Eco-Friendly Extraction of Green Coffee Oil for Industrial Applications: Its Antioxidant, Cytotoxic, Clonogenic, and Wound Healing Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Green Coffee Oil Extraction by Cold-Pressing
2.3. Spectroscopic Analysis
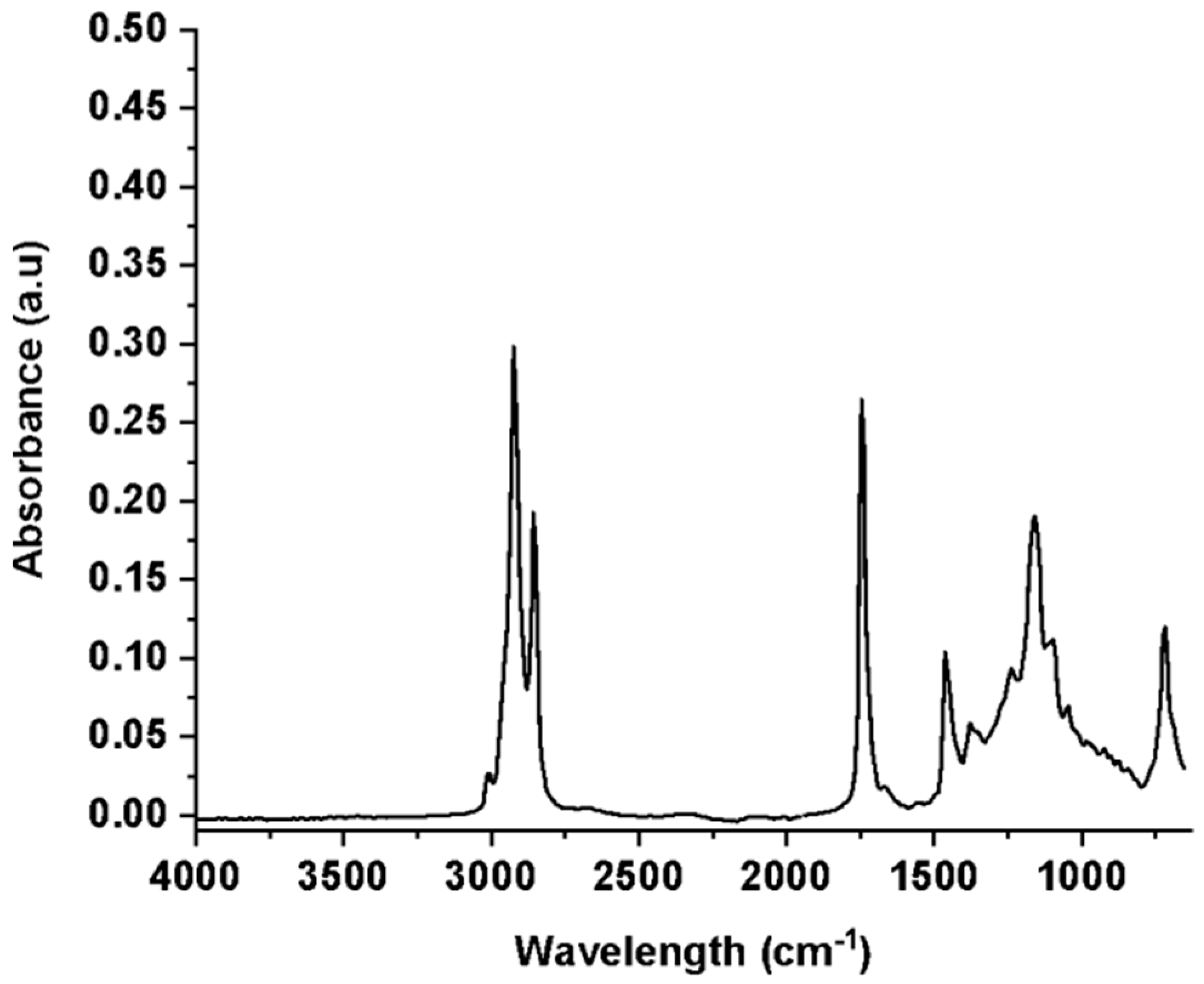
2.3.1. FT–IR
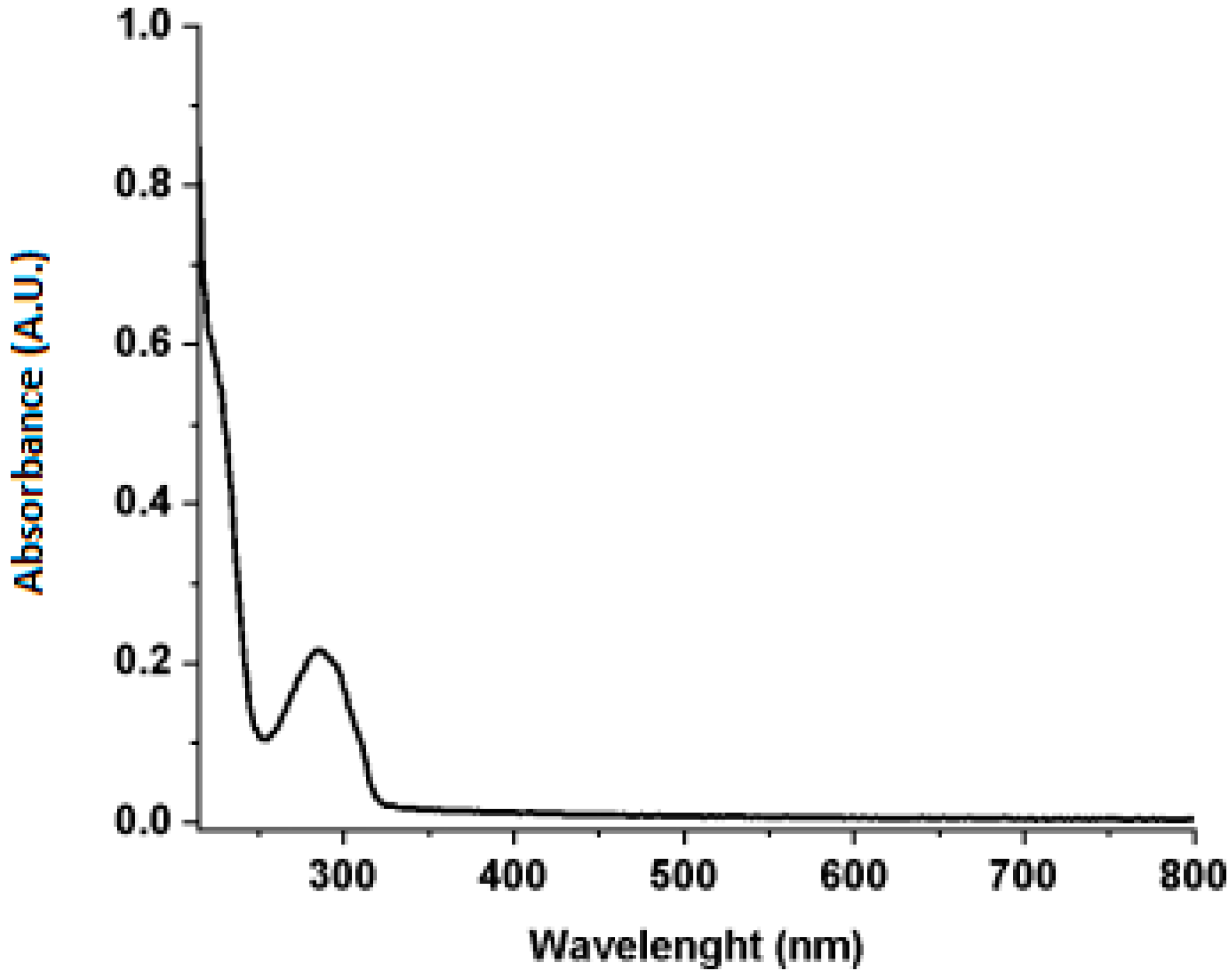
2.3.2. UV–Vis
2.3.3. Peroxide Test
2.3.4. Determination of the Fatty Acids Profile by GC–MS
2.4. Biological and Functional Evaluation of Green Coffee Oil
2.4.1. Antioxidant Activity
2.4.2. Cytotoxicity Assay
2.4.3. Clonogenic Efficiency
2.4.4. Wound Healing
2.5. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Characterization of Green Coffee Oil
3.2. Biological and Functional Evaluation of Green Coffee Oil
3.2.1. Antioxidant Activity
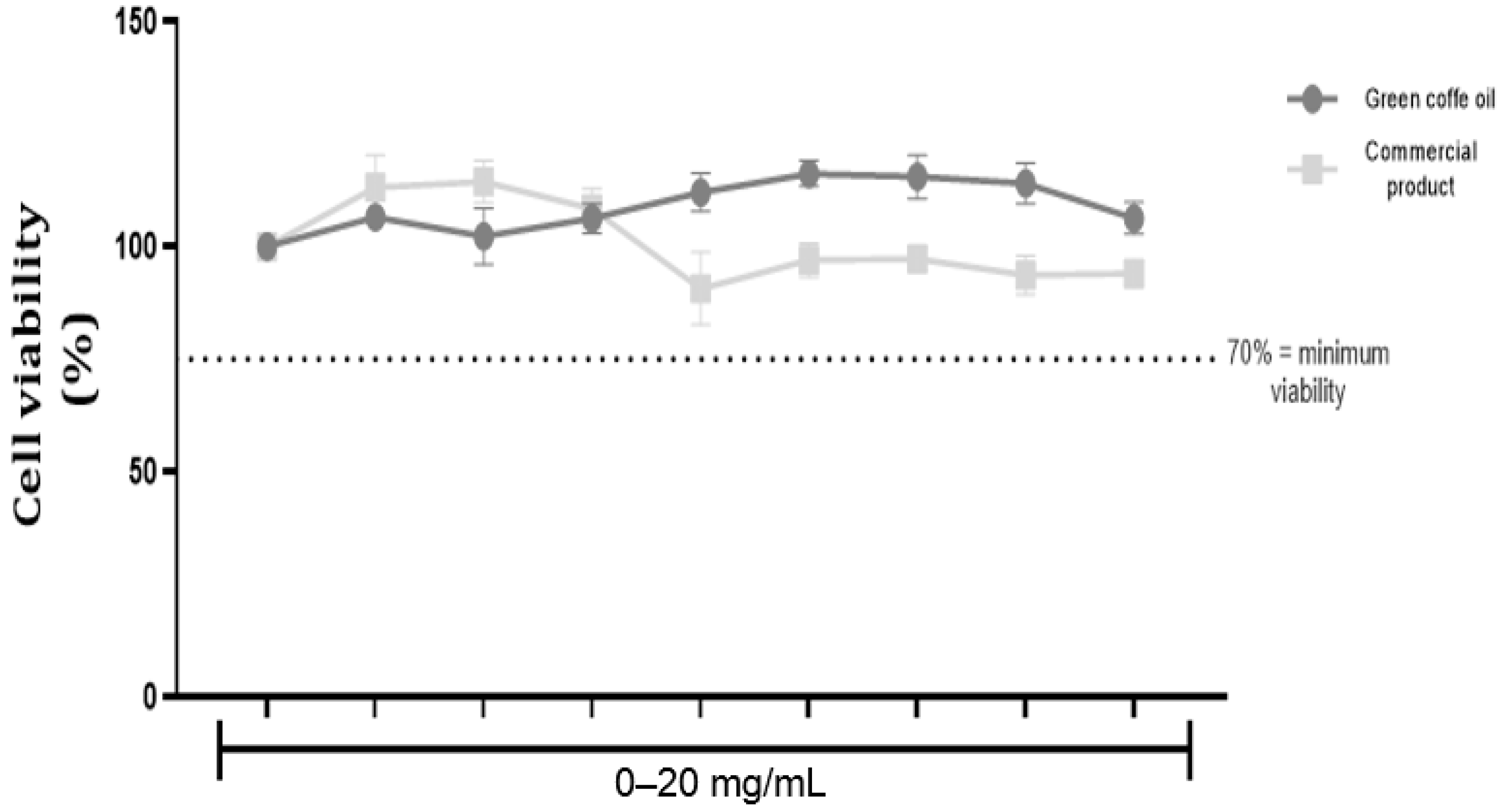
3.2.2. MTT Assay
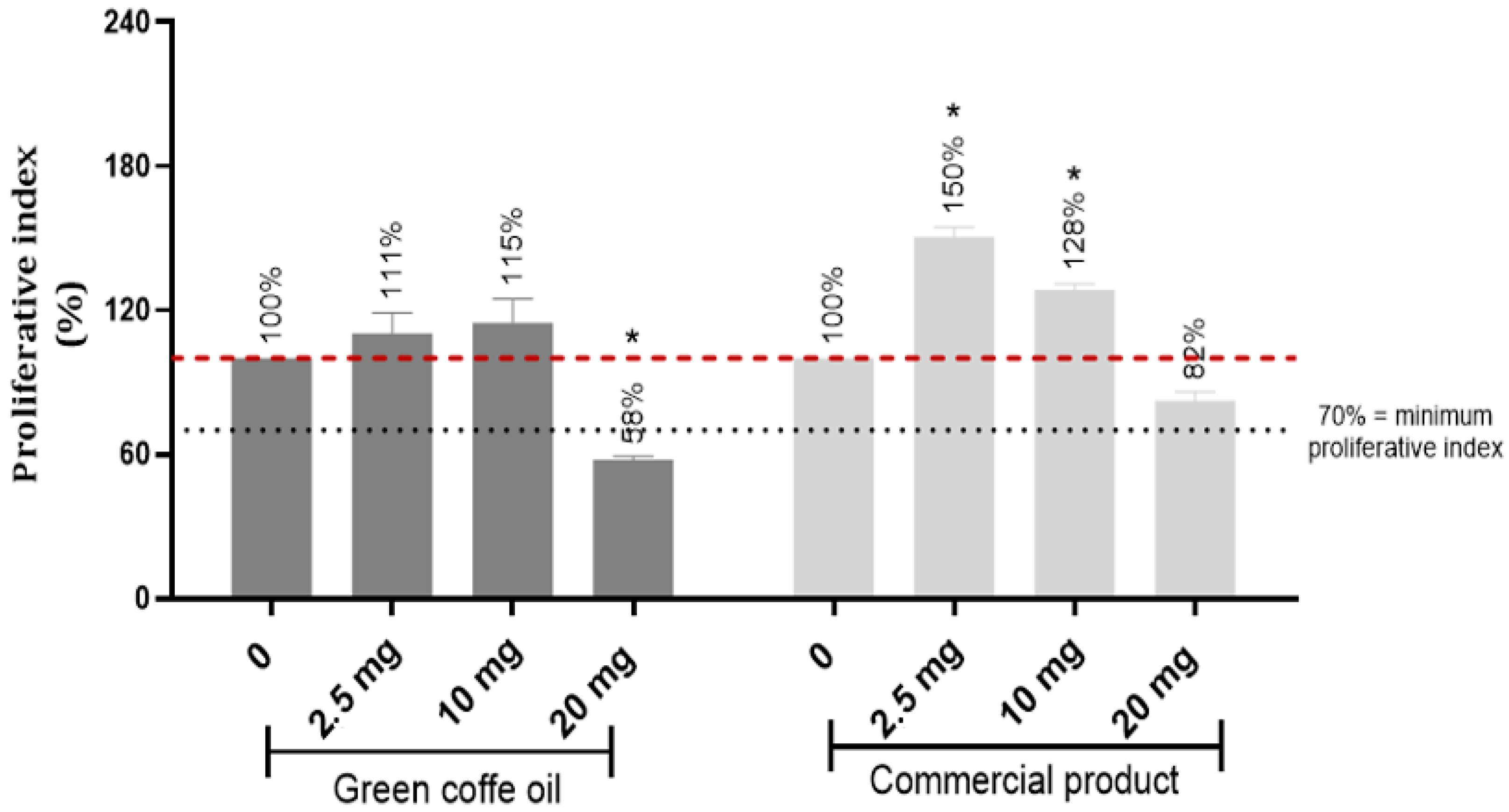
3.2.3. Clonogenic Efficiency
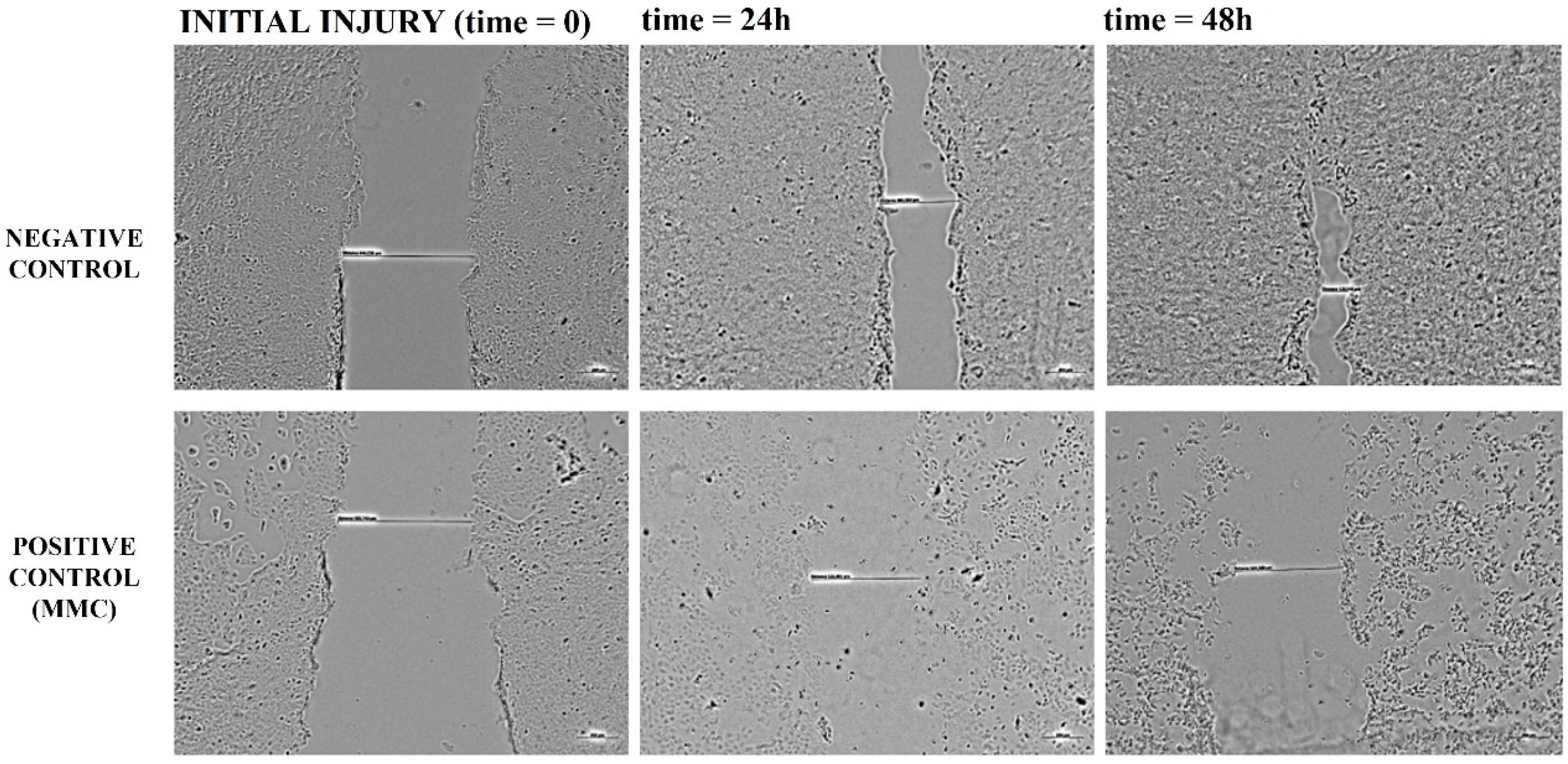
3.2.4. Wound Healing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coffee Production is Expected to Reach 55.7 million Bags in the 2022 Harvest. Available online: https://www.gov.br/en/government-of-brazil/latest-news/2022/coffee-production-is-expected-to-reach-55-7-million-bags-in-the-2022-harvest (accessed on 9 April 2023).
- dos Santos, E.M.; de Macedo, L.M.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.; Mazzola, P.G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Cui, C.H.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Wagemaker, T.A.L.; Carvalho, C.R.L.; Maia, N.B.; Baggio, S.R.; Guerreiro, O. Sun protection factor, content and composition of lipid fraction of green coffee beans. Ind. Crops Prod. 2011, 33, 469–473. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M.; Kaluzna-Czaplinska, J.; Rosiak, A.; Chrzescijanska, E. Antioxidant Properties of Green Coffee Extract. Forests 2020, 11, 557. [Google Scholar] [CrossRef]
- Almeida, F.S.; Dias, F.F.G.; Sato, A.C.K.; Bell, J. From solvent extraction to the concurrent extraction of lipids and proteins from green coffee: An eco-friendly approach to improve process feasibility. Food Bioprod. Process. 2021, 129, 144–156. [Google Scholar] [CrossRef]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Mediani, A.; Kamal, N.; Lee, S.Y.; Abas, F.; Farag, M.A. Green Extraction Methods for Isolation of Bioactive Substances from Coffee Seed and Spent. Sep. Purif. Rev. 2022, 52, 24–42. [Google Scholar] [CrossRef]
- Chiari, B.G.; Trovatti, E.; Pecoraro, E.; Correa, M.A.; Cicarelli, R.M.B.; Ribeiro, S.J.L.; Isaac, V.L.B. Synergistic effect of green coffee oil and synthetic sunscreen for health care application. Ind. Crops Prod. 2014, 52, 389–393. [Google Scholar] [CrossRef]
- de Oliveira, P.M.A.; de Almeida, R.H.; de Oliveira, N.A.; Bostyn, S.; Goncalves, C.B.; de Oliveira, A.L. Enrichment of diterpenes in green coffee oil using supercritical fluid extraction—Characterization and comparison with green coffee oil from pressing. J. Supercrit. Fluids 2014, 95, 137–145. [Google Scholar] [CrossRef]
- Dong, W.J.; Chen, Q.Y.; Wei, C.Q.; Hu, R.S.; Long, Y.Z.; Zong, Y.; Chu, Z. Comparison of the effect of extraction methods on the quality of green coffee oil from Arabica coffee beans: Lipid yield, fatty acid composition, bioactive components, and antioxidant activity. Ultrason. Sonochem. 2021, 74, 105578. [Google Scholar] [CrossRef]
- Araujo, J.M.A.; Sandi, D. Extraction of coffee diterpenes and coffee oil using supercritical carbon dioxide. Food Chem. 2007, 101, 1087–1094. [Google Scholar] [CrossRef]
- de Azevedo, A.B.A.; Kieckbush, T.G.; Tashima, A.K.; Mohamed, R.S.; Mazzafera, P.; de Melo, S. Extraction of green coffee oil using supercritical carbon dioxide. J. Supercrit. Fluids 2008, 44, 186–192. [Google Scholar] [CrossRef]
- Tsukui, A.; Santos, H.M.; Oigrnan, S.S.; de Souza, R.; Bizzo, H.R.; Rezende, C.M. Microwave-assisted extraction of green coffee oil and quantification of diterpenes by HPLC. Food Chem. 2014, 164, 266–271. [Google Scholar] [CrossRef]
- Kreuder, A.D.; House-Knight, T.; Whitford, J.; Ponnusamy, E.; Miller, P.; Jesse, N.; Rodenborn, R.; Sayag, S.; Gebel, M.; Aped, I.; et al. A Method for Assessing Greener Alternatives between Chemical Products Following the 12 Principles of Green Chemistry. ACS Sustain. Chem. Eng. 2017, 5, 2927–2935. [Google Scholar] [CrossRef]
- Raba, D.N.; Poiana, M.A.; Borozan, A.B.; Stef, M.; Radu, F.; Popa, M.V. Investigation on Crude and High-Temperature Heated Coffee Oil by ATR-FTIR Spectroscopy along with Antioxidant and Antimicrobial Properties. PLoS ONE 2015, 10, e0138080. [Google Scholar] [CrossRef]
- Vidal, O.L.; Santos, M.C.B.; Batista, A.P.; Andrigo, F.F.; Barea, B.; Lecomte, J.; Figueroa-Espinoza, M.C.; Gontard, N.; Villeneuve, P.; Guillard, V.; et al. Active packaging films containing antioxidant extracts from green coffee oil by-products to prevent lipid oxidation. J. Food Eng. 2022, 312, 110744. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dluzewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Zbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef]
- Brühl, L. Official Methods and Recommended Practices of the American Oil Chemist’s Society, Physical and Chemical Characteristics of Oils, Fats and Waxes; AOCS Press: Champaign, IL, USA, 1996. [Google Scholar]
- Marquele-Oliveira, F.; Fonseca, Y.M.; Georgetti, S.R.; Vicentini, F.; Bronzati, V.; Fonseca, M.J.V. Evaluation of the antioxidant activity as an additional parameter to attain the functional quality of natural extracts. Lat. Am. J. Pharm. 2008, 27, 325–332. [Google Scholar]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell-growth and survival—modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Munari, C.C.; de Oliveira, P.F.; Lima, I.M.D.; Martins, S.D.L.; da Costa, J.D.; Bastos, J.K.; Tavares, D.C. Evaluation of cytotoxic, genotoxic and antigenotoxic potential of Solanum lycocarpum fruits glicoalkaloid extract in V79 cells. Food Chem. Toxicol. 2012, 50, 3696–3701. [Google Scholar] [CrossRef]
- Borges, G.A.; Elias, S.T.; da Silva, S.M.M.; Magalhaes, P.O.; Macedo, S.B.; Ribeiro, A.P.D.; Guerra, E.N.S. In vitro evaluation of wound healing and antimicrobial potential of ozone therapy. J. Cranio-Maxillofac. Surg. 2017, 45, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.P.; Franca, A.S.; Oliveira, L.S. Evaluation of the potential of FTIR and chemometrics for separation between defective and non-defective coffees. Food Chem. 2012, 132, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.; Milea, M.S.; Boran, S.; Nitu, S.V.; Mosoarca, G.E.; Vancea, C.; Lazau, R.I. Rapid adulteration detection of cold pressed oils with their refined versions by UV-Vis spectroscopy. Sci. Rep. 2020, 10, 16100. [Google Scholar] [CrossRef] [PubMed]
- Didham, M.; Truong, V.K.; Chapman, J.; Cozzolino, D. Sensing the Addition of Vegetable Oils to Olive Oil: The Ability of UV-VIS and MIR Spectroscopy Coupled with Chemometric Analysis. Food Anal. Methods 2020, 13, 601–607. [Google Scholar] [CrossRef]
- Navarra, G.; Moschetti, M.; Guarrasi, V.; Mangione, M.R.; Militello, V.; Leone, M. Simultaneous Determination of Caffeine and Chlorogenic Acids in Green Coffee by UV/Vis Spectroscopy. J. Chem. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Steele, R. Understanding and Measuring the Shelf-Life of Food; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Wagemaker, T.A.L.; Rijo, P.; Rodrigues, L.M.; Campos, P.; Fernandes, A.S.; Rosado, C. Integrated approach in the assessment of skin compatibility of cosmetic formulations with green coffee oil. Int. J. Cosmet. Sci. 2015, 37, 506–510. [Google Scholar] [CrossRef]
- Esquivel, P.; Jimenez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Schmidt, E.; Bail, S.; Buchbauer, G.; Stoilova, I.; Atanasova, T.; Stoyanova, A.; Krastanov, A.; Jirovetz, L. Chemical Composition, Olfactory Evaluation and Antioxidant Effects of Essential Oil from Mentha x piperita. Nat. Prod. Commun. 2009, 4, 1107–1112. [Google Scholar] [CrossRef]
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado Seed: A Comparative Study of Antioxidant Content and Capacity in Protecting Oil Models from Oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef]
- Rodriguez, L.G.; Wu, X.; Guan, J.-L. Wound-healing assay. Methods Mol. Biol. 2005, 294, 6. [Google Scholar]
- Poljsak, N.; Kreft, S.; Glavac, N.K. Vegetable butters and oils in skin wound healing: Scientific evidence for new opportunities in dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.D.V.; Dieamant, G.D.; Eberlin, S.; Nogueira, C.; Colombi, D.; Di Stasi, L.C.; Queiroz, M.L.D. Effect of green Coffea arabica L. seed oil on extracellular matrix components and water-channel expression in in vitro and ex vivo human skin models. J. Cosmet. Dermatol. 2009, 8, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Lania, B.G.; Morari, J.; de Souza, A.L.; da Silva, M.N.; de Almeida, A.R.; Veira-Damiani, G.; Alegre, S.M.; Cesar, C.L.; Velloso, L.A.; Cintra, M.L.; et al. Topical use and systemic action of green and roasted coffee oils and ground oils in a cutaneous incision model in rats (Rattus norvegicus albinus). PLoS ONE 2017, 12, e0188779. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.; Moura, N.G.; Motoyama, A.B. A review of the potential therapeutic and cosmetic use of propolis in topical formulations. J. Appl. Pharm. Sci. 2019, 10, 131–141. [Google Scholar] [CrossRef]



| Composition in Fatty Acids (% w/w) | Green Coffee | |
|---|---|---|
| C 12:0 | lauric | - |
| C 14:0 | myristic | - |
| C 15:0 | pentadecanoic | - |
| C16:0 | palmitic | 35.4 |
| C 16:1 | palmitoleic | 1.5 |
| C 17:0 | margaric | - |
| C 18:0 | stearic | 8.1 |
| C 18:1 | oleic | 9.4 |
| C 18:2 trans | t-linolenic | - |
| C 18:2 | linoleic | 44.8 |
| C 18:3 | linolenic | 1.9 |
| C 20:0 | arachidic | 3.2 |
| C 20:1 | eicosenoic | - |
| C 22:0 | behenic | - |
| C 24:0 | lignoceric | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, L.D.; Carbinatto, F.M.; Almeida, I.d.S.; Blanco, K.C.; Marquele-Oliveira, F.; Munari, C.C.; Bagnato, V.S. Eco-Friendly Extraction of Green Coffee Oil for Industrial Applications: Its Antioxidant, Cytotoxic, Clonogenic, and Wound Healing Properties. Fermentation 2023, 9, 370. https://doi.org/10.3390/fermentation9040370
Dias LD, Carbinatto FM, Almeida IdS, Blanco KC, Marquele-Oliveira F, Munari CC, Bagnato VS. Eco-Friendly Extraction of Green Coffee Oil for Industrial Applications: Its Antioxidant, Cytotoxic, Clonogenic, and Wound Healing Properties. Fermentation. 2023; 9(4):370. https://doi.org/10.3390/fermentation9040370
Chicago/Turabian StyleDias, Lucas D., Fernanda Mansano Carbinatto, Isabelle da Silveira Almeida, Kate C. Blanco, Franciane Marquele-Oliveira, Carla Carolina Munari, and Vanderlei Salvador Bagnato. 2023. "Eco-Friendly Extraction of Green Coffee Oil for Industrial Applications: Its Antioxidant, Cytotoxic, Clonogenic, and Wound Healing Properties" Fermentation 9, no. 4: 370. https://doi.org/10.3390/fermentation9040370
APA StyleDias, L. D., Carbinatto, F. M., Almeida, I. d. S., Blanco, K. C., Marquele-Oliveira, F., Munari, C. C., & Bagnato, V. S. (2023). Eco-Friendly Extraction of Green Coffee Oil for Industrial Applications: Its Antioxidant, Cytotoxic, Clonogenic, and Wound Healing Properties. Fermentation, 9(4), 370. https://doi.org/10.3390/fermentation9040370






