Abstract
Carrot pomace (CP) which is generated in a large volume in the juice production process, is rich in cellulose, hemicellulose, sugars, pectin, and minerals. However, in many previous investigations, only cellulose was purified and utilized while other components of CP were discarded as waste. Here, CP was valorized into fungal biomass and cellulose with the aim of utilizing all the CP components. Enzymatic pretreatments were applied to solubilize the digestible fraction of CP including hemicellulose, pectin, sucrose, and other sugars for fungal cultivation, while cellulose remained intact in the solid fraction. The dissolved fraction was utilized as a substrate for the cultivation of an edible fungus (Rhizopus delemar). Fungal cultivation was performed in shake flasks and bench-scale bioreactors. The highest fungal biomass concentration was obtained after pretreatment with invertase (5.01 g/L) after 72 h of cultivation (36 and 42% higher than the concentrations obtained after hemicellulase and pectinase treatments, respectively). Invertase pretreatment resulted in the hydrolysis of sucrose, which could then be taken up by the fungus. Carbohydrate analysis showed 28–33% glucan, 4.1–4.9% other polysaccharides, 0.01% lignin, and 2.7–7% ash in the CP residues after enzymatic pretreatment. Fourier transform infrared spectroscopy and thermogravimetric analysis also confirmed the presence of cellulose in this fraction. The obtained fungal biomass has a high potential for food or feed applications, or as a raw material for the development of biomaterials. Cellulose could be purified from the solid fraction and used for applications such as biobased-textiles or membranes for wastewater treatment, where pure cellulose is needed.
1. Introduction
Large quantities of organic waste remain after the processing of fruits and vegetables. Food and vegetable waste generation is increasing, which results in a global problem with several environmental, economic, and social drawbacks [1]. According to directive 2018/851, the European Union prohibits the landfill of this waste without valorization due to economic and environmental reasons [2]. Additionally, incineration is not efficient due to the high moisture content of fruit and vegetable residues. The global production of carrot pomace was around 45 million tons in 2019. Carrot pomace (CP), which comprises a significant portion of carrot, is discarded during the industrial juice extraction process [3]. CP contains various biopolymers including cellulose (10–28%), hemicellulose (5–20%), lignin (2.5–7.7%), and pectin (2–8%). Moreover, free sugars such as sucrose (14.3–47.2%), glucose (7.9–30.44%), and fructose (5.4–14.2%) are other major components of CP [4]. CP has a lower degree of recalcitrance compared to wood. Therefore, the processing and isolation of cellulose from CP and its application in different final products is expected to require less energy compared to the cellulose extracted from wood [5].
Only a small portion of carrot pomace, around 10–15%, is used as animal feed and fertilizer, while the rest is discarded [6]. To valorize the CP, two scenarios are suggested. The first common approach is to isolate and purify the cellulose from CP, which can be transformed into nanocellulose [7]. During the cellulose purification process, valuable compounds such as sugars, pectin, hemicellulose, and minerals are released to the wastewater where they have a good potential to be used as nutrients for the cultivation of microorganisms [8]. The second scenario is the utilization of CP nutrients in fermentation processes using different microorganisms for ethanol production [9]. To convert the CP components to fermentable sugars, hydrothermal, acidic, alkaline, and enzymatic hydrolysis have been applied [10].
To improve the nutrient accessibility for microorganisms, enzymatic hydrolysis is performed on CP, which does not require high temperatures, high pressures, and chemicals. After a hydrolysis step, CP is used as a substrate to develop value-added products by fermentation. A lipid-rich microalgae and the fungus Penicillium chrysogenum were grown on pretreated CP where the products were used as feedstock for biodiesel production and the inulinase enzyme for medical applications, respectively [11,12]. However, simultaneous cellulose isolation and nutrient release for the cultivation of microorganisms has not been previously reported.
Zygomycetes fungi can grow on a low-cost substrate such as food waste. The biomass of these fungi is a rich source of proteins that can be used for food or feed applications. Furthermore, the cell wall of these fungi contain fibrous biopolymers such as chitin and chitosan, which can be used for different applications such as the production of textiles and packaging materials [13]. Recently, Svensson et al. and Köhnlein et al. used bread waste as a substrate for the cultivation of a zygomycete fungus called Rhizopus delemar. The obtained fungal biomass was used for the development of textile fibers and bioplastic films, respectively [14,15].
It is anticipated that CP waste could also be used as a substrate for the cultivation of fungus. Therefore, in this study, a sustainable approach for the valorization of CP into fungal biomass and cellulose was conducted to enable the complete utilization of CP. Simultaneous cellulose isolation and nutrient release were performed through the enzymatic pretreatment of CP using hemicellulase, pectinase, or invertase. The released nutrients, containing sugars, were then used for fungal cultivation in shake flasks and bench-scale bioreactors to produce fungal biomass. Cellulose remained intact in the insoluble fraction of CP.
2. Materials and Methods
2.1. Materials
The carrot pomace was kindly provided by Herrlijunga Cider AB (Herrlijunga, Sweden) after the juice extraction process. It was stored in a freezer at −18 °C until use. The zygomycete fungus R. delemar CBS 145, 940 was obtained from the Centraalbureau voor Schimmelcultutres (Westerdijk Institute, Utrecht, The Netherlands), which was originally isolated from banana leaves used in tempeh production [16]. Yeast extract, agar, hydrochloric acid (HCl), sodium hydroxide (NaOH), sodium carbonate (Na2CO3), sulfuric acid (H2SO4), peptone, glucose, hemicellulase from Aspergillus niger, pectinase from Aspergillus, and invertase from baker’s yeast (Saccharomyces. cerevisiae) were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.2. Preparation of Fungal Agar Plates
Agar plates containing 17.0 g/L agar, 20 g/L glucose, and 4 g/L peptone were prepared (pH 5.5, sterilization in autoclave, VX-95, Systec, Linden, Germany, at 121 °C for 20 min). The spore suspension was prepared by adding 20 mL of sterile water to a pre-made agar plate of R. delemar and scraping on the surface of the agar plate to release the spores. New agar plates were inoculated with 0.1 mL of the spore suspension and placed in an incubator (BINDER, KB 23, Tuttlingen, Germany) at 30 °C for 3 days to grow the fungus. Finally, agar plates were stored in a refrigerator at 4 °C until use [13].
2.3. Pretreatment of the Carrot Pomace
The carrot pomace was subjected to various pretreatments including hydrothermal and enzymatic pretreatments. Hydrothermal pretreatment was conducted in a rotary evaporator (Laborata 20; Heidolph Instruments GmbH & Co. KG, Nuremberg, Germany) at 80 °C for 1 h and 100 rpm. Enzymatic pretreatments (using hemicellulase, pectinase, and invertase) were applied to hydrolyze the hemicellulose, pectin, and sucrose fractions of CP, respectively. Pretreatments were performed in a 65-L kettle (Kegland Digiboil, China) with controlled heating at 40 °C. To facilitate separation of the soluble and insoluble fractions, the CP was kept inside a fabric polyester bag (210 microns, Reuseable BIAB bag) that was placed inside the kettle during the pretreatments, and hemicellulase (1 unit per g dry weight CP), pectinase (1 unit per g dry weight CP), and invertase (0.1 unit per g dry weight CP) were added to the carrot pomace suspension in water (4 dry wt. %) after adjusting the pH to 4.5 using 1 M NaOH and 1 M HCl. The suspension was mixed every 15 min using a mixer (Robot Coupe Inc., Mini MP 190 V.V, South Perkins, Ridgeland, MS, USA) during the 4 h pretreatment. Then, the pretreated CP was taken out from the kettle using the bag and pressed inside the bag using a mechanical fruit press (screw press 121) to extract the juice and separate the liquid fraction (LFCP) from the solid fraction (SFCP) of the pomace. These fractions were collected and the yields of SFCP after each enzyme treatment were measured. The abbreviations used throughout this paper are provided in Table 1.

Table 1.
A list of the abbreviations used in this article.
2.4. Fungal Growth in Shake Flasks
Fungal cultivation was conducted in 250 mL cotton plugged shake flasks containing 100 mL of LFCP. The liquid was supplemented with various concentrations of yeast extract (0–3 g/L) and the pH was adjusted to 5.5 before sterilization in an autoclave (VX-95, Systec, Linden, Germany). The flasks were then inoculated with a 2 mL spore suspension and fungal growth was performed at 35 °C in a water bath shaker (Grant Instruments Ltd., Cambridge, UK) at 100 rpm for 48 or 72 h. All the experiments were conducted in triplicate. The fungal biomass was harvested by filtration through a kitchen sieve (1.5 mm pore size), washed with water three times, dried at 70 °C, and weighed.
2.5. Fungal Cultivation in Bench-Scale Bioreactors
Fungal cultivation was scaled up to bench-scale bubble column bioreactors (Belach Bioteknik AB, Skogås, Sweden) with a working volume of 3.5 L, according to the conditions that resulted in the best fungal growth in the shake flask experiments. The bioreactors were filled with LFCP containing 0 or 1 g/L yeast extract at pH 5.5 and sterilized in an autoclave at 121 °C for 20 min. The reactors were inoculated with the spore suspension (0.02 mL/mL of the substrate). The fungal cultivation was performed at an aeration rate of 2 vvm (volume of air per volume of liquid per minute) at 35 °C for 72 h. During the cultivation, the pH was monitored and readjusted to 5.5 manually. The samples were taken every 24 h to analyze the sugar and ethanol contents. The fungal biomass was harvested and filtered using a 1.5 mm kitchen sieve and washed three times with tap water, and part of it was used to determine the yield. The rest was stored at −18 °C.
2.6. Analytical Methods
The moisture and total solid contents were measured in CP, LFCP, and SFCP (after each enzymatic pretreatment) to determine the yield of the pretreatments. The composition of the freeze-dried SFCP after hydrolysis was determined according to the standard methods published by the National Renewable Energy Laboratory (NREL). Accordingly, the total lignin, ash, and carbohydrate content of SFCP were measured. The treatment of the materials was performed with sulfuric acid in two steps, which converted carbohydrates to free sugars and lignin to two fractions of acid-soluble and acid-insoluble lignin. Sugars (glucan, xylan, arabinan, and galactan) were measured by HPLC using a lead (II)-based column (Aminex HPX-87P, Bio-Red, Hercules, CA, USA) operating at 85 °C, with 0.6 mL/ min ultrapure water as the eluent. Acid soluble lignin was estimated by measuring the absorbance of the hydrolysate at 240 nm by UV spectroscopy (Biochrom libra S50, Cambridge, UK) and acid-insoluble lignin was estimated based on the weight of the solid residue after two steps of hydrolysis with sulfuric acid [17].
Glucose, sugar mix, and ethanol concentration in the fermentation broth were analyzed by high-performance liquid chromatography (HPLC) with a hydrogen-based ion-exchange column (Aminex HPX-87H, Bio-Rad, Hercules, CA, USA) at 60 °C with 0.6 mL/min of 5 mM H2SO4 as an eluent. The sugar mix is representative of monosaccharides other than glucose.
2.6.1. Thermogravimetric Analysis (TGA)
The thermal decomposition behavior of CP after each enzymatic pretreatment was investigated by TGA analysis with a Q500 TA instrument supplied by Waters LLC (New Castle, DE, USA). A total of 10 mg of freeze-dried CP or SFCP was heated from room temperature to 800 °C with a heating rate of 20 °C/min in a nitrogen atmosphere. Graphs on the percentage weight loss against the temperature were obtained and the thermal stability of the materials after the treatments was studied.
2.6.2. Fluidscope™ Scanning (oCelloScope)
To observe and measure the dimension of hyphae of the fungus after cultivation, a Fluid Scope™ scanning analysis was performed using an oCelloScope (BioScience Solutions, Copenhagen, Denmark). To capture the images, the wet fungal biomass was dispersed in distilled water using a kitchen blender (Bosch, MMBM7G3M, Plymouth, MI, USA) for 1 min. For each image, the default setting for a 24-well plate was used.
2.6.3. Fourier Transform Infrared (FTIR) SPECTROSCOPY
FTIR analysis was performed by a Nicolet iS10 (Thermo Fisher Scientific, Waltham, MA, USA) to determine the composition of CP and SFCP. The spectra were recorded for 32 scans with a resolution of 4 cm−1.
2.6.4. Statistical Analysis
All the experiments were performed in duplicate unless otherwise stated and the average values were reported. Statistical analysis was conducted using the analysis of variance (ANOVA) and post hoc Tukey test. p-values smaller than 0.05 were considered as significant differences.
3. Results and Discussion
Carrot pomace is a by-product of the juice production process that is often discarded in large volumes. However, the nutrients available in carrot pomace can serve as a suitable substrate for fungal cultivation. Other fractions of CP contain cellulose that can be purified and used for different applications. This research aimed toward the simultaneous isolation of cellulose and the release of nutrients for fungal growth from carrot pomace. The cultivation of zygomycete fungus R. delemar was conducted first in shake flasks and then scaled to 4.5 L bioreactors with the liquid fraction as the substrate containing the released nutrients. The effect of different pretreatments on fungal growth was investigated. Moreover, the characteristics of the solid fraction that remained after enzymatic pretreatments were evaluated, confirming that cellulose stayed intact during the pretreatment of CP.
3.1. Effect of Pretreatments on CP and SFCP
The initial moisture content of CP was 90.5%. The yield of SFCP was 71.7, 75.5, and 75.9% after pectinase, hemicellulase, and invertase pretreatments, respectively. The composition of CP and SFCP after each enzymatic pretreatment is shown in Table 2. The ash content was around 2.7–7.0% and less than 1% of lignin was found in CP and enzymatically treated CP, which is in agreement with previous studies [8]. The glucan value was 30.6 ± 0.2%, while these values reached 26–33 after pretreatment. In the same way, the content of the hemicellulose components such as xylan, arabinan, and galactan were around 10–12%. Chojnaki et al. [18] reported that CP contained 20% of cellulose and 12% of hemicellulose, respectively. Likewise, comparable values were recorded in this study. It should be mentioned that some of the glucose detected in the carbohydrate analysis does not have a glucan origin and originates from glucose, which exists as a free sugar or is released after the hydrolysis of sucrose. Ramos et al. expressed that the water extractive of CP contained 47% sucrose, which is released during the pretreatment [19]. According to previous research, the carbohydrate content of CP is approximately 53% of the total dry matter. The carbohydrate fraction consists of soluble sugars such as glucose, fructose, and sucrose (which are extractable by hot water) as well as polysaccharides. A major part of polysaccharides is glucan, which is in the form of cellulose, since starch is a minor fraction of carrot pomace. Hemicellulose and pectin made up 5.4 and 3.7% of CP, respectively, according to Vrije et al. [20].

Table 2.
The composition of CP and the solid fraction of CP after hydrothermal and enzymatic treatment. All values are given in wt. (%).
3.2. Effect of Pretreatments on Fungal Growth in Shake Flasks
The pretreatment of CP had a significant impact on the efficiency of the fungal cultivation process. After hydrothermal treatment, the concentration of soluble solids in the hydrolysate was 1.6%. However, this value increased to 2.9, 2.9, and 4.5% after the hemicellulase, invertase, and pectinase pretreatments (Figure 1), respectively. To determine the best condition of scale-up, various parameters such as the type of enzyme, nitrogen source supplementation, and harvesting time were investigated for the fungal growth in shake flasks to find the best condition for scale-up. The details of the trials are presented in Table 3. The growth of fungus in the liquid fraction obtained by the hydrothermal treatment resulted in a biomass concentration of 1.8 g/L, which was below the concentration obtained after all enzymatic pretreatments. This was mainly due to the low concentration of nutrients present in this liquid. Therefore, this pretreatment was not considered in the rest of the experiments. Sucrose is one of the main free sugars available in CP [20]. Souza et al. [21] reported the presence of 24.97 g/L sucrose in potato protein liquor, which was not directly consumed by the zygomycetes fungi. Since the fungus used in this study belongs to the same family, cleavage of this sugar to glucose and fructose by treatment with invertase was expected to increase the fungal growth. The enzymatic pretreatments were applied to increase the accessible nutrient concentration for the fungal cultivation. Accordingly, the pretreatment of CP with pectinase, hemicellulase, and invertase resulted in fungal biomass concentrations of 2.3, 2.8, and 4.0 g/L after 72 h, respectively. The addition of a yeast extract (1 g/L) as the nitrogen source improved the fungal biomass concentration by 3–13%, and a further increase in the yeast extract concentration did not significantly improve the fungal growth after the hemicellulase and pectinase pretreatments.
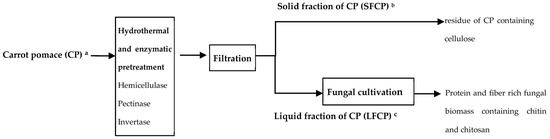
Figure 1.
Process flow diagram for the valorization of carrot pomace. (a) The initial dry weight of CP was 800 g for all pretreatments. (b) Respective dry weight of the solid fraction after pectinase, hemicellulase, and invertase pretreatments of 660, 570, and 607 g. (c) The concentration of soluble solids (dry solid) in LFCP was 1.6, 2.9, 4.5, and 2.9 % after the hydrothermal, hemicellulase, pectinase, and invertase pretreatments, respectively.

Table 3.
Summary of the pretreatment and cultivation circumstances (shake flask scale).
The addition of the yeast extract was not effective in the case of invertase pretreatment. However, extending the cultivation time to 72 h enhanced the concentration of fungal biomass in some of the pretreatments. The concentration of fungal biomass obtained after invertase pretreatment was higher than that of the other pretreatments. This was because of the higher concentration of glucose in the LFCP after invertase treatment, which was caused by the ability of this enzyme to hydrolyze the sucrose to glucose and fructose. The yield of fungal biomass (g dry weight per g soluble solids in the medium) was higher for the invertase treatment compared to the other enzymes, confirming that the fungus could not directly consume the sucrose in the LFCP after the hemicellulase and pectinase treatments. The use of the pectinase and hemicellulase enzymes simultaneously decreased the fungal growth. The concentration of soluble solids (dry solid) in LFCP after simultaneous pretreatment with pectinase and hemicellulase was 3.9%, lower than the value obtained after pectinase treatment (Figure 1). This could be due to the inhibitory effect of the two enzymes on each other when they were mixed.
3.3. Scale-Up of the Fungal Cultivation
The hydrolysates obtained from hemicellulase, pectinase, and invertase pretreatments were used and the cultivation was scaled-up to bench-scale bioreactors (4.5 L). The impact of the yeast extract supplementation on fungal growth was also investigated. Table 4 shows that the presence of the yeast extract significantly improved the concentration of fungal biomass after the hemicellulase pretreatment from 3.26 to 3.6 g/L (p = 0.045). The same phenomenon was observed for the hydrolysate obtained after the pectinase pretreatment where the supplementation of the yeast extract significantly improved the fungal growth (p = 0.001) and the biomass concentration reached 4.01 g/L. Miao et al. reported that the yeast extract enhanced the growth of Arthrinium c.f. saccharicola fungus by providing nitrogenous nutrients that were more important for mycelial growth compared to glucose as a carbon source, which was more important for the production of bioactive compounds [22]. The highest biomass growth was achieved after the pretreatment of the CP with invertase, where the biomass concentration reached 5 g/L after 72 h of cultivation. This is explained by the sucrose hydrolysis, which enhanced the concentration of sugars that are digestible for R. delemar. Adding the yeast extract did not have a significant effect on the fungal growth for the pectinase pretreatment.

Table 4.
Substrate consumption as well as the fungal biomass concentration and yield obtained during the fungal cultivation in bench-scale bioreactors.
Table 4 indicates that the highest and lowest average fungal biomass yields (5.01 and 2.9 g/L) were obtained after the invertase and pectinase pretreatments, respectively. The pectinase treatment resulted in the highest concentration of soluble solids in the liquid fraction, but a significant part of the soluble solids (around 50%) remained at the end of cultivation for this pretreatment. The remaining soluble solids may contain sucrose and products of pectin hydrolysis, which cannot be consumed by the fungus. Moreover, a significant fraction of soluble solids remained intact at the end of cultivation in the hemicellulase pretreatment. This can also contain the sucrose that was not taken up by the fungus. In contrast, in the case of the invertase pretreatment, only around 26% of the soluble solids remained intact at the end of cultivation, which is an indication of the successful sugar uptake by the fungus after sucrose hydrolysis.
To follow the fungal growth and nutrient consumption during the cultivation, samples were collected from the bioreactor and the concentration of glucose, sugar mix, and ethanol was measured using HPLC (Figure 2). The graphs illustrate that the glucose content of LFCP after pectinase treatment was less than the value obtained after pretreatment with other enzymes, since the main function of pectinase is pectin hydrolysis. Higher glucose concentration after the hemicellulase and invertase pretreatments is an indication of the respective hydrolysis of hemicellulase and sucrose and consequent sugar release. The treatment with invertase resulted in the release of 8.7 ± 0.6 and 10 ± 0.7 g/L glucose and sugar mix, respectively, due to the sucrose hydrolysis. The sugar mix represents monomeric sugars other than glucose (e.g., xylose, fructose, galactose, arabinose).
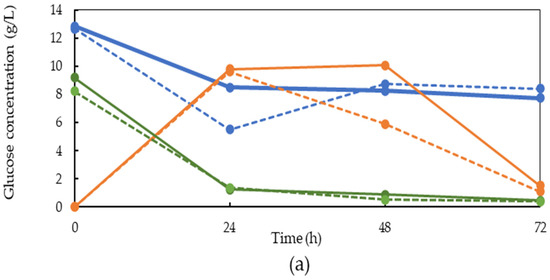
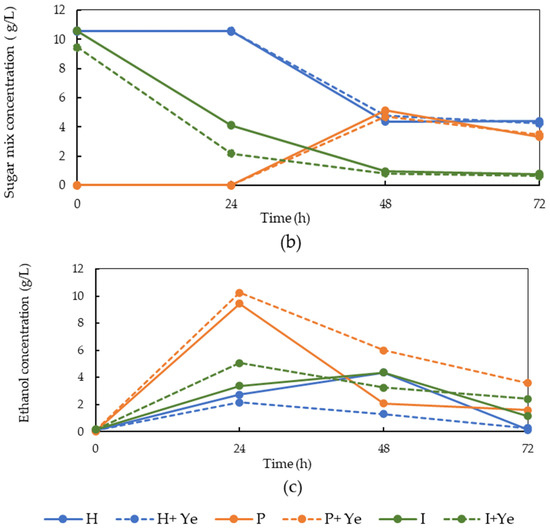
Figure 2.
Glucose, sugar mix, and ethanol concentration profiles during fungal cultivation on LFCP obtained after the hemicellulase (H), pectinase (P), and invertase (I) pretreatments in the absence and presence of the yeast extract (Ye) in a 4.5 L bench-scale bubble column bioreactor during 72 h. (a) Glucose concentration (b) ethanol concentration, and (c) sugar mix concentration. The standard deviations were 0.01–2.3%.
During the cultivation of the fungus in the LFCP obtained after the invertase treatment, a major fraction of the released sugars was consumed by the fungus within 24 h and the glucose and sugar mix concentrations reached 1.3 and 4.0 g/L, respectively. Prolonging the cultivation time to 72 h resulted in further sugar depletion, and the final glucose and sugar mix concentrations were 0.4 g/L and 0.6 g/L, respectively. The glucose profile was not significantly different for this pretreatment in the presence or absence of the yeast extract. Ethanol was formed as the main metabolite during the fungal growth after the invertase pretreatment. In the absence of the yeast extract, the ethanol concentration was increased to 4.3 g/L within 48 h. A reduction was observed in the ethanol concentration during the third day of cultivation when the final ethanol concentration was 1.1 g/L. The reduction in ethanol concentration may be due to the consumption of the ethanol by the fungus as well as ethanol evaporation. Supplementation of LFCP with the yeast extract after the invertase pretreatment increased the rate of ethanol production and the ethanol peak was already observed at 24 h (5.05 g/L). The ethanol concentration, however, decreased during the second and third day of cultivation. This may be an indication of enhancement in the fungal cell activity in the presence of growth factors provided by the yeast extract.
The glucose and sugar mix concentrations were 12.6 and 10.5 g/L, respectively, at the beginning of the fungal cultivation in LFCP obtained from the hemicellulase pretreatment. Glucose consumption was faster during the first 24 h in the presence of the yeast extract and reached 5.5 g/L (compared to 8.5 g/L in the absence of yeast extract). This indicates a positive effect of the yeast extract on the fungal activity. The yeast extract provided growth factors and nitrogen to the substrate, which improved the fungal growth. In the presence of the yeast extract, the glucose concentration was increased to 8.7 g/L at 48 h. Increase in the glucose concentration was due to the hydrolysis effect of fungal enzymes on the soluble carbohydrates available in the LFCP. A similar pattern was reported for the growth of the fungus in bread [14]. The fungus, however, was unable to consume a major fraction of the glucose during the third day. This might be due to the depletion of other nutrients necessary for fungal growth such as nitrogen, or the need for longer cultivation time. In the absence of the yeast extract, the glucose concentration was not increased. Furthermore, the fungus did not consume the available glucose during the second and third day of cultivation. This might also be due to the lack of other nutrients such as nitrogen components in the medium. The sugar mix concentration did not change within the first 24 h, but it decreased during the second day of cultivation and remained constant during the third day. This indicates that glucose is a preferred sugar for the fungus compared to other types of sugars such as fructose and pentoses that were available in the sugar mix. A similar phenomenon was reported by Sues et al. [23]. The concentration for the sugar mix decreased to 10.5 and 4.4 g/L after 24 and 48 h, respectively. Adding the yeast extract did not have any effect on the sugar mix consumption. The highest ethanol concentration was obtained in the absence of the yeast extract at 48 h (4.4 g/L).
The glucose and sugar mix profiles obtained after the pectinase pretreatment were different to those obtained after the pretreatments with the other enzymes. While the glucose and sugar mix concentrations were nearly zero at the beginning of the cultivation, the glucose concentration reached 9.6 g/L within 24 h of cultivation. The sugar mix concentration was zero until 24 h and increased to 5.13 g/L after 48 h. This was due to the hydrolysis of the soluble carbohydrates by the fungal enzymes. The fungus was able to consume nearly all the glucose during the second and third days. The sugar mix concentration was reduced to 3.3 g/l during the third day of cultivation. Glucose consumption was quicker in the presence of the yeast extract, which is an indication of higher cell activity. For this pretreatment, the ethanol concentration passed a peak at 24 h and reached 10.2 and 9.4 g/L in the presence and absence of the yeast extract, respectively. The ethanol concentration was reduced during the second and third days of cultivation. Some previous studies have been conducted on the cultivation of fungi in carrot waste for bioethanol production [9,24,25,26]. In the previous investigations, the effect of different nitrogen sources on the bioethanol production by S. cerevisiae was conducted using 120 g/L CP at pH 6 and 72 h, and 6.9 g/L of bioethanol and 0.2 g/L of yeast cells were obtained in the presence of (NH4)2SO4 as the nitrogen source [27].
The morphology of the fungal biomass grown in the bioreactor is shown in Figure 3 where a branched microfibrillar structure is visible. The diameter of the microfibers was between 6 and 10 µm. This is similar to the findings of Wijayarathna et al., who reported an average diameter of hyphae of around 6.9 ± 0.9 µm when R. delemar was cultivated on bread waste [13]. In another study performed on bread waste, the diameter of the mycelium of the same strain was reported to be 4–8 µm [15].

Figure 3.
(a–c) Fungal cultivation in bubble column bioreactors using LFCP after the hemicellulase, pectinase, and invertase pretreatments, respectively; (d) harvested biomass from the hemicellulase (top), pectinase (middle), and invertase (bottom) pretreatments; (e) fungal mycelium obtained after the hemicellulase pretreatment (image was captured by oCelloScope).
The fungal strain used in this study belongs to the family of zygomycetes. The cell wall of the fungus, grown on bread, was analyzed in a previous study [28]. The cell wall contained 36% glucosamine and 23% N-acetyl glucosamine, representing chitosan and chitin, respectively. These biopolymers have valuable properties such as biocompatibility, biodegradability, non-toxicity, and antibacterial effect. For the fungus grown on bread waste, the protein concentration of the fungal biomass was 35% and eight of the nine essential amino acids were recognized in the fungal biomass. The fat value of the biomass was recorded at 15.9% and palmitic acid, linoleic acid, and stearic acid were the major fatty acids in this biomass [14]. It is expected that the fungal biomass prepared from CP in this study will have a comparable profile of chitin, chitosan, amino acids, and fatty acids.
3.4. Thermal Characterization of CP and SFCP after the Enzymatic Pretreatments
The thermal degradation behavior of SFCP after each enzymatic treatment was studied by thermogravimetric (TG) and derivative thermogravimetric (DTG) analyses (Figure 4). Thermal degradation occurred in several steps due to the presence of different components in the CP and SFCP. The initial decrease in weight in Figure 4a can be attributed to the evaporation of the moisture. In Figure 4b, hemicellulose decomposition can be observed in the region of 150–300 °C [29]. Cellulose degradation was observed in the temperature region of 300–340 °C. The thermal degradation in this region was more prominent in the solid residue of CP after the enzymatic pretreatments (SFCP). This confirms that the pretreatment increased the amount of cellulose in the carrot residue [30]. However, further purification steps are needed to purify cellulose from SFCP. Furthermore, Figure 4a,b shows that the enzymatically treated CP exhibited better thermal stability compared to CP.
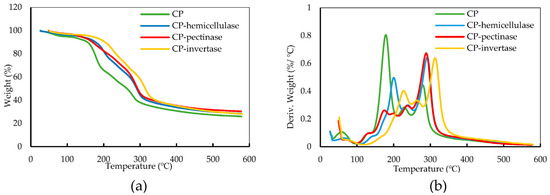
Figure 4.
(a) Thermogravimetric analysis and (b) derivative thermogravimetric analysis of CP and the solid residue obtained after the enzymatic pretreatment.
3.5. Chemical Composition Analyzed by Fourier Transform Infrared Spectroscopy
FTIR spectroscopy was utilized to study the chemical composition of CP and its derivatives after the enzymatic pretreatments. According to Figure 5, a broad peak between 3500 and 3000 cm−1 was observed, which was associated with the stretching of –OH groups and the intensity of this peak was higher in CP. Moreover, the peaks around 2800–3000 cm−1 were dedicated to the –CH stretching vibrations of the CH, CH2, and CH3 groups, which were present in the cellulose, hemicellulose, and lignin structures. The intensity in this region was lower in the pretreated CP, which indicates that some parts of the products were solubilized and removed from CP during the pretreatments. C–O stretching at 1733 cm−1 was found in the CP and SFCPs. This peak is related to the stretching of the carbonyl groups, which could be present in the different PC components (e.g., lignin, pectin, and hemicellulose), and the intensity was approximately the same for all specimens [7]. The peak 1607 cm−1 was related to the stretching vibration of the carbonyl group of the carboxylate ion (COO–), corresponding to the hemicellulose, and especially the pectin structure. Decreasing the intensity of this peak compared to CP showed that the extraction of pectin occurred during all pretreatments. The vibration peaks at 1367 cm−1 were related to cellulose as reported by Sucheta et al. [31]. The intensity of the peaks in the region 900–800 cm−1 connected to the aromatic compound vibrations was higher for CP compared to the enzymatically treated CP [32]. Cellulose can be further purified from the residue of the CP through a combination of hot water treatment (at 85 °C), sodium hydroxide pretreatment (at 80 °C), and bleaching with NaClO2, according to Berglund et al. [5].
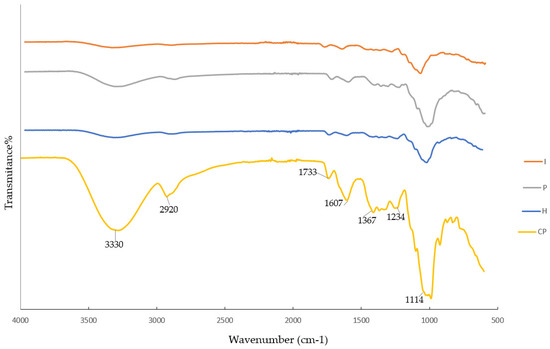
Figure 5.
Fourier transform infrared spectroscopy of carrot pomace (CP) and its derivatives after hemicellulase (H), pectinase (P), and invertase (I) pretreatments.
4. Conclusions
A sustainable process was successfully developed to valorize carrot pomace (CP). The CP was subjected to various enzymatic pretreatments to release its nutrients, which were then utilized as a substrate for fungal cultivation. Moreover, pretreatments did not negatively influence the possibility for cellulose recovery and purification. Fungal cultivation resulted in a fiber- and protein-rich fungal biomass. Simultaneously, cellulose was recovered in the insoluble fractions of CP after the pretreatments. Among the different enzymatic pretreatments, the invertase pretreatment resulted in the highest fungal biomass production in bench-scale bioreactors. The produced fungal biomass can be used for food and feed application. Furthermore, the presence of chitin and chitosan in the fungal biomass makes it a good candidate for biomaterial development. The recovered cellulose fraction of the CP can be further purified and used for the development of cellulose-based materials.
Author Contributions
Conceptualization, A.Z. and S.N.M.; Methodology, A.Z. and S.N.M.; Software, S.N.M.; Formal analysis, S.N.M.; Investigation, A.Z. and S.N.M.; Data curation, S.N.M.; Writing—original draft preparation, S.N.M.; Writing—review and editing, A.Z., M.H., A.M.S., S.K.R. and M.P.; Visualization, A.Z. and S.N.M.; Supervision, A.Z.; Project administration, A.Z.; Funding acquisition, A.Z. and A.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the ÅForsk Foundation, grant number 21-78.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, C.; Wu, H.; Cai, M.; Li, Y.; Guo, C.; Han, Y.; Zhang, Y.; Song, B. Valorization of food waste digestate to ash and biochar composites for high performance adsorption of methylene blue. J. Clean. Prod. 2023, 397, 136612. [Google Scholar] [CrossRef]
- Ibarruri, J.; Hernández, I. Valorization of cheese whey and orange molasses for fungal biomass production by submerged fermentation with Rhizopus sp. Bioprocess Biosyst. Eng. 2019, 42, 1285–1300. [Google Scholar] [CrossRef]
- Rovai, D.; Ortgies, M.; Amin, S.; Kuwahara, S.; Schwartz, G.; Lesniauskas, R.; Garza, J.; Lammert, A. Utilization of Carrot Pomace to Grow Mealworm Larvae (Tenebrio molitor). Sustainability 2021, 13, 9341. [Google Scholar] [CrossRef]
- Amoroso, L.; De France, K.J.; Milz, C.I.; Siqueira, G.; Zimmermann, T.; Nyström, G. Sustainable Cellulose Nanofiber Films from Carrot Pomace as Sprayable Coatings for Food Packaging Applications. ACS Sustain. Chem. Eng. 2021, 10, 342–352. [Google Scholar] [CrossRef]
- Berglund, L.; Noël, M.; Aitomäki, Y.; Öman, T.; Oksman, K. Production potential of cellulose nanofibers from industrial residues: Efficiency and nanofiber characteristics. Ind. Crops Prod. 2016, 92, 84–92. [Google Scholar] [CrossRef]
- Zambelli, R.A.; Pontes, B.C.V.; Pontes, E.R.; Silva, M.L.; Junior, E.C.S.; Pinto, L.I.F.; Melo, C.A.L.; Farias, M.M.; da Costa, C.S.; da Silva, A.C. Broccoli and carrot industrial solid waste characterization and application in the bread food matrix. Int. J. Nutr. Food Sci. 2017, 6, 9–15. [Google Scholar] [CrossRef]
- Ramos-Andrés, M.; Díaz-Cesteros, S.; Majithia, N.; García-Serna, J. Pilot-scale biorefinery for the production of purified biopolymers based on hydrothermal treatment in flow-through reactor cycles. Chem. Eng. J. 2022, 437, 135123. [Google Scholar] [CrossRef]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple pomace as a functional and healthy ingredient in food products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Jiang, B.-H.; Duan, K.-J. Production of bioethanol from carrot pomace using the thermotolerant yeast Kluyveromyces marxianus. Energies 2013, 6, 1794–1801. [Google Scholar] [CrossRef]
- Roukas, T.; Kotzekidou, P. From food industry wastes to second generation bioethanol: A review. Rev. Environ. Sci. Biotechnol. 2022, 21, 299–329. [Google Scholar] [CrossRef]
- Singh, R.S.; Chauhan, K.; Singh, J.; Pandey, A.; Larroche, C. Solid-state fermentation of carrot pomace for the production of inulinase by Penicillium oxalicum BGPUP-4. Food Technol. Biotechnol. 2018, 56, 31–39. [Google Scholar] [CrossRef]
- Çakır, Z.B.; Yılmaz, H.; Ertan, F.; Tanrıseven, A.; Özkan, M. Carrot pomace alone supports heterotrophic growth and lipid production of Auxenochlorella protothecoides. Biomass Convers. Biorefin. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Wijayarathna, E.R.K.B.; Mohammadkhani, G.; Soufiani, A.M.; Adolfsson, K.H.; Ferreira, J.A.; Hakkarainen, M.; Berglund, L.; Heinmaa, I.; Root, A.; Zamani, A. Fungal textile alternatives from bread waste with leather-like properties. Resour. Conserv. Recycl. 2022, 179, 106041. [Google Scholar] [CrossRef]
- Svensson, S.E.; Bucuricova, L.; Ferreira, J.A.; Souza Filho, P.F.; Taherzadeh, M.J.; Zamani, A. Valorization of bread waste to a fiber-and protein-rich fungal biomass. Fermentation 2021, 7, 91. [Google Scholar] [CrossRef]
- Köhnlein, M.B.M.; Abitbol, T.; Oliveira, A.O.; Magnusson, M.S.; Adolfsson, K.H.; Svensson, S.E.; Ferreira, J.A.; Hakkarainen, M.; Zamani, A. Bioconversion of food waste to biocompatible wet-laid fungal films. Mater. Des. 2022, 216, 110534. [Google Scholar] [CrossRef]
- Wikandari, R.; Millati, R.; Lennartsson, P.R.; Harmayani, E.; Taherzadeh, M.J. Isolation and characterization of zygomycetes fungi from tempe for ethanol production and biomass applications. Biotechnol. Appl. Biochem. 2012, 167, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M.J. Starch and protein recovery from brewer’s spent grain using hydrothermal pretreatment and their conversion to edible filamentous fungi—A brewery biorefinery concept. Bioresour. Technol. 2021, 337, 125409. [Google Scholar] [CrossRef]
- Chojnacki, J.; Zdanowicz, A.; Ondruška, J.; Šooš, Ľ.; Smuga-Kogut, M. The Influence of Apple, Carrot and Red Beet Pomace Content on the Properties of Pellet from Barley Straw. Energies 2021, 14, 405. [Google Scholar] [CrossRef]
- Ramos-Andrés, M.; Aguilera-Torre, B.; García-Serna, J. Hydrothermal production of high-molecular weight hemicellulose-pectin, free sugars and residual cellulose pulp from discarded carrots. J. Clean. Prod. 2021, 290, 125179. [Google Scholar] [CrossRef]
- de Vrije, T.; Budde, M.A.W.; Lips, S.J.; Bakker, R.R.; Mars, A.E.; Claassen, P.A.M. Hydrogen production from carrot pulp by the extreme thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int. J. Hydrogen Energy 2010, 35, 13206–13213. [Google Scholar] [CrossRef]
- Souza Filho, P.F.; Zamani, A.; Taherzadeh, M.J. Production of edible fungi from potato protein liquor (PPL) in airlift bioreactor. Fermentation 2017, 3, 12. [Google Scholar] [CrossRef]
- Miao, L.; Kwong, T.F.N.; Qian, P.-Y. Effect of culture conditions on mycelial growth, antibacterial activity, and metabolite profiles of the marine-derived fungus Arthrinium c.f. saccharicola. Appl. Microbiol. Biotechnol. 2006, 72, 1063–1073. [Google Scholar] [CrossRef]
- Sues, A.; Millati, R.; Edebo, L.; Taherzadeh, M.J. Ethanol production from hexoses, pentoses, and dilute-acid hydrolyzate by Mucor indicus. FEMS Yeast Res. 2005, 5, 669–676. [Google Scholar] [CrossRef]
- Yesmin, M.N.; Azad, M.A.K.; Kamruzzaman, M.; Uddin, M.N. Bioethanol Production from Corn, Pumpkin and Carrot of Bangladesh as Renewable Source using Yeast. Acta Chem. Malays. 2020, 4, 45–54. [Google Scholar] [CrossRef]
- Aimaretti, N.R.; Ybalo, C.V.; Rojas, M.L.; Plou, F.J.; Yori, J.C. Production of bioethanol from carrot discards. Bioresour. Technol. 2012, 123, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Clementz, A.; Torresi, P.A.; Molli, J.S.; Cardell, D.; Mammarella, E.; Yori, J.C. Novel method for valorization of by-products from carrot discards. LWT 2019, 100, 374–380. [Google Scholar] [CrossRef]
- Demiray, E.; Karatay, S.E.; Dönmez, S.; Dönmez, G. The usage of carrot pomace for bioethanol production. J. Chil. Chem. 2016, 61, 2996–2998. [Google Scholar] [CrossRef]
- Svensson, S.E.; Ferreira, J.A.; Hakkarainen, M.; Adolfsson, K.H.; Zamani, A. Fungal textiles: Wet spinning of fungal microfibers to produce monofilament yarns. Sustain. Mater. Technol. 2021, 28, e00256. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Parthasarathy, P.; Mackey, H.R.; Al-Ansari, T.; Elhassan, O.; Mansour, S.; McKay, G. Biochar development from thermal TGA studies of individual food waste vegetables and their blended systems. Biomass Convers. Biorefin. 2022, 12, 1–18. [Google Scholar] [CrossRef]
- Varanasi, S.; Henzel, L.; Sharman, S.; Batchelor, W.; Garnier, G. Producing nanofibres from carrots with a chemical-free process. Carbohydr. Polym. 2018, 184, 307–314. [Google Scholar] [CrossRef]
- Sucheta; Chaturvedi, K.; Yadav, S.K. Ultrasonication assisted salt-spices impregnation in black carrots to attain anthocyanins stability, quality retention and antimicrobial efficacy on hot-air convective drying. Carbohydr. Polym. 2019, 58, 104661. [Google Scholar] [CrossRef] [PubMed]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).