Fermentation Properties and Bacterial Community Composition of Mixed Silage of Mulberry Leaves and Smooth Bromegrass with and without Lactobacillus plantarum Inoculation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Silage Preparation
2.2. Analyses of Fermentation Products, Chemical Composition and Microbial Population
2.3. Analyses of Bacterial Community
2.4. Statistical Analyses
3. Results and Discussion
3.1. Chemical Composition and Microbial Population of Raw Materials
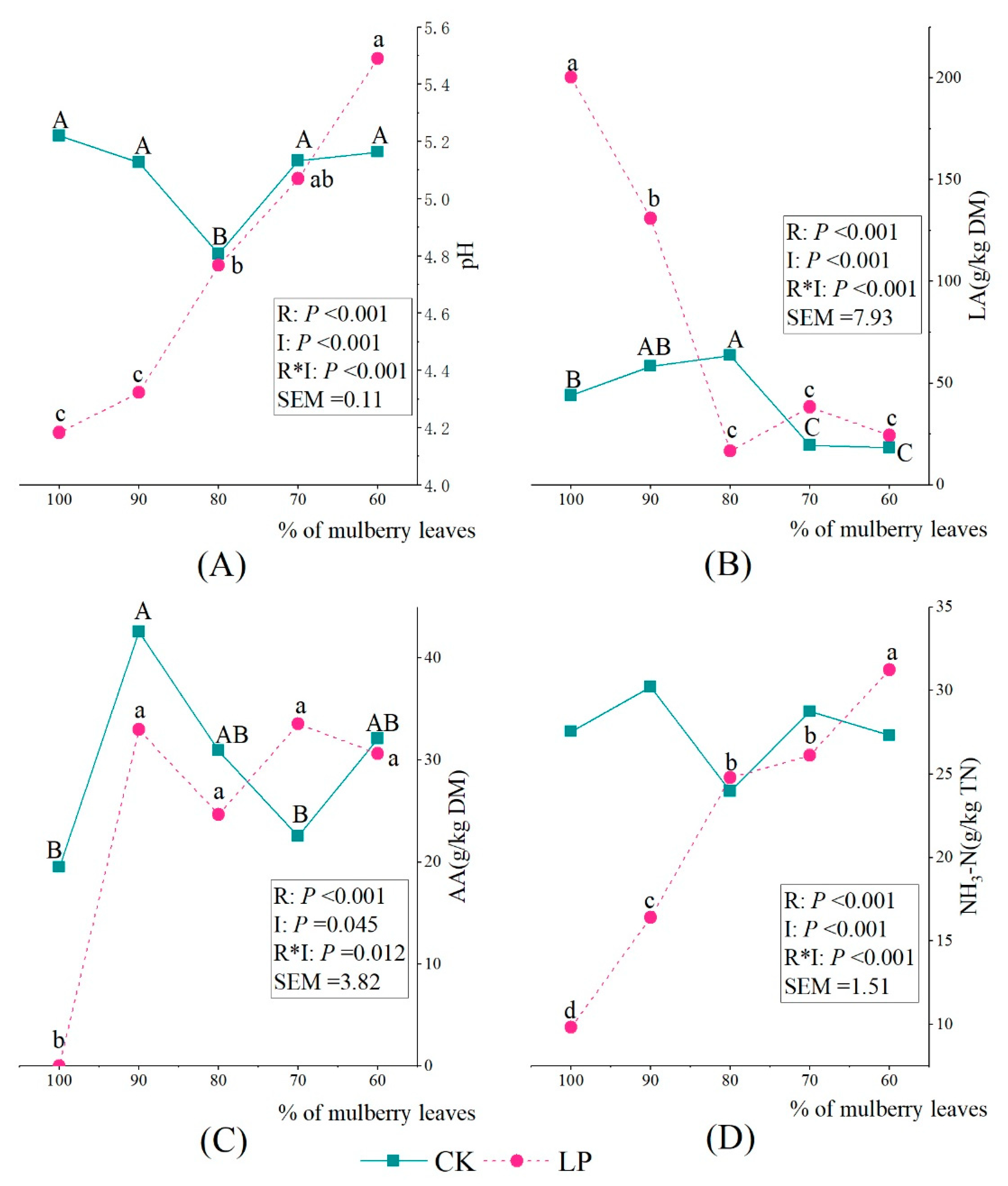
3.2. Chemical Composition and Fermentation Properties of Silage
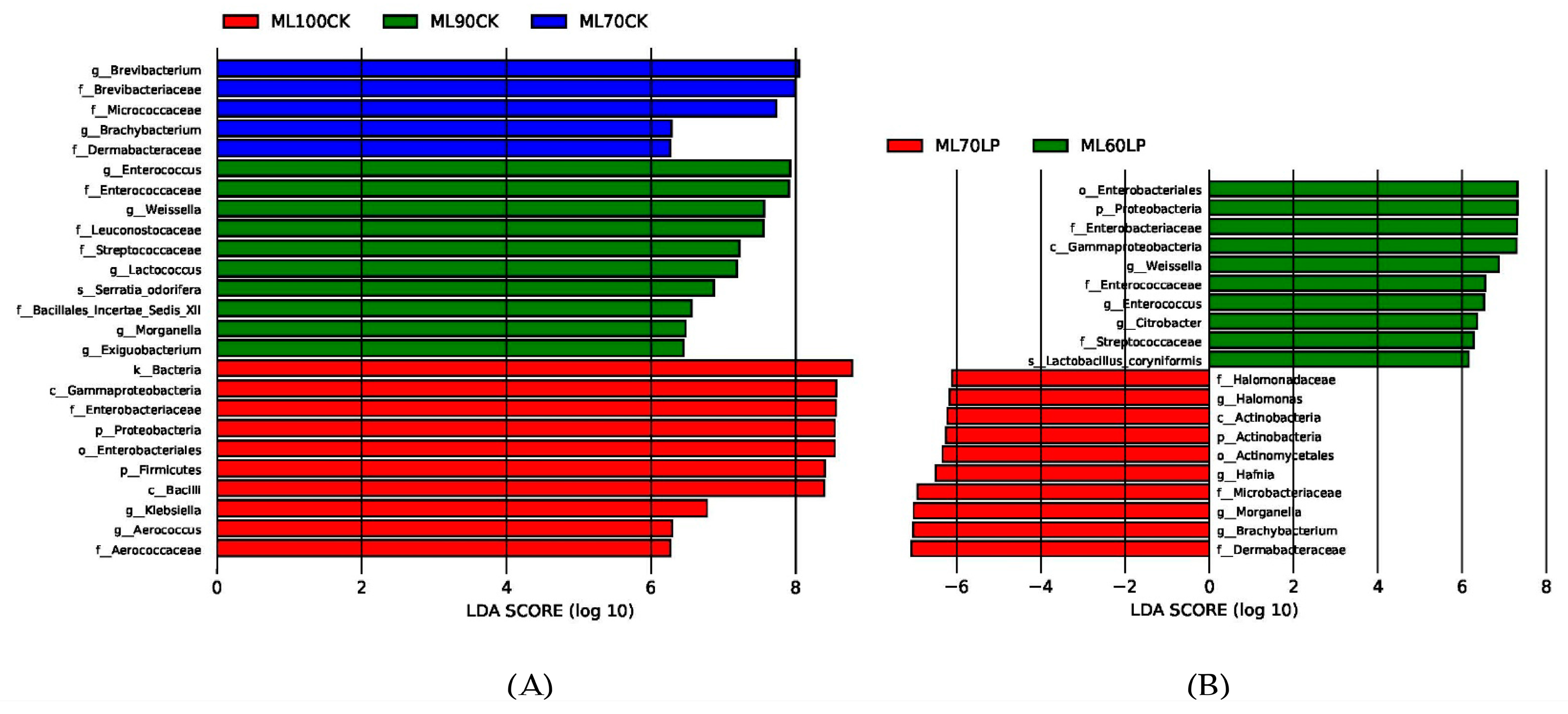
3.3. Bacterial Community Analyses of Silage
3.4. Spearman Correlations of Bacterial Community with Fermentation Properties in Silage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yao, F.; Ni, W.Z.; Yang, X.E. Genotype resources and ecological adaptation of mulberry plants (Morus indica L.) and their application foreground. Bull. Sci. Technol. 2004, 20, 289–297. [Google Scholar] [CrossRef]
- Islam, M.R.; Siddiqui, M.N.; Khatun, A.; Siddiky, M.N.A.; Rahman, M.Z.; Bostami, A.B.M.R. Dietary effect of mulberry leaf (Morus alba) meal on growth performance and serum cholesterol level of broiler chickens. SAARC J. Agric 2014, 12, 79–89. [Google Scholar] [CrossRef]
- Kandylis, K.; Hadjigeorgiou, I.; Harizanis, P. The nutritive value of mulberry leaves (Morus alba) as a feed supplement for sheep. J. Trop. Anim. Health Prod. 2009, 41, 17–24. [Google Scholar] [CrossRef]
- Liu, J.X.; Yao, J.; Yan, B.; Yu, J.Q.; Shi, Z.Q. Effects of mulberry leaves to replace rapeseed meal on performance of sheep feeding on ammoniated rice straw diet. Small Rumin. Res. 2001, 39, 131–136. [Google Scholar] [CrossRef]
- Martínez, M.; Motta, W.; Cervera, C.; Pla, M. Feeding mulberry leaves to fattening rabbits: Effects on growth, carcass characteristics and meat quality. J. Anim. Sci. 2005, 80, 275–281. [Google Scholar] [CrossRef]
- Zhao, X.; Li, L.; Luo, Q.; Ye, M.; Luo, G.; Kuang, Z. Effects of mulberry (Morus alba L.) leaf polysaccharides on growth performance, diarrhea, blood parameters, and gut microbiota of early-weanling pigs. Livest. Sci. 2015, 177, 88–94. [Google Scholar] [CrossRef]
- Dong, M.Y.; Li, Q.Q.; Xu, F.Q.; Wang, S.Y.; Chen, J.H.; Li, W.J. Effects of microbial inoculants on the fermentation characteristics and microbial communities of sweet sorghum bagasse silage. Sci. Rep. 2020, 10, 837. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.W.; Xing, Y.Q.; Zhou, W.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 2019, 284, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, L.W.; Xing, Y.Q.; Zhou, W.; Pian, R.Q.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Bacterial diversity and fermentation quality of Moringa oleifera leaves silage prepared with lactic acid bacteria inoculants and stored at different temperatures. Bioresour. Technol. 2019, 284, 349–358. [Google Scholar] [CrossRef]
- Yang, F.Y.; Wang, Y.P.; Zhao, S.S.; Wang, Y. Lactobacillus plantarum inoculants delay spoilage of high moisture alfalfa silages by regulating bacterial community composition. Front. Microbiol. 2020, 11, 1989. [Google Scholar] [CrossRef]
- Ni, K.K.; Wang, F.F.; Zhu, B.G.; Yang, J.X.; Zhou, G.A.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Zeng, T.; Li, X.; Guan, H.; Yang, W.; Yan, Y. Dynamic microbial diversity and fermentation quality of the mixed silage of corn and soybean grown in strip intercropping system. Bioresour. Technol. 2020, 313, 123655. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Y.; Gou, W.; Cheng, Q.; Cai, Y. Silage fermentation and bacterial community of bur clover, annual ryegrass and their mixtures prepared with microbial inoculant and chemical additive. Anim. Feed Sci. Technol. 2018, 247, 285–293. [Google Scholar] [CrossRef]
- Xie, K.Y.; Xiang-Lin, L.I.; Feng, H.E.; Zhang, Y.J.; Wan, L.Q.; Hannaway, D.B. Effect of nitrogen fertilization on yield, n content, and nitrogen ifxation of alfalfa and smooth bromegrass grown alone or in mixture in greenhouse pots. J. Integr. Agric. 2015, 14, 1864–1876. [Google Scholar] [CrossRef]
- Wang, H.L.; Xu, C.C. Utilization of tea grounds as feedstuff for ruminant. J. Anim. Sci. Biotechnol. 2013, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Wang, Y.; Li, D.; Cai, Y.; Pang, H. Characterization, identification and application of lactic acid bacteria isolated from forage paddy rice silage. PLoS ONE 2015, 10, e0121967. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.J.; Goncalves, M.C.M.; Vyas, D.; et al. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef]
- Zhao, S.S.; Wang, Y.P.; Yang, F.Y.; Wang, Y.; Zhang, H. Screening a Lactobacillus plantarum strain for good adaption in alfalfa ensiling and demonstrating its improvement of alfalfa silage quality. J. Appl. Microbiol. 2020, 129, 233–242. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990.
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Murphy, R.P. A method for the extraction of plant samples and the determination of total soluble carbohydrates. J. Sci. Food Agric. 1958, 9, 714–717. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, S.; Wang, Y.; Fan, X.; Wang, Y.; Feng, C. Assessment of bacterial community composition and dynamics in alfalfa silages with and without Lactobacillus plantarum inoculation using absolute quantification 16S rRNA sequencing. Front. Microbiol. 2021, 11, 629894. [Google Scholar] [CrossRef]
- Smets, W.; Leff, J.W.; Bradford, M.A.; Mcculley, R.L.; Lebeer, S.; Fierer, N. A method for simultaneous measurement of soil bacterial abundances and community composition via 16S rRNA gene sequencing. Soil Biol. Biochem. 2016, 96, 145–151. [Google Scholar] [CrossRef]
- Tkacz, A.; Hortala, M.; Poole, P.S. Absolute quantitation of microbiota abundance in environmental samples. Microbiome 2018, 6, 110. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Yu, Y.; Li, H.; Zhang, B.; Wang, J.; Shi, X.; Huang, J.Z.; Yang, J.; Zhang, Y.; Deng, Z. Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials. Int. J. Food Prop. 2018, 21, 1495–1507. [Google Scholar] [CrossRef]
- Hao, J.Y.; Wan, Y.; Yao, X.H.; Zhao, W.G.; Hu, R.Z.; Chen, C.; Li, L.; Zhang, D.Y.; Wu, G.H. Effect of different planting areas on the chemical compositions and hypoglycemic and antioxidant activities of mulberry leaf extracts in Southern China. PLoS ONE 2018, 13, e0198072. [Google Scholar] [CrossRef]
- Neto, A.F.G.; Da Silva, J.; Do Nascimento, E.M.; Lourenço, J.C.S.; Fernandes, S.R. Nutritional value and physical and chemical characteristics of white mulberry tree using different conservation methods for ruminant feed. Semin. Cienc. Agrar. 2018, 39, 771–786. [Google Scholar] [CrossRef]
- Zheng, S.; Zeng, W.; Han, L.; Liu, C.; Yu, M.; Xiang, Z.; Zhao, A. Comprehensive evaluation of nutritional quality of leaves from 45 mulberry germplasms and varieties. Food Sci. 2017, 38, 159–163. [Google Scholar] [CrossRef]
- He, L.W.; Chen, N.; Lv, H.J.; Wang, C.; Zhou, W.; Chen, X.Y.; Zhang, Q. Gallic acid influencing fermentation quality, nitrogen distribution and bacterial community of high-moisture mulberry leaves and stylo silage. Bioresour. Technol. 2020, 295, 122255. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.K.; Zhao, J.Y.; Zhu, B.G.; Su, R.N.; Pan, Y.; Ma, J.K.; Zhou, G.A.; Tao, Y.; Liu, X.R.; Zhong, J. Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.Z.; Zheng, M.L.; Zuo, S.S.; Jiang, D.; Xu, C.C. Effects of maize meal and limestone on the fermentation profile and aerobic stability of smooth bromegrass silage. Grass Forage Sci. 2018, 3, 622–629. [Google Scholar] [CrossRef]
- Gao, C.H.; Cao, H.; Ju, F.; Xiao, K.Q.; Huang, Q. Emergent transcriptional adaption facilitates convergent succession within a synthetic community. ISME J. 2021, 1, 46. [Google Scholar] [CrossRef]
- Hu, W.; Schmidt, R.J.; Mcdonell, E.E.; Klingerman, C.M.; Kung, L., Jr. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J. Dairy Sci. 2009, 92, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Gerlach, K.; Sudekum, K.H. Biogenic amines and gamma-amino butyric acid in silages: Formation, occurrence and influence on dry matter intake and ruminant production. Anim. Feed Sci. Technol. 2015, 210, 1–16. [Google Scholar] [CrossRef]
- Lima, R.; Lourenço, M.; Díaz, R.F.; Castro, A.; Fievez, V. Effect of combined ensiling of sorghum and soybean with or without molasses and lactobacilli on silage quality and in vitro rumen fermentation. Anim. Feed Sci. Technol. 2010, 155, 122–131. [Google Scholar] [CrossRef]
- Mu, L.; Xie, Z.; Hu, L.X.; Chen, G.H.; Zhang, Z.F. Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 2020, 315, 123772. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Zhao, S.; Feng, C.; Fan, X. Dynamics of the fermentation products, residual non-structural carbohydrates, and bacterial communities of wilted and non-wilted alfalfa silage with and without Lactobacillus plantarum inoculation. Front. Microbiol. 2022, 12, 824229. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Oude Elferink, S.J.W.H.; Spoelstra, S.F. Microbiology of ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA; Crop Science Society of America Inc.: Madison, WI, USA; Soil Science Society of America, Inc. Publications: Madison, WI, USA, 2003; pp. 31–93. [Google Scholar]
- Zheng, M.L.; Niu, D.Z.; Jiang, D.; Zuo, S.S.; Xu, C.C. Dynamics of microbial community during ensiling direct-cut alfalfa with and without LAB inoculant and sugar. J. Appl. Microbiol. 2017, 122, 1456–1470. [Google Scholar] [CrossRef]
- Guan, H.; Yan, Y.H.; Li, X.L.; Li, X.M.; Shuai, Y.; Feng, G.Y.; Ran, Q.F.; Cai, Y.M.; Li, Y.; Zhang, X.Q. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 2018, 265, 282–290. [Google Scholar] [CrossRef]
- Yang, L.L.; Yuan, X.J.; Li, J.F.; Dong, Z.H.; Shao, T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 2019, 275, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Duniere, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thevenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Pang, H.L.; Qin, G.Y.; Tan, Z.F.; Li, Z.W.; Wang, Y.P.; Cai, Y.M. Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst. Appl. Microbiol. 2011, 34, 235–241. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, N.; Heron, S. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Bucks, UK, 1991. [Google Scholar]
- Kung, L., Jr.; Taylor, C.C.; Lynch, M.P.; Neylon, J.M. The effect of treating alfalfa with Lactobacillus buchneri 40788 on silage fermentation, aerobic stability, and nutritive value for lactating dairy cows. J. Dairy Sci. 2003, 86, 336–343. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Muck, R.E. New trends in development and use of inoculants for silage. FEMS Microbiol. Lett. 1996, 19, 53–68. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Jiang, Y.; Cervantes, A.A.P.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef]
- Brabb, T.; Denise, N.; Andrew, B.; Martha, H. Chapter 23–Infectious Diseases. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents, 1st ed.; Suckow, M.A., Stevens, K.A., Wilson, R.P., Eds.; Academic Press/American College of Laboratory Animal Medicine: Cambridge, MA, USA, 2012; pp. 637–683. [Google Scholar] [CrossRef]
- Gundogan, N. Klebsiella. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 383–388. [Google Scholar] [CrossRef]



| Mulberry Leaves | Smooth Bromegrass | |
|---|---|---|
| Dry matter (%) | 26.67 | 14.79 |
| Crude protein (% DM) | 25.51 | 16.47 |
| Neutral detergent fiber (% DM) | 19.29 | 49.18 |
| Acid detergent fiber (% DM) | 13.82 | 25.83 |
| Water-soluble carbohydrate (% DM) | 3.90 | 7.45 |
| Lactic acid bacteria (lg cfu/g FM) | 3.85 | 5.69 |
| Coliform bacteria (lg cfu/g FM) | 5.15 | 6.79 |
| Mold (lg cfu/g FM) | 3.85 | 3.65 |
| Inoculation | Item | Ratio of Mulberry Leaves | p-Value | SEM | ||||
|---|---|---|---|---|---|---|---|---|
| ML100 | ML90 | ML80 | ML70 | ML60 | ||||
| CK | DM (% FM) | 29.66 ab | 27.62 b | 30.48 a | 30.08 a | 23.52 c | p < 0.001 | 0.73 |
| CP (% DM) | 26.69 a | 26.98 a | 25.05 b | 23.41 c | 23.20 c | p < 0.001 | 0.30 | |
| NDF (% DM) | 23.11 d | 25.18 c | 29.18 b | 28.62 b | 31.02 a | p < 0.001 | 0.39 | |
| ADF (% DM) | 20.53 c | 22.73 b | 21.53 bc | 24.70 a | 24.60 a | p < 0.001 | 0.39 | |
| LP | DM (% FM) | 31.53 a | 30.02 a | 30.28 a | 26.78 b | 25.21 b | 0.001 | 0.82 |
| CP (% DM) | 27.34 a | 25.26 b | 24.48 b | 24.30 b | 23.12 c | p < 0.001 | 0.31 | |
| NDF (% DM) | 23.27 c | 26.12 b | 28.34 a | 27.78 ab | 27.58 ab | 0.001 | 0.58 | |
| ADF (% DM) | 19.97 c | 19.97 c | 24.53 b | 23.47 b | 25.90 a | p < 0.001 | 0.40 | |
| pH | LA | AA | NH3-N | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Correlations of fermentation properties with alpha diversity indices | ||||||||
| Observed | 0.59 | 0.001 | −0.50 | 0.005 | 0.40 | 0.028 | 0.55 | 0.002 |
| Chao 1 | 0.59 | 0.001 | −0.50 | 0.005 | 0.40 | 0.028 | 0.54 | 0.002 |
| Shannon | 0.66 | <0.001 | −0.42 | 0.019 | 0.22 | 0.237 | 0.58 | 0.001 |
| Correlations of fermentation properties with the top 10 most abundant genera | ||||||||
| Lactobacillus | 0.46 | 0.011 | −0.37 | 0.045 | 0.01 | 0.966 | 0.64 | <0.001 |
| Unassigned | 0.75 | <0.001 | −0.35 | 0.056 | 0.11 | 0.573 | 0.78 | <0.001 |
| Enterobacter | 0.71 | <0.001 | −0.38 | 0.036 | 0.08 | 0.678 | 0.73 | <0.001 |
| Enterococcus | 0.74 | <0.001 | −0.39 | 0.035 | 0.25 | 0.185 | 0.70 | <0.001 |
| Weissella | 0.69 | <0.001 | −0.43 | 0.017 | 0.30 | 0.108 | 0.65 | <0.001 |
| Pediococcus | 0.62 | <0.001 | −0.29 | 0.124 | 0.15 | 0.432 | 0.52 | 0.003 |
| Lactococcus | 0.71 | <0.001 | −0.41 | 0.026 | 0.23 | 0.233 | 0.67 | <0.001 |
| Clostridium sensu stricto | 0.53 | 0.003 | −0.15 | 0.445 | −0.08 | 0.660 | 0.43 | 0.017 |
| Klebsiella | 0.70 | <0.001 | −0.28 | 0.131 | 0.10 | 0.585 | 0.73 | <0.001 |
| Citrobacter | 0.53 | 0.003 | −0.36 | 0.054 | 0.41 | 0.026 | 0.57 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Yang, F.; Feng, C.; Zhao, S.; Zhang, X.; Wang, Y. Fermentation Properties and Bacterial Community Composition of Mixed Silage of Mulberry Leaves and Smooth Bromegrass with and without Lactobacillus plantarum Inoculation. Fermentation 2023, 9, 279. https://doi.org/10.3390/fermentation9030279
Yang W, Yang F, Feng C, Zhao S, Zhang X, Wang Y. Fermentation Properties and Bacterial Community Composition of Mixed Silage of Mulberry Leaves and Smooth Bromegrass with and without Lactobacillus plantarum Inoculation. Fermentation. 2023; 9(3):279. https://doi.org/10.3390/fermentation9030279
Chicago/Turabian StyleYang, Weihan, Fengyuan Yang, Changsong Feng, Shanshan Zhao, Xueying Zhang, and Yanping Wang. 2023. "Fermentation Properties and Bacterial Community Composition of Mixed Silage of Mulberry Leaves and Smooth Bromegrass with and without Lactobacillus plantarum Inoculation" Fermentation 9, no. 3: 279. https://doi.org/10.3390/fermentation9030279
APA StyleYang, W., Yang, F., Feng, C., Zhao, S., Zhang, X., & Wang, Y. (2023). Fermentation Properties and Bacterial Community Composition of Mixed Silage of Mulberry Leaves and Smooth Bromegrass with and without Lactobacillus plantarum Inoculation. Fermentation, 9(3), 279. https://doi.org/10.3390/fermentation9030279





