Effect of Lipid Type on the Acidogenic Performance of Food Waste
Abstract
1. Introduction
| Origin | Saturated Fatty Acid (%) | Unsaturated Fatty Acid (%) | Refs. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14:0 | 16:0 | 17:0 | 18:0 | 20:0 | 22:0 | 24:0 | Total | 16:1 | 18:1 | 18:2 | 18:3 | 20:1 | Total | |||
| Animal origin | Beef tallow | 2–6 | 20–37 | 0–1 | 16–40 | 40–55 | 2–8 | 35–50 | 0.5–6 | 45–60 | [22,26,27,28] | |||||
| Muttonfat | 3–6 | 25–28 | 1–2 | 30–34 | 60–70 | 1–2 | 30–35 | 1–2 | 30–40 | [27,28] | ||||||
| Plant origin | Soybean oil | 10–14 | 2–5 | 13–19 | 22–26 | 47–57 | 7–8 | 80–85 | [22,29,30,31] | |||||||
| Peanutoil | 9–14 | 2–5 | 1–2 | 2–3 | 1–2 | 17–20 | 45–55 | 23–33 | 0–2 | 0–1 | >80 | [22,26,29,32] | ||||
| Rapeseed oil | 0–5 | 0–2 | 7–8 | 60–65 | 15–20 | 5–10 | 1–2 | >90 | [22,29,32] | |||||||
2. Materials and Methods
2.1. Substrate and Inoculum
2.2. General Procedure and Experimental Design
2.3. Analytical Methods
3. Results and Analysis
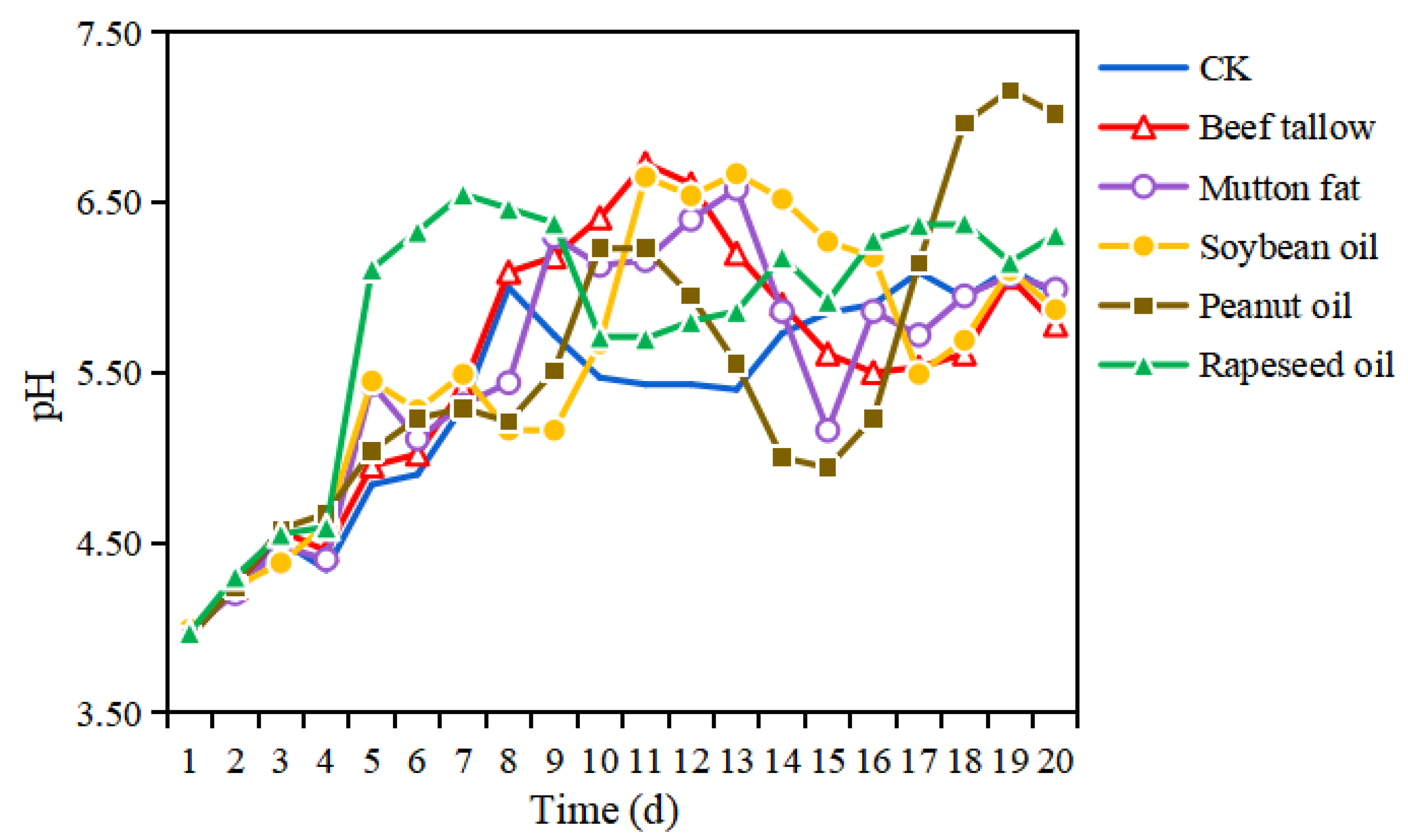
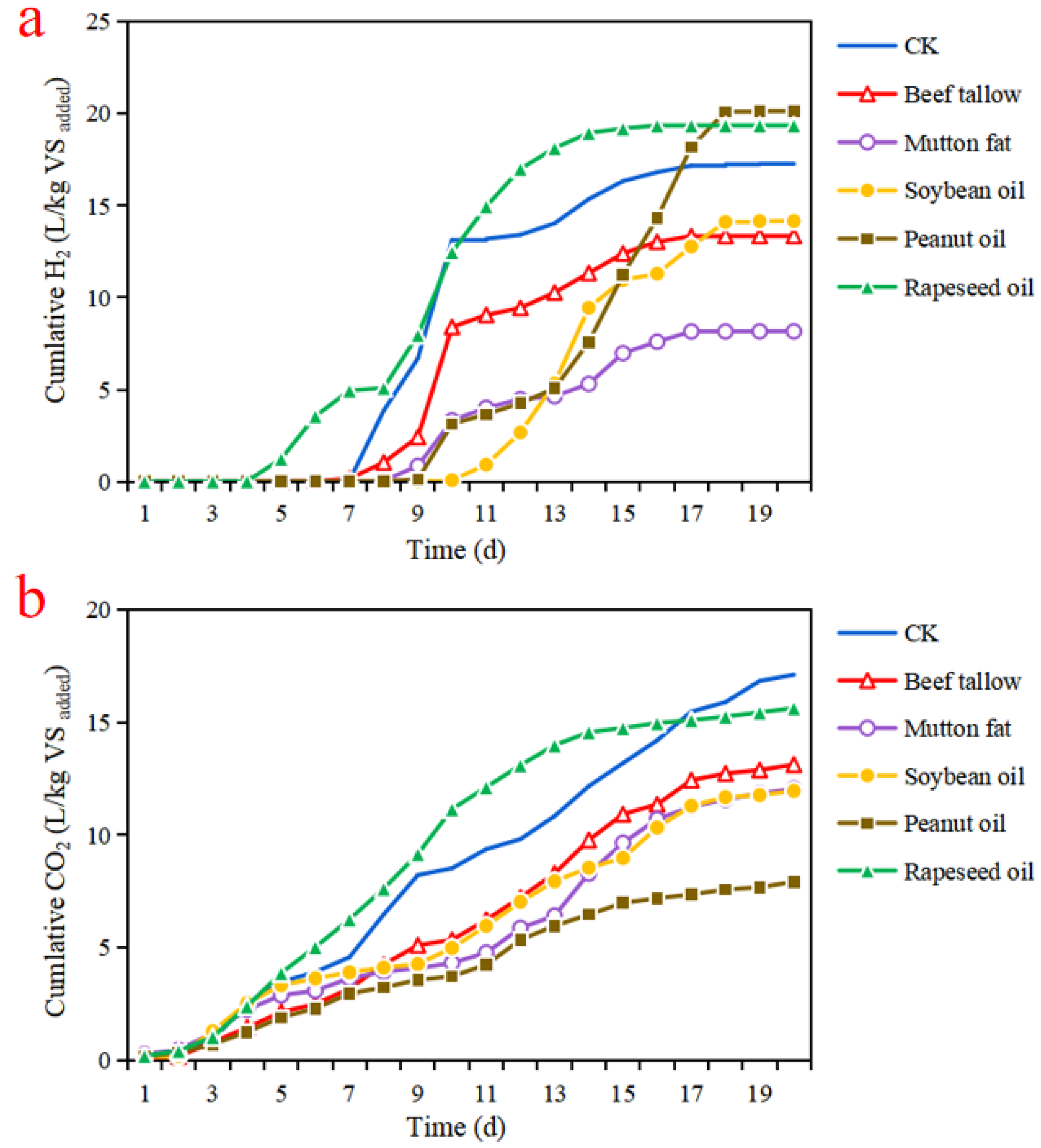
3.1. Effect of Different Lipid Types on the Variations of pH and Chemical Oxygen Demand
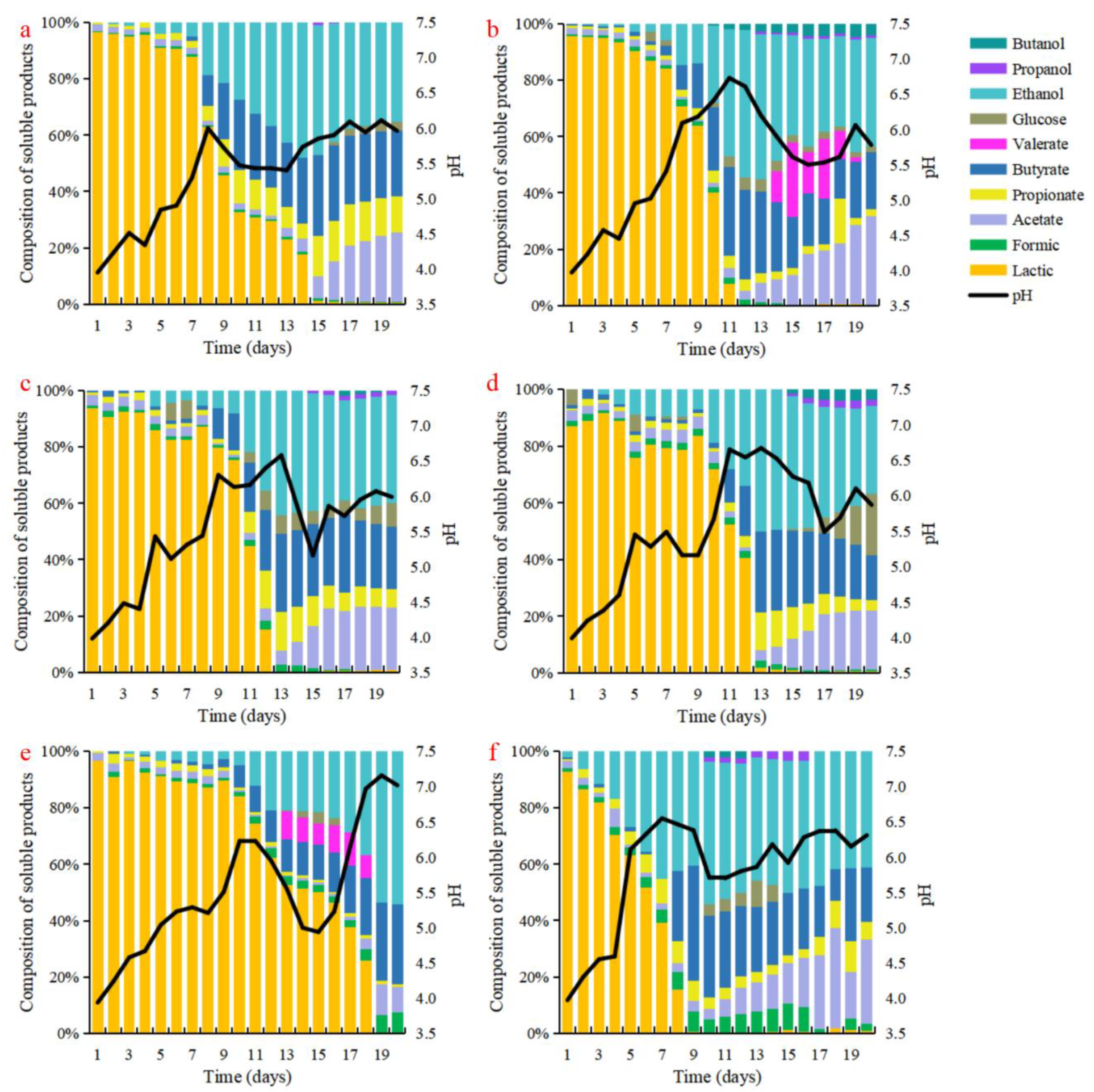
3.2. Effect of Different Lipid Types on the Production of Soluble Products
4. Discussion
4.1. Effect of Lipid Type on the Acidogenic Fermentation Process of Food Waste
4.2. Effect of Lipid Type on the Generation of Acidogenic Product
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Notations
| COD | Chemical oxygen demand | CK | Blank |
| LCFAs | Long-chain fatty acids | T1 | Beef tallow |
| TN | Total nitrogen | T2 | Mutton fat |
| TOC | Total organic carbon | T3 | Soybean oil |
| TS | Total solids | T4 | Peanut oil |
| VFAs | Volatile fatty acids | T5 | Rapeseed oil |
| VS | Volatile solids |
References
- Capson-Tojo, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.-P.; Delgenès, J.-P.; Escudié, R. Dry anaerobic digestion of food waste and cardboard at different substrate loads, solid contents and co-digestion proportions. Bioresour. Technol. 2017, 233, 166–175. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Tabatabaei, M.; Aghbashlo, M. Biogas production from food wastes: A review on recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 7, 100202. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Dehhaghi, M.; Guillemin, G.J.; Gupta, V.K.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Bioethanol production from food wastes rich in carbohydrates. Curr. Opin. Food Sci. 2022, 43, 71–81. [Google Scholar] [CrossRef]
- Pervez, N.; Bilgiç, B.; Mahboubi, A.; Uwineza, C.; Zarra, T.; Belgiorno, V.; Naddeo, V.; Taherzadeh, M.J. Double-stage membrane-assisted anaerobic digestion process intensification for production and recovery of volatile fatty acids from food waste. Sci. Total. Environ. 2022, 825, 154084. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Sun, S.; Yang, D.; Sheng, W.; Ma, Y.; He, W.; Li, G. Anaerobic digestion: An alternative resource treatment option for food waste in China. Sci. Total. Environ. 2021, 779, 146397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yu, M.; Wang, Q.; Song, N.; Che, S.; Wu, C.; Sun, X. Effect of Ethanol and Lactic Acid Pre-fermentation on Putrefactive Bacteria Suppression, Hydrolysis, and Methanogenesis of Food Waste. Energy Fuels 2016, 30, 2982–2989. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Sheng, L. Study on the comprehensive utilization of city kitchen waste as a resource in China. Energy 2019, 173, 263–277. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Zhang, H.; Zhang, D.; Chadwick, D.; Li, G.; Wang, G.; Chi, M.; Yang, F. Effects of adding bulking agents on the biodrying of kitchen waste and the odor emissions produced. J. Environ. Sci. 2018, 67, 344–355. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Li, X. Biogas Production from Anaerobic Co-digestion of Food Waste with Dairy Manure in a Two-Phase Digestion System. Appl. Biochem. Biotechnol. 2009, 160, 643–654. [Google Scholar] [CrossRef]
- Yan, B.H.; Selvam, A.; Wong, J.W. Application of rumen microbes to enhance food waste hydrolysis in acidogenic leach-bed reactors. Bioresour. Technol. 2014, 168, 64–71. [Google Scholar] [CrossRef]
- Shen, F.; Yuan, H.; Pang, Y.; Chen, S.; Zhu, B.; Zou, D.; Liu, Y.; Ma, J.; Yu, L.; Li, X. Performances of anaerobic co-digestion of fruit & vegetable waste (FVW) and food waste (FW): Single-phase vs. two-phase. Bioresour. Technol. 2013, 144, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Salama, E.-S.; Saha, S.; Kurade, M.B.; Dev, S.; Chang, S.W.; Jeon, B.-H. Recent trends in anaerobic co-digestion: Fat, oil, and grease (FOG) for enhanced biomethanation. Prog. Energy Combust. Sci. 2018, 70, 22–42. [Google Scholar] [CrossRef]
- Wang, H.; Fotidis, I.A.; Angelidaki, I. Ammonia effect on hydrogenotrophic methanogens and syntrophic acetate-oxidizing bacteria. FEMS Microbiol. Ecol. 2015, 91, fiv130. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Li, J. Influence of thermal hydrolysis on composition characteristics of fatty acids in kitchen waste. Energy 2016, 102, 139–147. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lang, Q.; Fang, M.; Li, X.; Bah, H.; Dong, H.; Dong, R. Combined effect of crude fat content and initial substrate concentration on batch anaerobic digestion characteristics of food waste. Bioresour. Technol. 2017, 232, 304–312. [Google Scholar] [CrossRef]
- Cirne, D.; Paloumet, X.; Björnsson, L.; Alves, M.; Mattiasson, B. Anaerobic digestion of lipid-rich waste—Effects of lipid concentration. Renew. Energy 2007, 32, 965–975. [Google Scholar] [CrossRef]
- Palatsi, J.; Affes, R.; Fernandez, B.; Pereira, M.; Alves, M.; Flotats, X. Influence of adsorption and anaerobic granular sludge characteristics on long chain fatty acids inhibition process. Water Res. 2012, 46, 5268–5278. [Google Scholar] [CrossRef]
- Awe, O.W.; Lu, J.; Wu, S.; Zhao, Y.; Nzihou, A.; Lyczko, N.; Minh, D.P. Effect of Oil Content on Biogas Production, Process Performance and Stability of Food Waste Anaerobic Digestion. Waste Biomass Valorization 2018, 9, 2295–2306. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Yin, X.-B.; Li, Q.; Li, X.; Zhang, Y.-F.; Deng, Y. Insights into biomethane production and microbial community succession during semi-continuous anaerobic digestion of waste cooking oil under different organic loading rates. AMB Express 2018, 8, 92. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, J. Influence of feed/inoculum ratios and waste cooking oil content on the mesophilic anaerobic digestion of food waste. Waste Manag. 2018, 73, 156–164. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Wang, H.; Yang, K. Direct interspecies electron transfer stimulated by granular activated carbon enhances anaerobic methanation efficiency from typical kitchen waste lipid-rapeseed oil. Sci. Total. Environ. 2019, 704, 135282. [Google Scholar] [CrossRef]
- Sobon-Mühlenbrock, E.; Schlienz, M.; Greger, M. Mesophilic and Thermophilic Anaerobic Digestion of Model Kitchen Waste with Variation of Fat Content. Chem. Ing. Tech. 2020, 92, 1840–1850. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, L.; Mu, L.; Ma, J.; Li, C.; Li, A. Comprehensive investigation of soybean oil-derived LCFAs on anaerobic digestion of organic waste: Inhibitory effect and transformation. Biochem. Eng. J. 2019, 151, 107314. [Google Scholar] [CrossRef]
- Lu, J.; Jia, Z.; Wang, P.; Yang, X.; Lin, P.; Ren, L.; Farghali, M. Restoration of acidified dry anaerobic digestion of food waste: Bioaugmentation of butyric acid-resistant microbes. J. Environ. Chem. Eng. 2022, 10, 106935. [Google Scholar] [CrossRef]
- De Schrijver, R.; Vermeulen, D.; Viaene, E. Lipid Metabolism Responses in Rats Fed Beef Tallow, Native or Randomized Fish Oil and Native or Randomized Peanut Oil. J. Nutr. 1991, 121, 948–955. [Google Scholar] [CrossRef]
- Grompone, M.A. Characteristics of Uruguayan mutton tallow. J. Am. Oil Chem. Soc. 1990, 67, 980. [Google Scholar] [CrossRef]
- Marikkar, N.; Alinovi, M.; Chiavaro, E. Analytical approaches for discriminating native lard from other animal fats. Ital. J. Food Sci. 2021, 33, 106–115. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, P.; Liu, B.; Meng, X. Effect of traditional Chinese cooking methods on fatty acid profiles of vegetable oils. Food Chem. 2017, 233, 77–84. [Google Scholar] [CrossRef]
- Minami, I.; Nakamura, Y.; Todoriki, S.; Murata, Y. Effect of γ Irradiation on the Fatty Acid Composition of Soybean and Soybean Oil. Biosci. Biotechnol. Biochem. 2012, 76, 900–905. [Google Scholar] [CrossRef]
- Kabir, Y.; Ide, T. Effect of Dietary Soybean Phospholipid and Fats Differing in the Degree of Unsaturation on Fatty Acid Synthesis and Oxidation in Rat Liver. J. Nutr. Sci. Vitaminol. 1995, 41, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Konuskan, D.B.; Arslan, M.; Oksuz, A. Physicochemical properties of cold pressed sunflower, peanut, rapeseed, mustard and olive oils grown in the Eastern Mediterranean region. Saudi J. Biol. Sci. 2019, 26, 340–344. [Google Scholar] [CrossRef]
- He, X.; Guo, Z.; Lu, J.; Zhang, P. Carbon-based conductive materials accelerated methane production in anaerobic digestion of waste fat, oil and grease. Bioresour. Technol. 2021, 329, 124871. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, X.; Horita, J.; Fang, X.; Zheng, J.; Li, X.; Meng, Q. Evaluation of total organic carbon contents in carbonate source rocks by modified acid treatment method and the geological significance of acid-soluble organic matters. Energy Explor. Exploit. 2019, 37, 219–229. [Google Scholar] [CrossRef]
- Liu, C.; Ren, L.; Yan, B.; Luo, L.; Zhang, J.; Awasthi, M.K. Electron transfer and mechanism of energy production among syntrophic bacteria during acidogenic fermentation: A review. Bioresour. Technol. 2021, 323, 124637. [Google Scholar] [CrossRef]
- Elsamadony, M.; Mostafa, A.; Fujii, M.; Tawfik, A.; Pant, D. Advances towards understanding long chain fatty acids-induced inhibition and overcoming strategies for efficient anaerobic digestion process. Water Res. 2020, 190, 116732. [Google Scholar] [CrossRef]
- Dasa, K.T.; Westman, S.Y.; Millati, R.; Cahyanto, M.N.; Taherzadeh, M.J.; Niklasson, C. Inhibitory Effect of Long-Chain Fatty Acids on Biogas Production and the Protective Effect of Membrane Bioreactor. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, J. Valorisation of food waste using salt to alleviate inhibition by animal fats and vegetable oils during anaerobic digestion. Biomass Bioenergy 2020, 143, 105826. [Google Scholar] [CrossRef]
- Wu, L.-J.; Kobayashi, T.; Kuramochi, H.; Li, Y.-Y.; Xu, K.-Q.; Lv, Y. High loading anaerobic co-digestion of food waste and grease trap waste: Determination of the limit and lipid/long chain fatty acid conversion. Chem. Eng. J. 2018, 338, 422–431. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Awasthi, M.K.; Taherzadeh, M. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Amani, T.; Nosrati, M.; Mousavi, S.M.; Kermanshahi, R.K. Study of syntrophic anaerobic digestion of volatile fatty acids using enriched cultures at mesophilic conditions. Int. J. Environ. Sci. Technol. 2011, 8, 83–96. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Worm, P.; Schink, B.; Stams, A.J.M.; Plugge, C.M. Syntrophic butyrate and propionate oxidation processes: From genomes to reaction mechanisms. Environ. Microbiol. Rep. 2010, 2, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, S.; Ruggeri, B.; Saracco, G. Development of a Photosynthetic Microbial Electrochemical Cell (PMEC) Reactor Coupled with Dark Fermentation of Organic Wastes: Medium Term Perspectives. Energies 2015, 8, 399–429. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, J.; Tan, M.; Du, J.; Yan, B.; Wong, J.W.; Zhang, Y. Enhanced carboxylic acids production by decreasing hydrogen partial pressure during acidogenic fermentation of glucose. Bioresour. Technol. 2017, 245, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Hafner, S.D.; De Laclos, H.F.; Koch, K.; Holliger, C. Improving Inter-Laboratory Reproducibility in Measurement of Biochemical Methane Potential (BMP). Water 2020, 12, 1752. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffiere, P.; Carballa, M.; de Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Khounani, Z.; Hosseinzadeh-Bandbafha, H.; Gupta, V.K.; Amiri, H.; Lam, S.S.; Morosuk, T.; Tabatabaei, M. Exergoenvironmental analysis of bioenergy systems: A comprehensive review. Renew. Sustain. Energy Rev. 2021, 149, 111399. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Hosseinzadeh-Bandbafha, H.; Shahbeik, H.; Tabatabaei, M. The role of sustainability assessment tools in realizing bioenergy and bioproduct systems. Biofuel Res. J. 2022, 9, 1697–1706. [Google Scholar] [CrossRef]



| Parameters | Food Waste | Inoculum |
|---|---|---|
| pH | 5.79 ± 0.14 | 6.86 ± 0.10 |
| VS (%) | 43.40 ± 0.95 | 2.26 ± 0.11 |
| TS (%) | 44.70 ± 0.34 | 2.96 ± 0.15 |
| TOC (%) | 40.25 ± 1.43 | 8.50 ± 0.31 |
| TN (%) | 2.13 ± 0.32 | 0.21 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, S.; Niu, H.; Yang, H.; Tan, J.; Zhang, J.; Ren, L.; Yan, B. Effect of Lipid Type on the Acidogenic Performance of Food Waste. Fermentation 2023, 9, 348. https://doi.org/10.3390/fermentation9040348
Liu C, Li S, Niu H, Yang H, Tan J, Zhang J, Ren L, Yan B. Effect of Lipid Type on the Acidogenic Performance of Food Waste. Fermentation. 2023; 9(4):348. https://doi.org/10.3390/fermentation9040348
Chicago/Turabian StyleLiu, Chao, Sheng Li, Hongyu Niu, Haijun Yang, Ju Tan, Jiachao Zhang, Liheng Ren, and Binghua Yan. 2023. "Effect of Lipid Type on the Acidogenic Performance of Food Waste" Fermentation 9, no. 4: 348. https://doi.org/10.3390/fermentation9040348
APA StyleLiu, C., Li, S., Niu, H., Yang, H., Tan, J., Zhang, J., Ren, L., & Yan, B. (2023). Effect of Lipid Type on the Acidogenic Performance of Food Waste. Fermentation, 9(4), 348. https://doi.org/10.3390/fermentation9040348






