Enhancement of Enzymatic Hydrolysis of Sugarcane Bagasse by the Combination of Delignification Pretreatment and Polysorbate 80
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment and Enzymatic Hydrolysis
2.3. Analytical Methods
2.4. Characterization of Native and Pretreated Material
3. Results and Discussion
3.1. Compositional Analysis of Pretreated Substrates
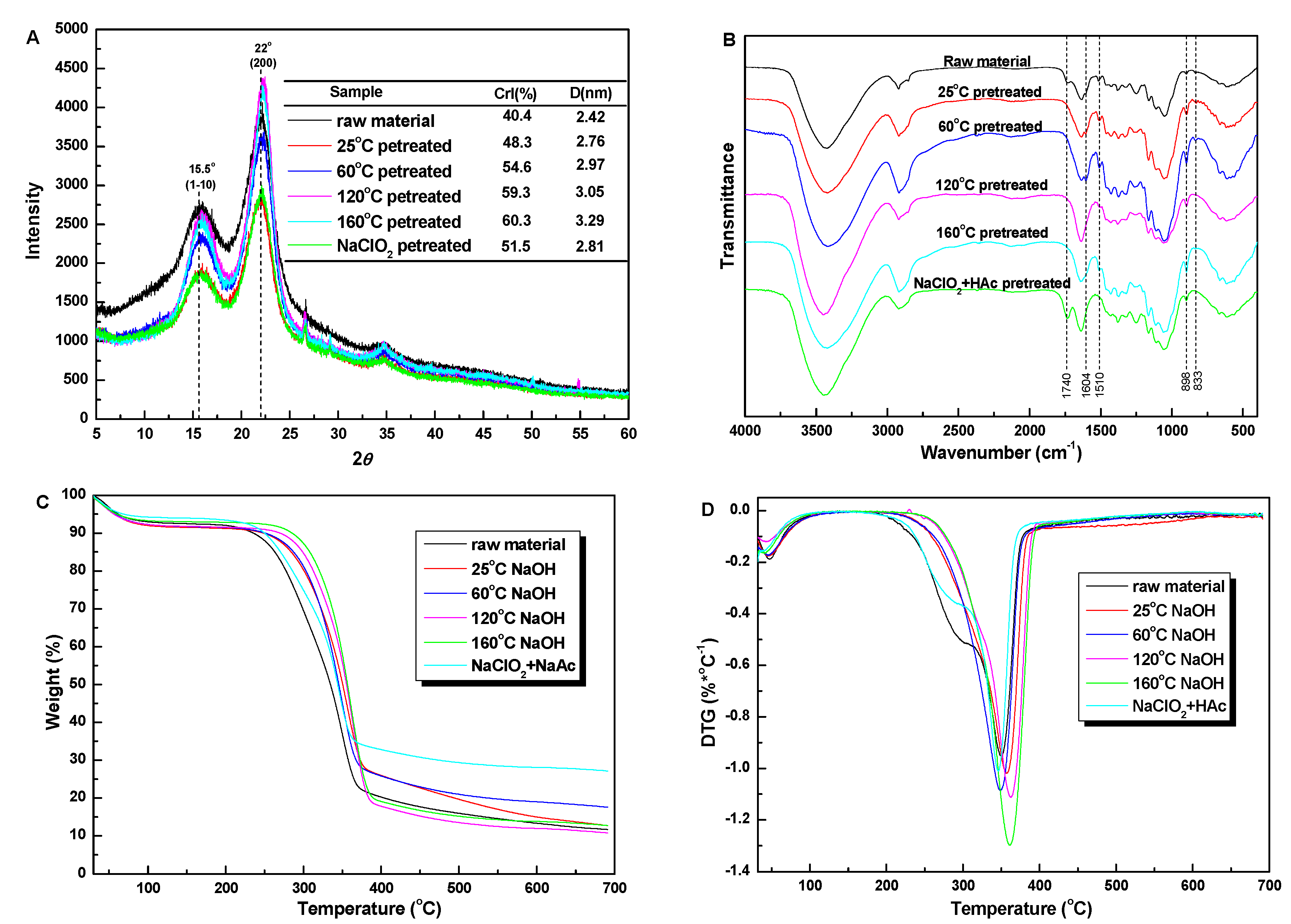
3.2. Characterization of Untreated and Pretreated Sugarcane Bagasse
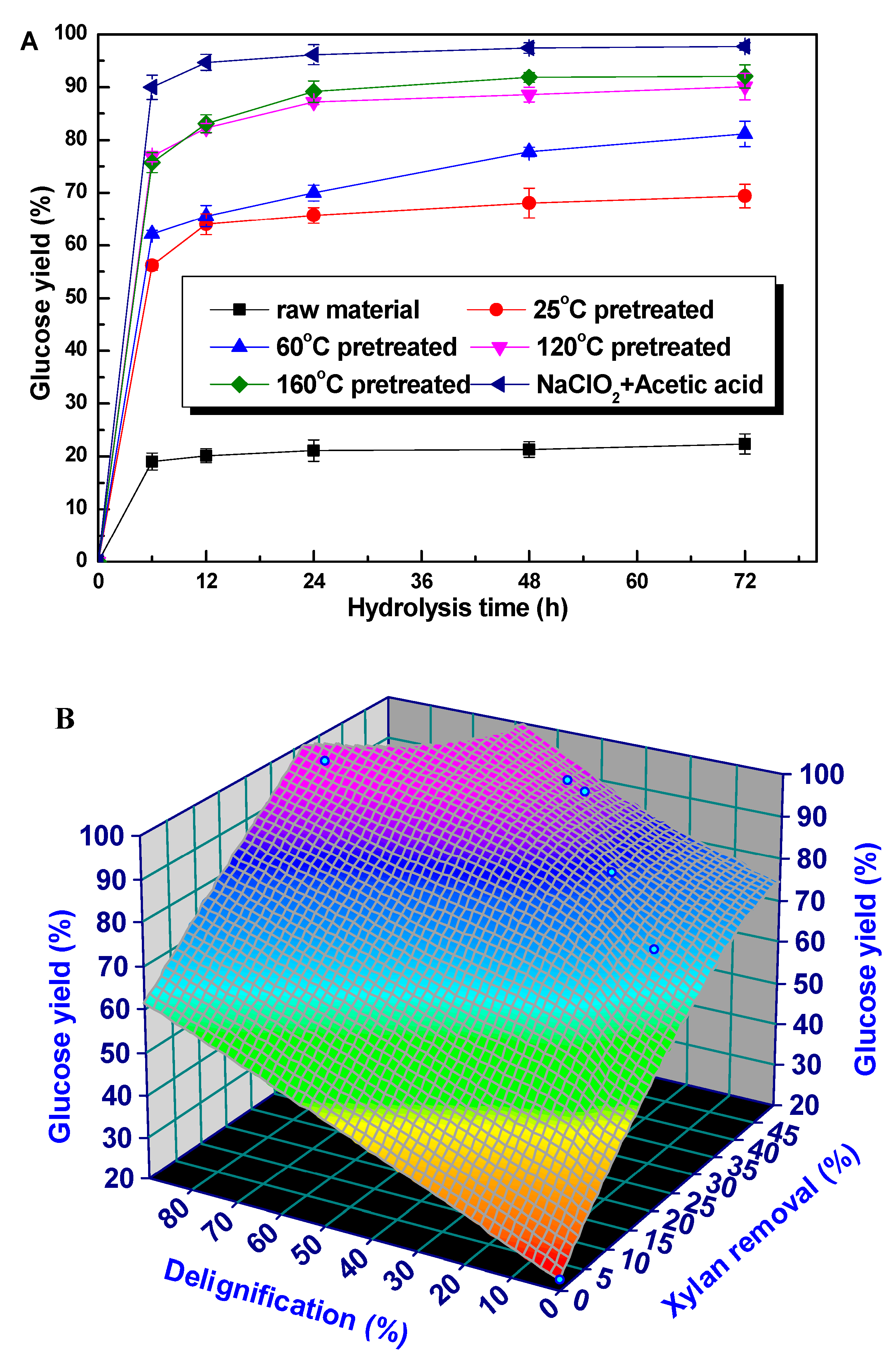
3.3. The Enzymatic Hydrolysis of Retained Cellulose
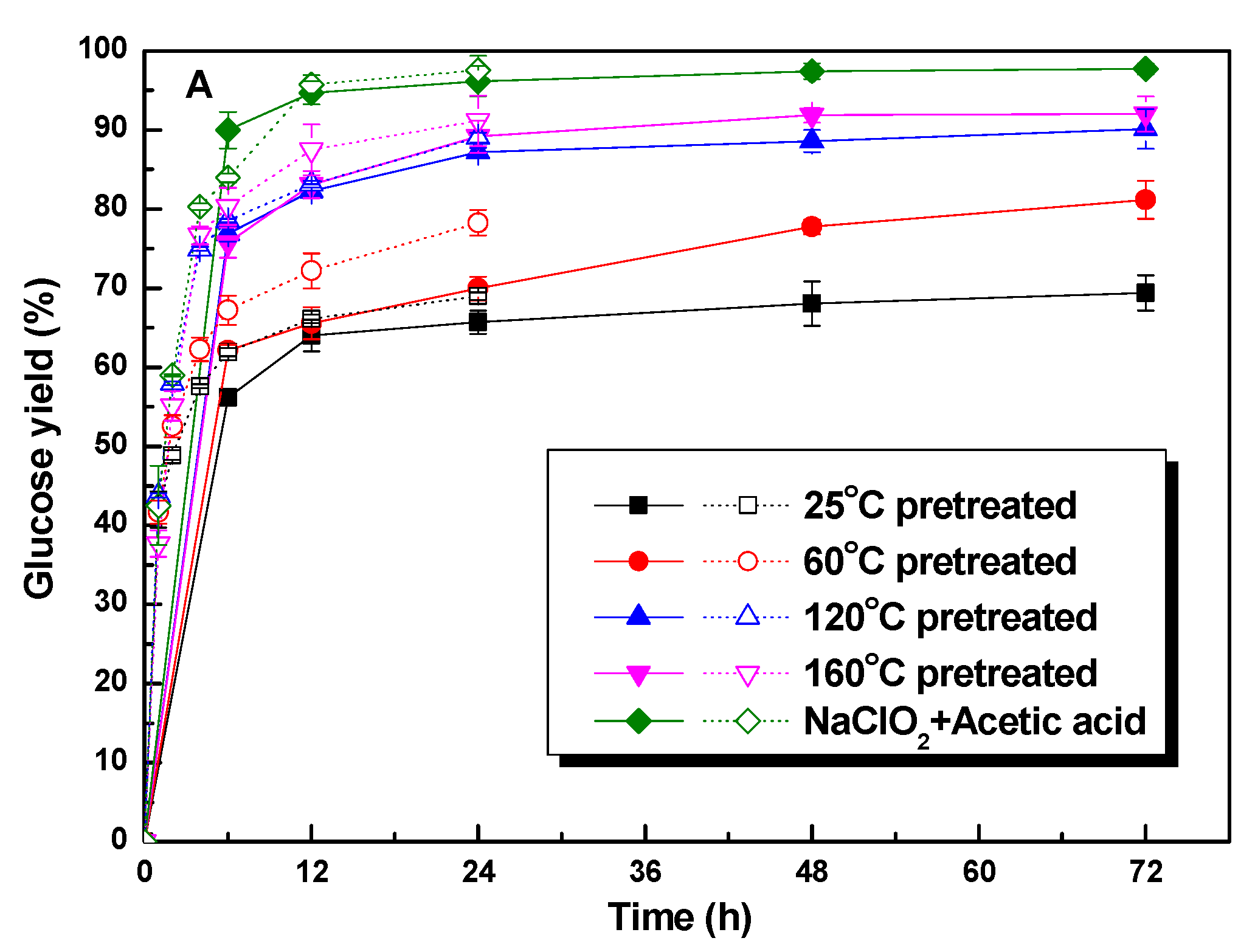
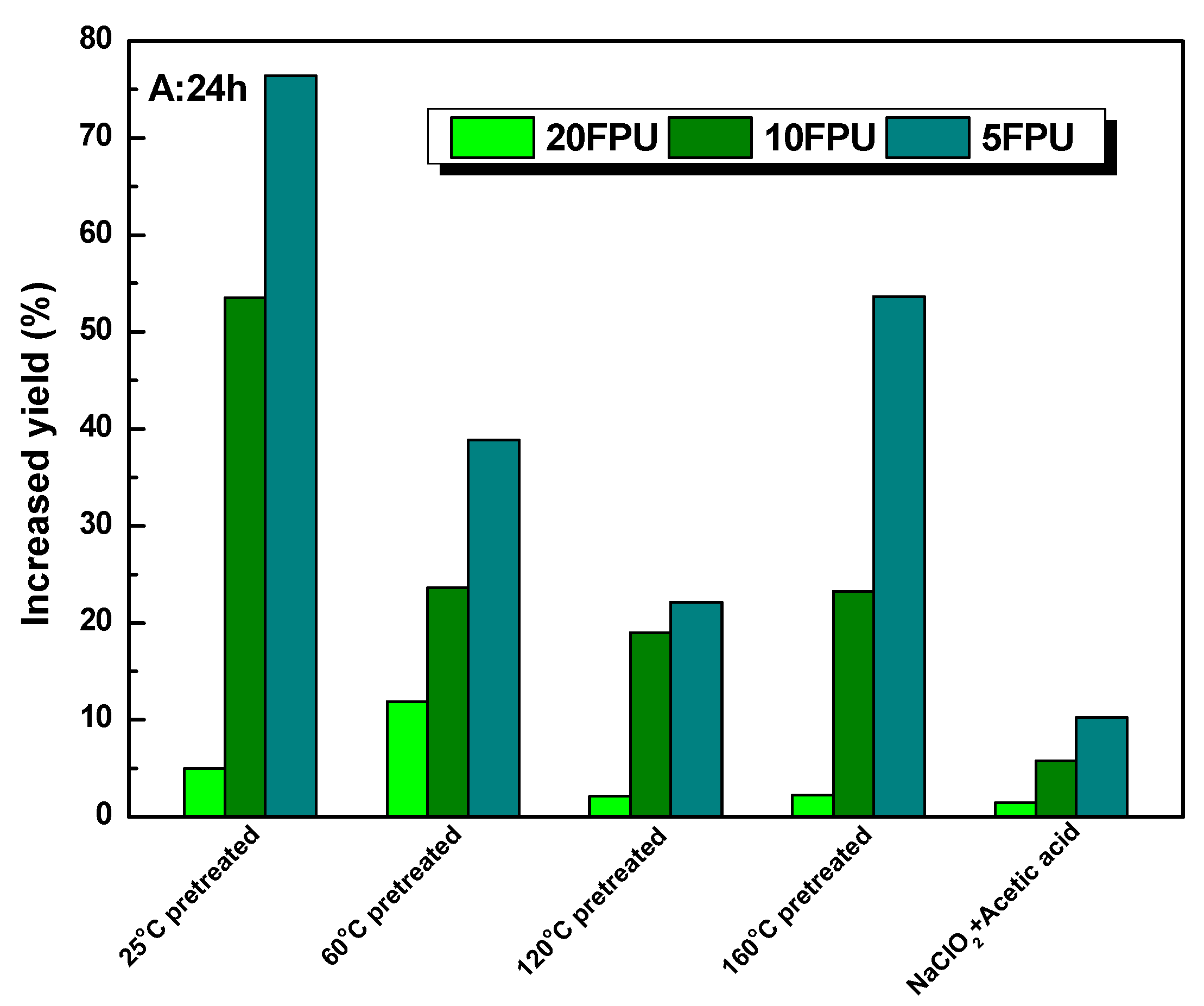
3.4. The Enhancement of Polysorbate 80 on the Glucose Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, A.K.; Al Makishah, N.H.; Wen, Z.Q.; Gupta, G.; Pandit, S.; Prasad, R. Recent developments in lignocellulosic biofuels, a renewable source of bioenergy. Fermentation 2022, 8, 161. [Google Scholar] [CrossRef]
- Vieira, S.; Barros, M.V.; Sydney, A.C.N.; Piekarski, C.M.; de Francisco, A.C.; Vandenberghe, L.P.D.; Sydney, E.B. Sustainability of sugarcane lignocellulosic biomass pretreatment for the production of bioethanol. Bioresour. Technol. 2020, 299, 122635. [Google Scholar] [CrossRef] [PubMed]
- Dimos, K.; Paschos, T.; Louloudi, A.; Kalogiannis, K.G.; Lappas, A.A.; Papayannakos, N.; Kekos, D.; Mamma, D. Effect of various pretreatment methods on bioethanol production from cotton stalks. Fermentation 2019, 5, 5. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Xu, J.L.; Zhang, Y.; Liang, C.Y.; He, M.C.; Yuan, Z.H.; Xie, J. Reinforced alkali-pretreatment for enhancing enzymatic hydrolysis of sugarcane bagasse. Fuel Process. Technol. 2016, 143, 1–6. [Google Scholar] [CrossRef]
- Xu, H.F.; Li, B.; Mu, X.D. Review of alkali-based pretreatment to enhance enzymatic saccharification for lignocellulosic biomass conversion. Ind. Eng. Chem. Res. 2016, 55, 8691–8705. [Google Scholar] [CrossRef]
- Chen, X.W.; Kuhn, E.; Jennings, E.W.; Nelson, R.; Tao, L.; Zhang, M.; Tucker, M.P. DMR (deacetylation and mechanical refining) processing of corn stover achieves high monomeric sugar concentrations (230 g L−1) during enzymatic hydrolysis and high ethanol concentrations (>10% v/v) during fermentation without hydrolysate purification or concentration. Energy Environ. Sci. 2016, 9, 1237–1245. [Google Scholar]
- Lai, C.H.; Tang, S.; Yang, B.; Gao, Z.Q.; Li, X.; Yong, Q. Enhanced enzymatic saccharification of corn stover by in situ modification of lignin with poly (ethylene glycol) ether during low temperature alkali pretreatment. Bioresour. Technol. 2017, 244, 92–99. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Chang, S.; Zhang, L.; Mao, Y.; Cui, T. Bioethanol production using the sodium hydroxide pretreated sweet sorghum bagasse without washing. Fuel 2016, 175, 20–25. [Google Scholar] [CrossRef]
- Isaac, A.; de Paula, J.; Viana, C.M.; Henriques, A.B.; Malachias, A.; Montoro, L.A. From nano- to micrometer scale: The role of microwave-assisted acid and alkali pretreatments in the sugarcane biomass structure. Biotechnol. Biofuels 2018, 11, 73. [Google Scholar] [CrossRef]
- Mohapatra, S.; Pattathil, S.; Thatoi, H. Structural and functional characterization of two pennisetum sp. biomass during ultrasono-assisted alkali pretreatment and enzymatic hydrolysis for understanding the mechanism of targeted delignification and enhanced saccharification. ACS Sustain. Chem. Eng. 2017, 5, 6486–6497. [Google Scholar] [CrossRef]
- Kumar, L.; Arantes, V.; Chandra, R.; Saddler, J. The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour. Technol. 2012, 103, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ye, G.; Wei, Y.; Li, X.; Zhang, A.; Xie, J. Enhanced enzymatic hydrolysis of sugarcane bagasse with ferric chloride pretreatment and surfactant. Bioresour. Technol. 2017, 229, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, C.W.; Wang, Y.T.; Zhang, H.J. Investigation on mechanism of intensifying coal fly ash froth flotation by pretreatment of non-ionic surfactant. Fuel 2019, 254, 115601. [Google Scholar] [CrossRef]
- Chen, Y.A.; Zhou, Y.; Qin, Y.; Liu, D.; Zhao, X. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: Pretreatment, hydrolysis conditions and ligninstructure. Bioresour. Technol. 2018, 269, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Martin, J.; Martinez-Bernal, C.; Perez-Cobas, Y.; Reyes-Sosa, F.M.; Garcia, B.D. Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2017, 244, 48–56. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, M.; Li, X.; Zhang, A.; Xie, J. Enhancing enzymatic hydrolysis of sugarcane bagasse by ferric chloride catalyzed organosolv pretreatment and Tween 80. Bioresour. Technol. 2018, 258, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Natl. Renew. Energy Lab. 2008, 1617, 1–16. [Google Scholar]
- Siqueira, G.; Varnai, A.; Ferraz, A.; Milagres, A.M.F. Enhancement of cellulose hydrolysis in sugarcane bagasse by the selective removal of lignin with sodium chlorite. Appl. Energy 2013, 102, 399–402. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Yue, W.; Wang, W.; Wang, Y.; Yuan, T.Q.; Sun, R.C. Valorization of lignin and cellulose in acid-steam-exploded corn stover by a moderate alkaline ethanol post-treatment based on an integrated biorefinery concept. Biotechnol. Biofuels 2016, 9, 238. [Google Scholar] [CrossRef]
- Patri, A.S.; Mostofian, B.; Pu, Y.Q.; Ciaffone, N.; Soliman, M.; Smith, M.D.; Kumar, R.; Cheng, X.L.; Wyrnan, C.E.; Tetard, L.; et al. A multifunctional cosolvent pair reveals molecular principles of biomass deconstruction. J. Am. Chem. Soc. 2019, 141, 12545–12557. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Yuan, H.; Lyu, G.; Xie, J. FeCl3-catalyzed ethanol pretreatment of sugarcane bagasse boosts sugar yields with low enzyme loadings and short hydrolysis time. Bioresour. Technol. 2018, 249, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, L.; Martin, A.E. An empirical method forestimating the degree of crystallinity of native celluloseusing X-ray diffractometer. Text Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Zhang, H.D.; Wu, S.B. Efficient sugar release by acetic acid ethanol-based organosolv pretreatment and enzymatic saccharification. J. Agric. Food Chem. 2014, 62, 11681–11687. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.S.; Rabelo, S.C.; Ciesielski, P.N.; Roberto, I.C.; Rocha, G.J.M.; Driemeier, C. Multiscale alterations in sugar cane bagasse and straw submitted to alkaline deacetylation. ACS Sustain. Chem. Eng. 2018, 6, 3796–3804. [Google Scholar] [CrossRef]
- Ying, W.J.; Fang, X.; Xu, Y.; Zhang, J.H. Combined acetic acid and enzymatic hydrolysis for xylooligosaccharides and monosaccharides production from poplar. Biomass Bioenerg. 2022, 158, 106377. [Google Scholar] [CrossRef]
- Yu, Z.P.; Du, Y.L.; Shang, X.N.; Zheng, Y.; Zhou, J.H. Enhancing fermentable sugar yield from cassava residue using a two-step dilute ultra-low acid pretreatment process. Ind. Crop. Prod. 2018, 124, 555–562. [Google Scholar] [CrossRef]
- Wang, N.; Yuan, W.J.; Han, X.T.; Bai, F.W.; Xu, Y.P. Effect of lignocellulose degradation products on ethanol fermentations using glucose and xylose by Kluyveromyces marxianus. Chin. J. Bioprocess. Eng. 2012, 10, 17–22. [Google Scholar]
- Fu, S.Y.; Hu, J.F.; Liu, H. Inhibitory effects of biomass degradation products on ethanol fermentation and a strategy to overcome them. Bioresources 2014, 9, 4323–4335. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Wang, H.S.; Li, B.; Liu, C.; Yu, G.; Mu, X.D. Comparison of different alkali-based pretreatments of corn stover for improving enzymatic saccharification. Bioresour. Technol. 2012, 1225, 193–199. [Google Scholar] [CrossRef]
- Sun, F.B.; Wang, L.; Hong, J.P.; Ren, J.L.; Du, F.G.; Hu, J.G.; Zhang, Z.Y.; Zhou, B.W. The impact of glycerol organosolv pretreatment on the chemistry and enzymatic hydrolyzability of wheat straw. Bioresour. Technol. 2015, 187, 354–361. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Merino, D.; Athanassiou, A. Alkaline hydrolysis of biomass as an alternative green method for bioplastics preparation: In situ cellulose nanofibrillation. Chem. Eng. J. 2023, 454, 140171. [Google Scholar] [CrossRef]
- French, A.D. Increment in evolution of cellulose crystallinity analysis. Cellulose 2020, 27, 5445–5448. [Google Scholar] [CrossRef]
- Gurgel, L.V.A.; Marabezi, K.; Ramos, L.A.; Curvelo, A.A.D.S. Characterization of depolymerized residues from extremely low acid hydrolysis (ELA) of sugarcane bagasse cellulose: Effects of degree of polymerization, crystallinity and crystallite size on thermal decomposition. Ind. Crop. Prod. 2012, 36, 560–571. [Google Scholar] [CrossRef]
- Ravindran, R.; Sarangapani, C.; Jaiswal, S.; Cullen, P.J.; Jaiswal, A.K. Ferric chloride assisted plasma pretreatment of lignocellulose. Bioresour. Technol. 2017, 243, 327–334. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, xylan and lignininteractions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Phitsuwan, P.; Sakka, K.; Ratanakhanokchai, K. Structural changes and enzymatic response of Napier grass (Pennisetum purpureum) stem induced by alkaline pretreatment. Bioresour. Technol. 2016, 218, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.; Lin, F.; Zhang, H.Y.; Xiao, R. Structural elucidation of industrial bioethanol residual lignin from corn stalk: A potential source of vinyl phenolics. Fuel Process. Technol. 2018, 169, 50–57. [Google Scholar] [CrossRef]
- Yuan, T.Q.; Wang, W.; Zhang, L.M.; Sun, R.C. Reconstitution of cellulose and lignin after [C2mim][OAc] pretreatment and its relation to enzymatic hydrolysis. Biotechnol. Bioeng. 2013, 110, 729–736. [Google Scholar] [CrossRef]
- Huang, C.X.; Li, R.L.; Tang, W.; Zheng, Y.Y.; Meng, X.Z. Improve enzymatic hydrolysis of lignocellulosic biomass by modifying lignin structure via sulfite pretreatment and using lignin blockers. Fermentation 2022, 8, 558. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Rollin, J.; Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.H.P. Increasing cellulose accessibility is more important than removinglignin: A comparison of cellulose solvent-based lignocellulosesfractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2011, 108, 22–30. [Google Scholar] [CrossRef]
- Kim, W.; Gamo, Y.; Sani, Y.M.; Wusiman, Y.; Ogawa, S.; Karita, S.; Goto, M. Effect of Tween 80 on hydrolytic activity and substrate accessibility of carbohydrolase I (CBH I) from Trichoderma viride. Asian Austral. J. Anim. 2006, 19, 684–689. [Google Scholar] [CrossRef]
- Wang, W.; Zhuang, X.S.; Tan, X.S.; Wang, Q.; Chen, X.Y.; Yu, Q.; Qi, W.; Wang, Z.M.; Yuan, Z.H. Dual effect of nonionic surfactants on improving the enzymatic hydrolysis of lignocellulose. Energy Fuel 2018, 32, 5951–5959. [Google Scholar] [CrossRef]
- Huang, C.X.; Jiang, X.; Shen, X.J.; Hu, J.G.; Tang, W.; Wu, X.X.; Ragauskas, A.; Jameel, H.; Meng, X.Z.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Hao, X.X.; Li, Y.F.; Wang, J.Y.; Qin, Y.J.; Zhang, J.H. Adsorption and desorption of cellulases on/from lignin-rich residues from corn stover. Ind. Crop. Prod. 2019, 139, 111559. [Google Scholar] [CrossRef]
- Zhang, H.D.; Huang, S.H.; Wei, W.Q.; Zhang, J.J.; Xie, J. Investigation of alkaline hydrogen peroxide pretreatment and Tween 80 to enhance enzymatic hydrolysis of sugarcane bagasse. Biotechnol. Biofuels 2019, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Soam, S.; Kapoor, M.; Kumar, R.; Gupta, R.P.; Puri, S.K.; Ramakumar, S.S.V. Life cycle assessment and life cycle costing of conventional and modified dilute acid pretreatment for fuel ethanol production from rice straw in India. J. Clean. Prod. 2018, 197, 732–741. [Google Scholar] [CrossRef]
- Tu, M.; Saddler, J.N. Potential enzyme cost reduction with the addition of surfactant during the hydrolysis of pretreated softwood. Appl. Biochem. Biotechnol. 2010, 161, 274–287. [Google Scholar] [CrossRef] [PubMed]



| Pretreatment Conditions | Solid Recovery | Glucan (%) | Xylan (%) | Lignin (%) | |||
|---|---|---|---|---|---|---|---|
| Content | Recovery | Content | Removal | Content | Removal | ||
| Raw material | 100 | 41.2 | -- | 20.2 | -- | 25.2 | -- |
| NaOH pretreatment | |||||||
| 25 °C, 12 h | 88.0 | 44.5 | 95.2 | 15.7 | 31.6 | 22.5 | 10.6 |
| 60 °C, 4 h | 77.7 | 49.8 | 94.1 | 16.6 | 36.1 | 19.1 | 24.2 |
| 120 °C, 2 h | 67.9 | 56.7 | 93.6 | 16.1 | 46.0 | 15.1 | 40.1 |
| 160 °C, 1 h | 62.7 | 61.9 | 94.4 | 17.5 | 45.7 | 14.2 | 43.7 |
| NaClO2/HAc pretreatment | |||||||
| 75 °C, 4 h | 76.1 | 53.1 | 98.2 | 18.2 | 31.3 | 3.9 | 84.6 |
| Pretreatment Conditions | Sugar Analysis (g/100 g Raw Material) | Inhibitors (g·L−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Glucose mono. | Glucose oligo. | Total Glucose | Xylose mono. | Xylose oligo. | Total xylose | Formic Acid | Acetic Acid | |
| 25 °C, 12 h | -- | 0.47 | 0.47 | 0.31 | 3.25 | 3.55 | 0.11 | 4.00 |
| 60 °C, 4 h | -- | 0.24 | 0.24 | 0.61 | 1.18 | 1.78 | 0.25 | 3.88 |
| 120 °C, 2 h | -- | 0.28 | 0.28 | 0.69 | 1.89 | 2.58 | 0.99 | 4.27 |
| 160 °C, 1 h | -- | 0.37 | 0.37 | 0.46 | 4.73 | 5.19 | 1.28 | 4.36 |
| 75 °C, 4 h | -- | 6.14 | 6.14 | -- | 1.11 | 1.11 | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Dai, T.; Huang, S.; Xie, J. Enhancement of Enzymatic Hydrolysis of Sugarcane Bagasse by the Combination of Delignification Pretreatment and Polysorbate 80. Fermentation 2023, 9, 371. https://doi.org/10.3390/fermentation9040371
Zhang H, Dai T, Huang S, Xie J. Enhancement of Enzymatic Hydrolysis of Sugarcane Bagasse by the Combination of Delignification Pretreatment and Polysorbate 80. Fermentation. 2023; 9(4):371. https://doi.org/10.3390/fermentation9040371
Chicago/Turabian StyleZhang, Hongdan, Tao Dai, Shihang Huang, and Jun Xie. 2023. "Enhancement of Enzymatic Hydrolysis of Sugarcane Bagasse by the Combination of Delignification Pretreatment and Polysorbate 80" Fermentation 9, no. 4: 371. https://doi.org/10.3390/fermentation9040371
APA StyleZhang, H., Dai, T., Huang, S., & Xie, J. (2023). Enhancement of Enzymatic Hydrolysis of Sugarcane Bagasse by the Combination of Delignification Pretreatment and Polysorbate 80. Fermentation, 9(4), 371. https://doi.org/10.3390/fermentation9040371





