Succinic Production from Source-Separated Kitchen Biowaste in a Biorefinery Concept: Focusing on Alternative Carbon Dioxide Source for Fermentation Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Enzymatic Hydrolysis
2.3. Succinic Acid Fermentation in Bioreactors
2.4. Anaerobic Digestion (AD)
2.5. Calculations
2.5.1. Enzymatic Hydrolysis
2.5.2. Succinic Fermentation
2.6. Analytical Methods
3. Results and Discussion
3.1. Characterization of the Organic Fraction of Household Kitchen Waste (OFHKW)
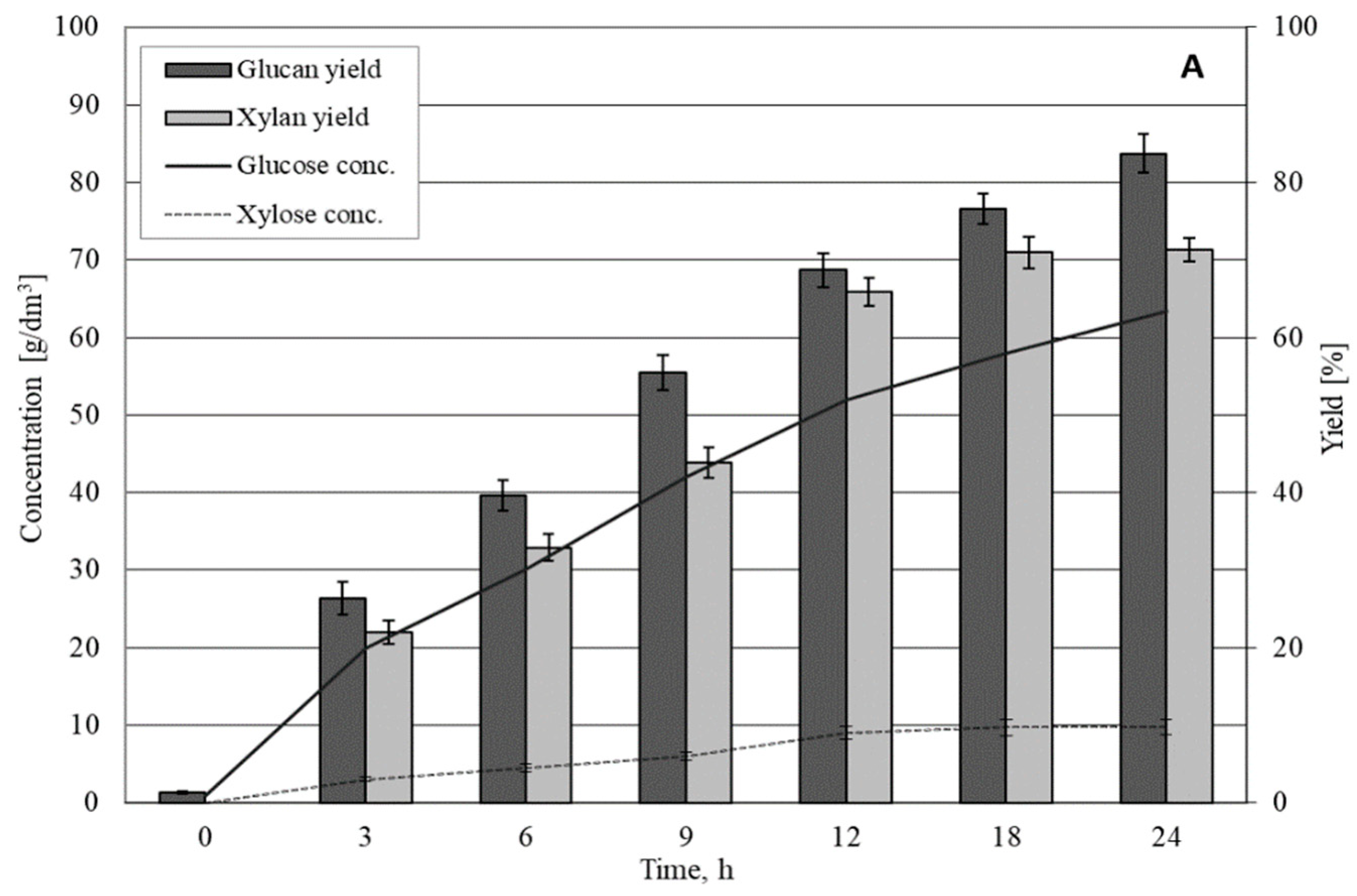
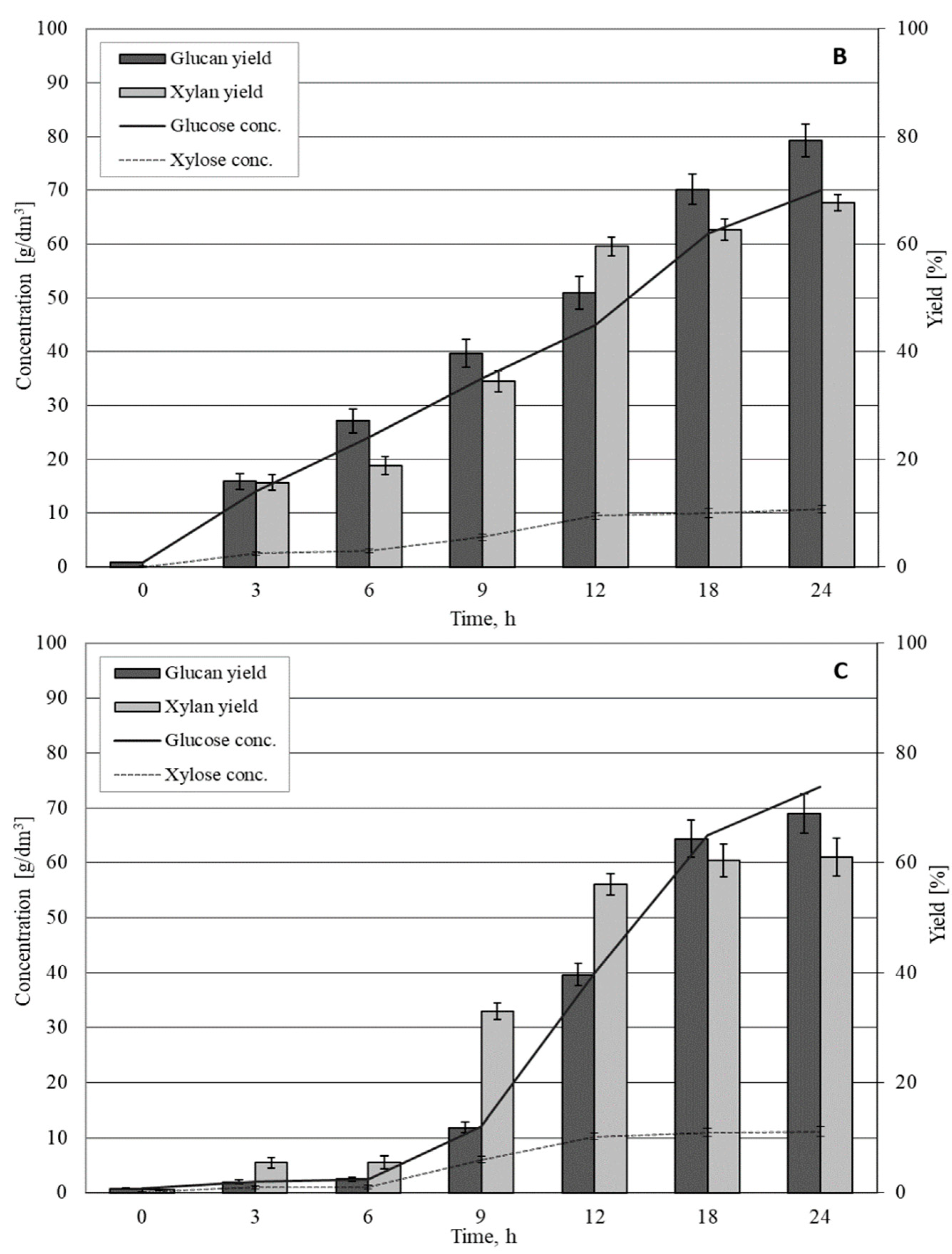
3.2. Enzymatic Hydrolysis of Solid Fraction from OFHKW
| Assay | Initial Glucose and Xylose b, g/dm3 | After 24 h of Enzymatic Hydrolysis | |||||
|---|---|---|---|---|---|---|---|
| Glucose, g/dm3 | Glucan Yield, % | Xylose, g/dm3 | Xylan Yield, % | Total Sugar, g/dm3 | Total Yield, % | ||
| Wet substrate loading: 20% (% w/v) Dry matter loading: 6.3% ± 0.6 | 29.7 ± 0.9 | 23.6 ± 1.1 | 93.3 ± 1.5 | 3.75 ± 0.2 | 82.3 ± 2.5 | 27.3 ± 1.2 | 91.7 ± 1.1 |
| Wet substrate loading: 40% (% w/v) Dry matter loading: 12.5% ± 1.2 | 59.6 ± 1.9 | 47.0 ± 3.0 | 93.0 ± 3.6 | 7.40 ± 0.5 | 81.0 ± 2.6 | 54.4 ± 3.4 | 91.2 ± 3.3 |
| Wet substrate loading: 60% (% w/v) Dry matter loading: 18.9% ± 1.9 | 89.4 ± 2.9 | 63.4 ± 3.8 | 83.6 ± 2.5 | 9.75 ± 0.5 | 71.3 ± 1.5 | 73.2 ± 4.2 | 81.8 ± 2.3 |
| Wet substrate loading: 70% (% w/v) Dry matter loading: 22.0% ± 2.2 | 104 ± 3.4 | 70.0 ± 3.1 | 79.3 ± 3.0 | 10.8 ± 0.7 | 67.6 ± 2.5 | 80.8 ± 3.6 | 77.5 ± 2.7 |
| Wet substrate loading: 80% (% w/v) Dry matter loading: 25.0% ± 2.5 | 119 ± 3.8 | 73.9 ± 6.1 | 69.0 ± 3.6 | 11.1 ± 0.9 | 61.0 ± 3.6 | 85.0 ± 6.8 | 71.3 ± 3.4 |
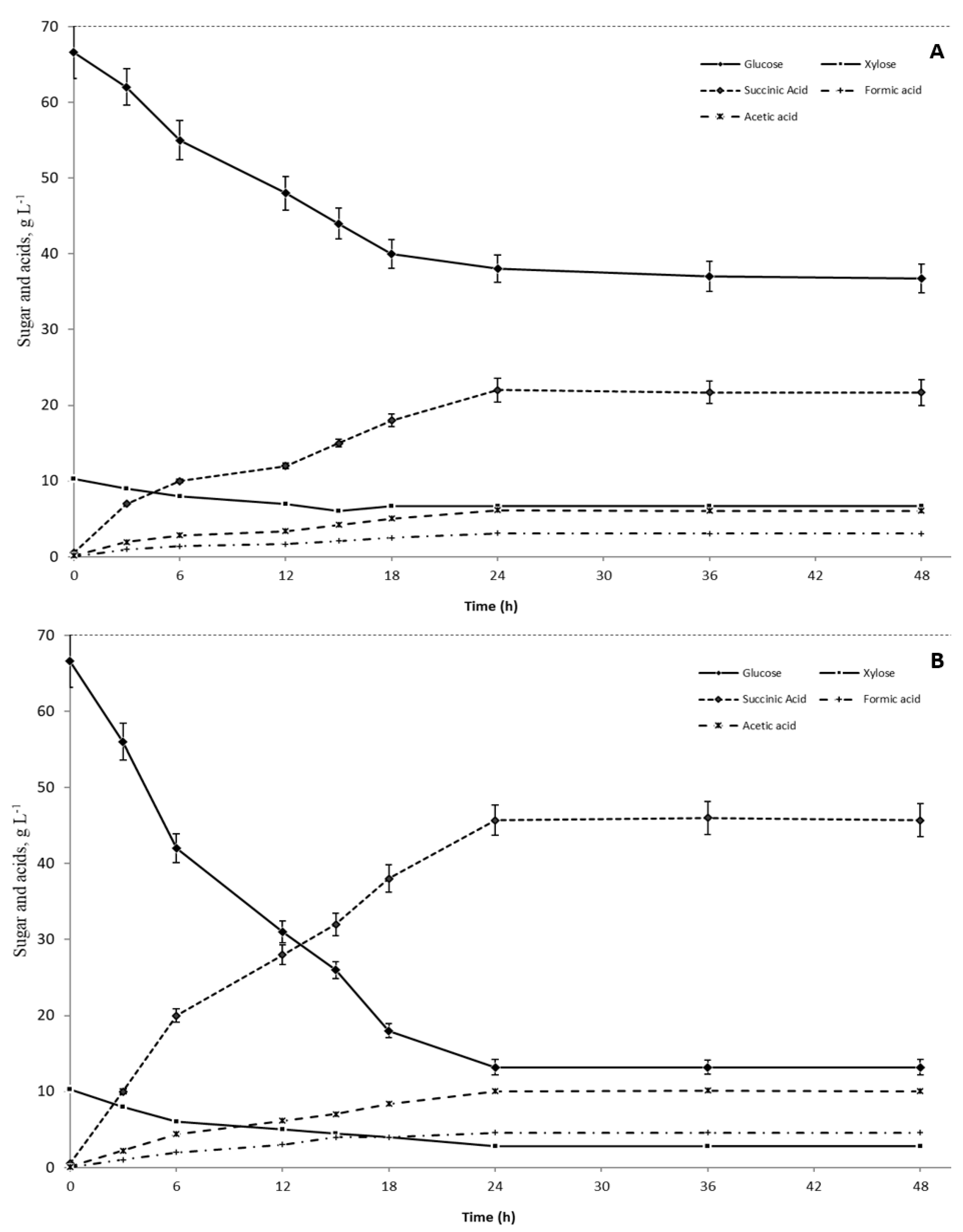
3.3. Succinic Fermentation Using Different Carbon Sources
| CO2 Source | Nutrients for Ferm. a | After Succinic Fermentation (48 h) | Biogas after Succinic Production g | |||||
|---|---|---|---|---|---|---|---|---|
| Glucose, g/dm3 | Xylose, g/dm3 | Sugar Utiliz., % | Succinic Acid, g/dm3 | Succinic Yield (Equation (3)), % f | CH4 % vol. | CO2 % vol. | ||
| MgCO3 (85–86 g/dm3) b | + | 13.8 ± 1.9 | 2.79 ± 0.3 | 78.5 ± 3.2 | 43.7 ± 3.0 | 72.4 ± 2.8 | - | - |
| MgCO3 (85–86 g/dm3) b | - | 14.1 ± 1.3 | 2.62 ± 0.4 | 78.1 ± 1.8 | 42.3 ± 2.5 | 70.5 ± 2.4 | - | - |
| Biogas as CO2 source | + | 36.9 ± 3.5 | 6.67 ± 0.8 | 42.4 ± 3.4 | 21.7 ± 1.7 | 63.0 ± 4.4 | 83.5 ± 1.6 | 17.2 ± 1.0 |
| MgCO3 (30–31 g/dm3) c + Biogas | - | 11.9 ± 2.2 | 2.40 ± 0.5 | 81.1 ± 3.6 | 46.3 ± 2.0 | 74.9 ± 3.3 | 87.5 ± 1.4 | 11.1 ± 1.0 |
| MgCO3 (20–21 g/dm3) d + Biogas | - | 13.2 ± 1.0 | 2.84 ± 0.3 | 79.0 ± 1.5 | 45.7 ± 2.5 | 75.4 ± 4.8 | 91.2 ± 1.5 | 8.65 ± 0.9 |
| MgCO3 (14–15 g/dm3) e + Biogas | - | 24.5 ± 2.1 | 2.92 ± 0.5 | 64.4 ± 2.7 | 34.3 ± 1.2 | 69.5 ± 2.1 | 90.2 ± 1.4 | 9.25 ± 0.8 |
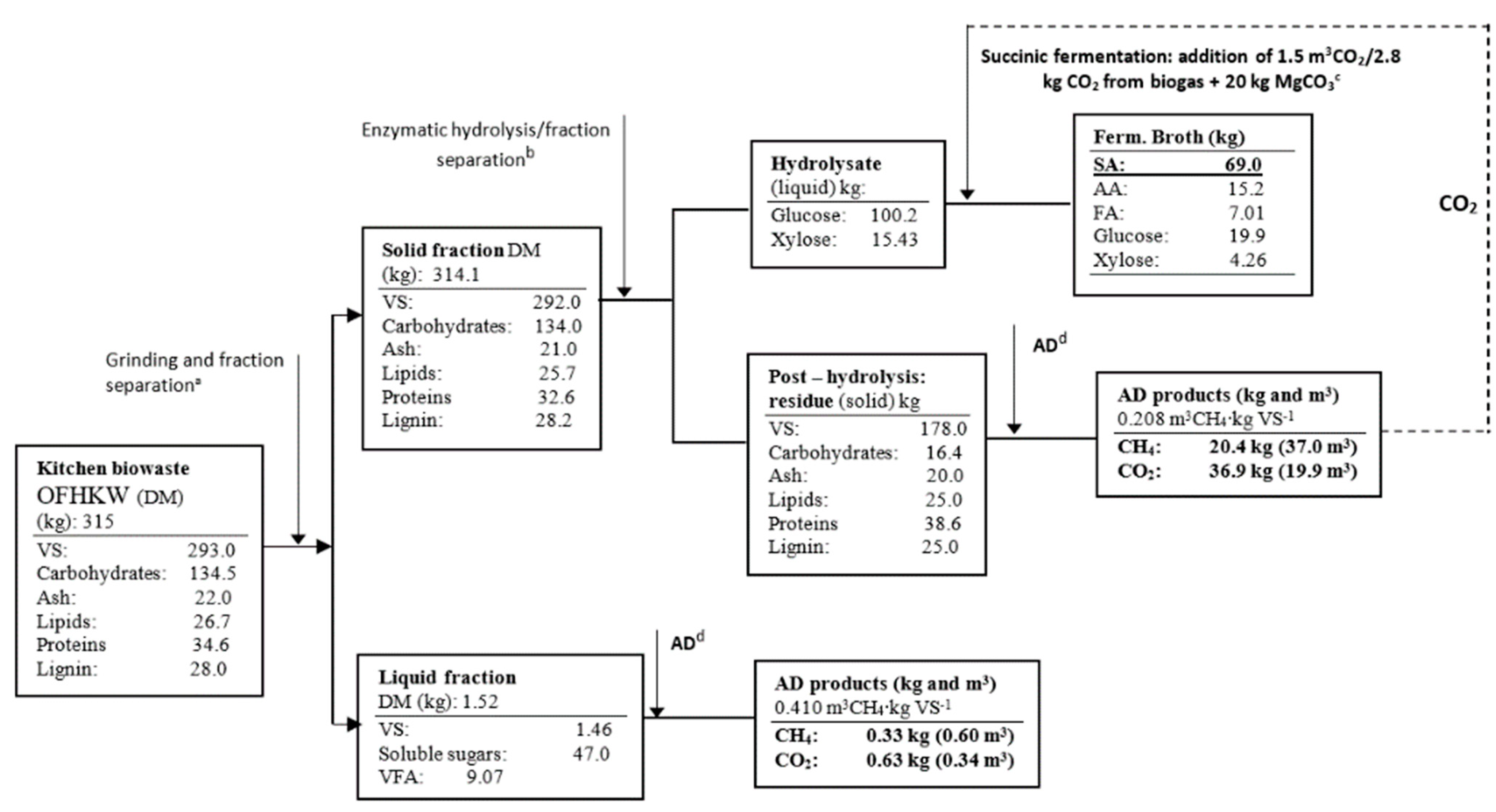
3.4. Anaerobic Digestion (AD) of Succinic By-Products
3.5. Biorefinery Concept
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pateraki, C.; Patsalou, M.; Vlysidis, A.; Kopsahelis, N.; Webb, C.; Koutinas, A.A.; Koutinas, M. Actinobacillus succinogenes: Advances on succinic acid production and prospects for development of integrated biorefineries. Biochem. Eng. J. 2016, 112, 285–303. [Google Scholar] [CrossRef]
- Dessie, W.; Xin, F.; Zhang, W.; Jiang, Y.; Wu, H.; Ma, J.; Jiang, M. Opportunities, challenges, and future perspectives of succinic acid production by Actinobacillus succinogenes. Appl. Microbiol. Biotechnol. 2018, 102, 9893–9910. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Zhao, X.B.; Zeng, J.; Zhang, J.A. Biotechnological production of succinic acid: Current state and perspectives. Biofuels Bioprod. Biorefin. 2012, 6, 302–318. [Google Scholar] [CrossRef]
- Gunnarsson, I.B.; Alvarado-Morales, M.; Angelidaki, I. Utilization of CO2 fixating bacterium Actinobacillus succinogenes 130Z for simultaneous biogas upgrading and biosuccinic acid production. Environ. Sci. Technol. 2014, 48, 12464–12468. [Google Scholar] [CrossRef]
- Zou, W.; Zhu, L.W.; Li, H.M.; Tang, Y.J. Significance of CO2 donor on the production of succinic acid by Actinobacillus succinogenes ATCC 55618. Microb. Cell Fact. 2011, 10, 87. [Google Scholar] [CrossRef]
- Herselman, J.; Bradfield, M.F.A.; Vijayan, U.; Nicol, W. The effect of carbon dioxide availability on succinic acid production with biofilms of Actinobacillus succinogenes. Biochem. Eng. J. 2017, 117A, 218–225. [Google Scholar] [CrossRef]
- Tan, J.P.; Luthfi, A.A.I.; Manaf, S.F.A.; Wu, T.Y.; Jahim, J.M. Incorporation of CO2 during the production of succinic acid from sustainable oil palm frond juice. J. CO2 Util. 2018, 26, 595–601. [Google Scholar] [CrossRef]
- Babaei, M.; Tsapekos, P.; Alvarado-Morales, M.; Hosseini, M.; Ebrahimi, S.; Niaei, A.; Angelidaki, I. Valorization of organic waste with simultaneous biogas upgrading for the production of succinic acid. Biochem. Eng. J. 2019, 147, 136–145. [Google Scholar] [CrossRef]
- Lyager, J.K.; Anthonisen, S. Valorisation of Sugar-Containing Wastes to Produce 2G Succinic Acid in Biorefinery Concept; Graduation Project; Technical University of Denmark: Lyngby, Denmark, 2018. [Google Scholar]
- Stenmark, Å.; Jensen, C.; Quested, T.; Moates, G. Estimates of European Food Waste Levels; Report; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016. [Google Scholar] [CrossRef]
- Mohanty, A.; Mankoti, M.; Rout, P.R.; Meena, S.S.; Dewan, S.; Kalia, B.; Varjani, S.; Won, J.W.C.; Banu, J.R. Sustainable utilization of food waste for bioenergy production: A step towards circular bioeconomy. Int. J. Food Microbiol. 2022, 365, 109538. [Google Scholar] [CrossRef]
- González-Torre, P.L.; Coque, J. From Food Waste to Donations: The Case of Marketplaces in Northern Spain. Sustainability 2016, 8, 575. [Google Scholar] [CrossRef]
- Pleissner, D.; Lam, W.C.; Sun, Z.; Lin, C.S.K. Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour. Technol. 2013, 137, 139–146. [Google Scholar] [CrossRef]
- Kim, M.S.; Na, J.G.; Lee, M.K.; Ryu, H.; Chang, Y.K.; Triolo, J.M.; Yun, Y.M.; Kim, D.H. More value from food waste: Lactic acid and biogas recovery. Water Res. 2016, 96, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Jones, R.J.; Oram, L.; Massanet-Nicolau, J.; Guwy, A. Intensification of Acidogenic Fermentation for the Production of Biohydrogen and Volatile Fatty Acids—A Perspective. Fermentation 2022, 8, 325. [Google Scholar] [CrossRef]
- Ntaikou, I.; Menis, N.; Alexandropoulou, M.; Antonopoulou, G.; Lyberatos, G. Valorization of kitchen biowaste for ethanol production via simultaneous saccharification and fermentation using co-cultures of the yeasts Saccharomyces cerevisiae and Pichia stipitis. Bioresour Technol. 2018, 263, 75–83. [Google Scholar] [CrossRef]
- Siqueira, J.G.W.; Teixeira, N.A.; Vandenberghe, L.P.S.; Oliveira, P.Z.; Soccol, C.R.; Rodrigues, C. Update and Revalidation of Ghose’s Cellulase Assay Methodology. Appl. Biochem. Biotechnol. 2020, 191, 1271–1279. [Google Scholar] [CrossRef]
- Dąbkowska, K.; Alvarado-Morales, M.; Kuglarz, M.; Angelidaki, I. Miscanthus straw as substrate for biosuccinic acid production: Focusing on pretreatment and downstream processing. Bioresour. Technol. 2019, 278, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, I.B.; Kuglarz, M.; Karakashev, D.; Angelidaki, I. Thermochemical pretreatments for enhancing succinic acid production from industrial hemp (Cannabis sativa L.). Bioresour. Technol. 2015, 182, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Procedure 2008, 1617, 1–16. [Google Scholar]
- Stylianou, E.; Pateraki, C.; Ladakis, D.; Cruz-Fernández, M.; Latorre-Sánchez, M.; Coll, C.; Koutinas, A. Evaluation of organic fractions of municipal solid waste as renewable feedstock for succinic acid production. Biotechnol. Biofuels 2020, 13, 72. [Google Scholar] [CrossRef]
- da Silva, A.S.; Espinheira, R.P.; Teixeira, R.S.S.; de Souza, M.F.; Ferreira-Leitão, V.; Bon, E.P.S. Constraints and advances in high-solids enzymatic hydrolysis of lignocellulosic biomass: A critical review. Biotechnol. Biofuels 2020, 13, 58. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, Z.; Leung, C.C.J.; Han, W.; Lau, K.Y.; Li, M.; Lin, C.S. Valorisation of bakery waste for succinic acid production. Green Chem. 2013, 15, 690–695. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Gao, Q.Q.C.; Lin, C.S.K. Mixed Food Waste as Renewable Feedstock in Succinic Acid Fermentation. Appl. Biochem. Biotechnol. 2014, 174, 1822–1833. [Google Scholar] [CrossRef]
- Wang, C.; Su, X.; Sun, W.; Zhou, S.; Zheng, J.; Zhang, M.; Sun, M.; Xue, J.; Liu, X.; Xing, J.; et al. Efficient production of succinic acid from herbal extraction residue hydrolysate. Bioresour. Technol. 2018, 265, 443–449. [Google Scholar] [CrossRef]
- Kuglarz, M.; Rom, M. Influence of carbon dioxide and nitrogen source on sustainable production of succinic acid from miscanthus hydrolysates. Int. J. Environ. Sci. Dev. 2019, 10, 362–367. [Google Scholar] [CrossRef]
- Rigaki, A.; Webb, C.; Theodoropoulos, C. Double substrate limitation model for the bio-based production of succinic acid from glycerol. Biochem. Eng. J. 2020, 153, 107391. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valor. 2017, 8, 267–283. [Google Scholar] [CrossRef]
- Breitenmoser, L.; Dhar, H.; Gross, T.; Bakre, M.; Huesch, R.; Hugi, C.; Wintgens, T.; Kumar, R.; Kumar, S. Methane potential from municipal biowaste: Insights from six communities in Maharashtra, India. Bioresour. Technol. 2018, 254, 224–230. [Google Scholar] [CrossRef]
- Moussa, H.I.; Elkamel, A.; Young, S.B. Assessing energy performance of bio-based succinic acid production using LCA. J. Clean. Prod. 2016, 139, 761–769. [Google Scholar] [CrossRef]
| Parameter | Unit | Value |
|---|---|---|
| Solid fraction of organic fraction of household kitchen waste (“OFHKW”, 85% of total sample weight)a | ||
| Total solids (TS) | g/kg | 315 ÷ 31 |
| Volatile solids (VS) | g/kg | 293 ÷ 29 |
| Total organic carbon (TOC) | %TS | 57.3 ÷ 2.5 |
| Carbohydrates | %TS | 42.7 ÷ 3.1 |
| Cellulose | %TS | 29.2 ÷ 2.4 |
| Starch | %TS | 7.10 ÷ 0.4 |
| Hemicellulose | %TS | 6.40 ÷ 0.3 |
| Nitrogen (TKN) | %TS | 11.0 ÷ 0.55 |
| Lipid content | % TS | 8.46 ÷ 0.65 |
| Lignin | % TS | 8.90 ÷ 0.80 |
| Ash | % TS | 2.20 ÷ 0.15 |
| Ca | g/kg TS | 9.15 ± 0.80 |
| Mg | g/kg TS | 1.25 ± 0.10 |
| P | g/kg TS | 3.05 ± 0.12 |
| S | g/kg TS | 2.10 ± 0.11 |
| Na | g/kg TS | 3.55 ± 0.21 |
| K | g/kg TS | 4.52 ± 0.20 |
| Fe | g/kg TS | 0.52 ± 0.03 |
| Mn | mg/kg TS | <1.0 |
| Ni | mg/kg TS | 1.20 ± 0.10 |
| Cu | mg/kg TS | 0.82 ± 0.2 |
| Cd | mg/kg TS | <1.0 |
| Cr | mg/kg TS | <1.0 |
| Hg | mg/kg TS | <0.1 |
| Ni | mg/kg TS | <1.0 |
| Pb | mg/kg TS | <2.0 |
| Zn | mg/kg TS | 3.2 ± 0.4 |
| Liquid fraction of organic fraction of household kitchen waste (“OFHKW”, 15% of total sample weight) a | ||
| pH | - | 4.8–5.2 |
| Total solids (TS) | g/dm3 | 15.0 ± 1.2 |
| Volatile solids (VS) | g/dm3 | 14.2 ± 1.2 |
| NH4+ | g/dm3 | 0.62 ± 0.10 |
| PO43− | g/dm3 | 0.11 ± 0.01 |
| VFA (acetic acid) | g/dm3 | 0.55 ± 0.03 |
| Soluble sugars | g/dm3 | 5.2 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuglarz, M.; Angelidaki, I. Succinic Production from Source-Separated Kitchen Biowaste in a Biorefinery Concept: Focusing on Alternative Carbon Dioxide Source for Fermentation Processes. Fermentation 2023, 9, 259. https://doi.org/10.3390/fermentation9030259
Kuglarz M, Angelidaki I. Succinic Production from Source-Separated Kitchen Biowaste in a Biorefinery Concept: Focusing on Alternative Carbon Dioxide Source for Fermentation Processes. Fermentation. 2023; 9(3):259. https://doi.org/10.3390/fermentation9030259
Chicago/Turabian StyleKuglarz, Mariusz, and Irini Angelidaki. 2023. "Succinic Production from Source-Separated Kitchen Biowaste in a Biorefinery Concept: Focusing on Alternative Carbon Dioxide Source for Fermentation Processes" Fermentation 9, no. 3: 259. https://doi.org/10.3390/fermentation9030259
APA StyleKuglarz, M., & Angelidaki, I. (2023). Succinic Production from Source-Separated Kitchen Biowaste in a Biorefinery Concept: Focusing on Alternative Carbon Dioxide Source for Fermentation Processes. Fermentation, 9(3), 259. https://doi.org/10.3390/fermentation9030259







