 D |
| BSRc HCl | 78.3 ± 19.2 AB | 21.7 ± 19.2 CD | BSRc HCl | 60.9 ± 20.3 ABCD | 39.1 ± 20.3 ABCD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quezada-Morales, D.L.; Campos-Guillén, J.; De Moure-Flores, F.J.; Amaro-Reyes, A.; Martínez-Martínez, J.H.; Chaparro-Sánchez, R.; Zavala-Gómez, C.E.; Flores-Macías, A.; Figueroa-Brito, R.; Rodríguez-Morales, J.A.; et al. Effect of Pretreatments on the Production of Biogas from Castor Waste by Anaerobic Digestion. Fermentation 2023, 9, 399. https://doi.org/10.3390/fermentation9040399
Quezada-Morales DL, Campos-Guillén J, De Moure-Flores FJ, Amaro-Reyes A, Martínez-Martínez JH, Chaparro-Sánchez R, Zavala-Gómez CE, Flores-Macías A, Figueroa-Brito R, Rodríguez-Morales JA, et al. Effect of Pretreatments on the Production of Biogas from Castor Waste by Anaerobic Digestion. Fermentation. 2023; 9(4):399. https://doi.org/10.3390/fermentation9040399
Chicago/Turabian StyleQuezada-Morales, Diana Laura, Juan Campos-Guillén, Francisco Javier De Moure-Flores, Aldo Amaro-Reyes, Juan Humberto Martínez-Martínez, Ricardo Chaparro-Sánchez, Carlos Eduardo Zavala-Gómez, Antonio Flores-Macías, Rodolfo Figueroa-Brito, José Alberto Rodríguez-Morales, and et al. 2023. "Effect of Pretreatments on the Production of Biogas from Castor Waste by Anaerobic Digestion" Fermentation 9, no. 4: 399. https://doi.org/10.3390/fermentation9040399
APA StyleQuezada-Morales, D. L., Campos-Guillén, J., De Moure-Flores, F. J., Amaro-Reyes, A., Martínez-Martínez, J. H., Chaparro-Sánchez, R., Zavala-Gómez, C. E., Flores-Macías, A., Figueroa-Brito, R., Rodríguez-Morales, J. A., & Ramos-López, M. A. (2023). Effect of Pretreatments on the Production of Biogas from Castor Waste by Anaerobic Digestion. Fermentation, 9(4), 399. https://doi.org/10.3390/fermentation9040399











