Abstract
Microalgae represent a promising solution in addressing the impacts associated with the current agricultural and manufacturing practices which are causing irreparable environmental damage. Microalgae have considerable biosynthetic potential, being a rich source of lipids, proteins, and high-value compounds. Under the scope of the H2020-BBI MULTI-STR3AM project, an innovative large-scale production system of valuable commodities for the food, feed, and fragrance sectors is being developed on the basis of microalgae, reducing costs, increasing the scale of production, and boosting value chain sustainability. In this work, we aimed to create a process model that can mimic an industrial plant to estimate mass and energy balances, optimize scheduling, and calculate production costs for a large-scale plant. Three autotrophic microalgae strains (Nannochloropsis sp., Dunaliella sp. and Spirulina sp.) were considered for this assessment, as well as the use of locally sourced CO2 (flue gas). The developed process model is a useful tool for obtaining the data required for techno-economic analysis (TEA) and life cycle assessment (LCA) of industrial biorefinery-based processes. Nannochloropsis sp. was the most economic option, whereas Dunaliella sp. was the most expensive strain to produce due to its lower productivity. Preliminary environmental assessments of the climate change impact category revealed that water recirculation and the use of flue gas could lead to values of 5.6, 10.6, and 9.2 kgCO2eq·kgAFDW−1 for Nannochloropsis sp., Dunaliella sp., and Spirulina sp., respectively, with electricity and NaCl as the main contributors. The obtained data allow for the quantification of the production costs and environmental impacts of the microalgal biomass fractions produced, which will be fundamental for future comparison studies and in determining if they are higher or lower than those of the replaced products. The process model developed in this work provides a useful tool for the evaluation and optimization of large-scale microalgae production systems.
1. Introduction
It is well established that increasing levels of carbon dioxide and associated compounds in the atmosphere are linked to climate change. To reduce the effects of climate change, it is necessary to invest in a more carbon-neutral society and industry and promote sustainable production systems that are capable of reducing the atmospheric concentration of compounds which can contribute to global warming [1]. Microalgae cultivation has been referred to by countless studies as a promising method for atmospheric carbon capture as usually this type of microorganism can use carbon dioxide as a raw material for growth [2]. These microorganisms can grow in diverse water sources (fresh, saline, or wastewater) with high photosynthetic efficiencies and growth rates [3]. Furthermore, this type of biomass can be harvested all year round and its cultivation can be undertaken on non-arable or contaminated land, therefore minimizing competition with other agricultural systems for arable land [3]. The commercial interest in microalgae relies primarily on their capacity to naturally produce a myriad of bioactive compounds such as polysaccharides, carotenoids, antioxidants, dietary fiber, sterols, etc. [4]. Of particular interest among their contents is their protein and lipid composition, the latter being rich in compounds such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are currently mainly obtained from animal sources, making the diversification of their origin highly desired [5]. One of the most interesting characteristics of microalgal production is their potential positive environmental impact. The possibility of coupling autotrophic production with a CO2-rich output stream derived from polluting industrial facilities makes microalgae production not only interesting from an economic standpoint but also a precious tool for flue gas treatment [6].
The major roadblock to microalgae cultivation remains the technological limitations, particularly those associated with the high energy costs of the production, harvesting, and dewatering stages [7]. According to Paladino and Neviani [8], microalgal downstream processing alone can account for approximately 60% of the total cost of the production process. Additionally, the harvesting procedure can be somewhat inefficient and must be coupled with recirculation systems to minimize water and biomass losses, while incentivizing nutrient depletion from the culture medium. Taking this into consideration, several improvements to the cultivation, harvesting, and downstream processing of microalgae have been reported in the literature, such as the alteration of growth parameters (feed system, temperature, carbon source, etc.), harvesting (centrifugation, electrocoagulation), or dewatering (solar drying, spray drying) [9]. It is also interesting to consider the replacement of defined media and purified/distilled water with undefined complex media. The environmental impact of microalgal cultivation might be reduced through the use of wastewaters, slurries, or other assorted waste streams as sources of micronutrients that are necessary for growth [9].
To guarantee that the changes to the process will have the intended effect, no modification or optimization measures should be applied without the assessment of the techno-economic and environmental impact of said modifications and comparisons with the conventional production system. Process modeling and simulation using software-based tools is a useful methodology for the evaluation of process feasibility at scales larger than laboratory scale, such as pilot and industrial scales. Microalgae-based biorefineries have been studied by research groups using these software tools (such as Aspen Plus and SuperPro Designer), concluding that these processes often involve high capital expenditure (CAPEX) and operational expenditure (OPEX) values [10,11,12,13,14,15,16,17]. Life-cycle assessment (LCA) is a mathematical process which allows for the quantification of the impacts associated with a given production process through the analysis of its inputs and outputs, reactants, energy, waste streams, etc., throughout the entire value chain. It can be employed at the planning and optimization stages as a decision-making tool, allowing for the quantification and comparison of the environmental impacts from various operational configurations, permitting one to identify bottlenecks in the process, such as increased energy demand or the use of compounds that are easily replaceable with more sustainable alternatives [18].
MULTI-STR3AM, www.multi-str3am.com (accessed on 21 January 2023), a Horizon 2020—Bio-based Industries Joint Undertaking (H2020-BBI)-funded project, aims to provide valuable microalgal products for large end-users in the food, feed, and fragrance sectors by reducing costs, increasing scale, and boosting sustainability. Its products will include lipids for edible spreads; protein, carbohydrates, and lipids for feed ingredients for poultry, pigs, and ruminants; and protein and small organic compounds as building blocks for the fragrance industry. Several chemical building blocks/products with interesting commercial potential were identified, along with the microalgae strains from which they could be easily and efficiently obtained, taking into consideration that each production system should yield more than one product. The criteria for this choice were based upon inherent biosynthetic potential, flexibility in growth mode (autotrophic, mixotrophic, heterotrophic, sequential hetero-autotrophic), chemical composition (nitrogenous or proteinaceous fractions), production feasibility, and the associated product yields of each strain. The three autotrophic strains chosen as the basis for this project are part of the production profile of the company: Nannochloropsis sp. (S#1), Dunaliella sp. (S#2), and Spirulina sp. (S#3). Nannochloropsis sp. are marine unicellular microalgae, and the commercial interest resides in their capacity to accumulate omega-3 (n-3) long-chain polyunsaturated eicosapentaenoic acid (ω-3, LC-PUFA, EPA), a substance that is commercially available in the food and feed industry as nutritional supplement. Dunaliella sp. is especially interesting due to its capability to accumulate both glycerol and β-carotene under cultivation conditions inducing high oxidative stress. This unicellular microalga can be rather advantageous in an industrial setting as it does not possess a cellular wall, but rather a more fragile, more easily degradable mucilaginous glycoprotein membrane [19]. Finally, the main industrial interest in Spirulina sp. (Arthrospira sp.) resides not only in its unusually high protein content, ranging from 60–70% dry weight [20], but also to the presence of several essential fatty acids, pigments, vitamins and minerals making it a well-rounded “superfood” [21].
Under the scope of the MULTI-STR3AM project, the main goal of this work was to develop the conceptual design of a microalgae production plant, as well as its harvesting, dewatering, cell disruption, and aqueous fraction processes, through process simulation. The process model was based upon experimental data obtained in a commercial-scale biomass production unit and demonstration-scale downstream processing for the recovery of added-value products. When complete, the model can be used to assess the economics, process integration, optimization, and equipment design of the whole multi-biorefinery demo plant. Furthermore, it allows for the evaluation of mass and heat integration (reducing OPEX and process waste streams), equipment sizing, and scaling-up effects. In the present study, we report the preliminary techno-economic and life-cycle assessments obtained from the autotrophic production pathways of three different strains, modeled using SuperPro Designer software (Intelligen, Inc.).
2. Materials and Methods
2.1. Feedstock Identification and Process Description
Focusing on the multi-product fractions to be obtained, three selected microalgae strains were produced autotrophically and partially fractionated. For this publication, the studied strains were Nannochloropsis sp. (S#1), Dunaliella sp. (S#2), and Spirulina sp. (S#3). In the scope of the present work, the analysis focuses on the production of each strain and potential constraints and bottlenecks to be surpassed.
Microalgae production was undertaken in two distinct cultivation systems: closed tubular photobioreactors (TPBR, S#2) and open cascade raceways (CRW, S#1 and S#3). Microalgae production data used in process simulations was collected from industrial TPBRs and CRWs. The cultivation conditions are summarized in Table 1.

Table 1.
Process conditions used for microalgae cultivation.
Following the cultivation stage, the microalgae biomass was harvested and subjected to cell disruption and dewatering. The resulting ruptured biomass was then processed to recover both aqueous and solid fractions.
2.2. Process Simulation
Figure 1 depicts a flowchart of the proposed integrated biomass production and biorefinery model. The production system varies according to the suited cultivation conditions of each strain and the necessary stages for their processing, i.e., cell rupture requirements, solid/liquid fractioning, etc. The model analysis was based on the autotrophic production of three microalgae strains. The production process was divided into three subsystems: SS1—Microalgae production; SS2—Harvesting and dewatering; and SS3—Aqueous fraction processing (AF). The subsystems SS4 and SS5 represent further fraction refining and processing, which, due to the preliminary stage of this analysis, were not considered in this study.
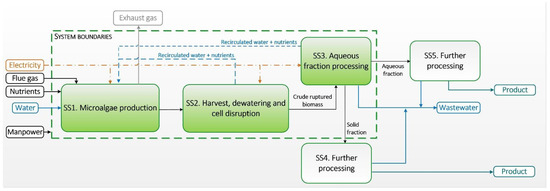
Figure 1.
Flowchart of microalgae production and processing into various fractions of interest.
Two different scales were considered for this process simulation study, 10 and 270 tonAFDW·year−1, corresponding to the MULTI-STR3AM demonstration scale and a model large-scale microalgae facility, respectively. The facilities of the partner company, A4F—Algae for Future, are located in Póvoa de Santa Iria, Portugal (ca. 10 km North from Lisbon); thus, all the technical, economic, and environmental assessments were performed in the Portuguese context.
Mass and energy balances were determined through simulation using SuperPro Designer v10 (Intelligen, Inc., Scotch Plains, NJ, United States of America) in order to quantify input requirements and predicted processual outputs. The process simulations of S#1, S#2, and S#3 production considered an autotrophic regimen during which flue gas from a nearby industrial facility provided the CO2 required for cellular growth. Culture medium data were obtained directly from the company A4F—Algae for Future (Póvoa de Santa Iria, Portugal), disregarding micronutrient content since its impact was relatively negligible on the overall analysis. Microalgae samples were submitted to elemental and proximal characterization analysis. The obtained data were used for calculation of the empirical molecular formula and the associated growth chemical simulations (depicted in Table 2). Minor elements such as chloride or sulfur were not considered for the simulation as their sum was quantified as <10% of the total dry biomass weight. CO2, NO3−, and H2O were considered as the sole sources of carbon, nitrogen, and hydrogen, respectively.

Table 2.
Chemical reactions expressing the conversions undertaken during the microalgae production stage.
Four scenarios were considered for analysis: (Scenario 1) no water recirculation applied and the use of industrial gaseous CO2 as a carbon source, which was the worst-case scenario for comparison purposes only and was not applied in the industrial plant; (Scenario 2) the use of flue gas as a carbon source; (Scenario 3) the application of water recirculation; and (Scenario 4) the use of flue gas as a carbon source with the application of water recirculation (optimized scenario, currently applied in the production facility). Each scenario was applied to the selected strains. A brief description of the considered subsystems follows.
2.2.1. Microalgae Production (SS1)
This subsystem included the culture medium preparation and the microalgae production system for each strain assessed (CRW, for S#1 and S#3; TPBR, for S#2). The cultivation conditions referred to in Section 2.1 were used during simulations. Additionally, two forms of carbon dioxide were also considered: food-grade CO2 and flue-gas-derived CO2 (22% CO2 mass composition), obtained from an adjacent sodium chlorate and hydrogen peroxide production plant. Both CO2 sources were added according to the stoichiometrically necessary quantity to produce a given biomass amount per month. Nitrogen and oxygen production were quantified according to biomass production equations (Table 2). The remaining nutrient consumption was defined as 20%. Water and nutrients were recycled into the system from SS3-AF and the consumed nutrients were replenished to their initial concentrations, as necessary.
2.2.2. Harvesting, Dewatering, and Cell Disruption (SS2)
The exhausted culture medium and produced biomass were collected from the bioreactors and processed through the harvesting unit for water and nutrient recuperation. Ninety percent of the stream was recirculated into the bioreactor. The concentrated biomass was conducted into cell disruption equipment as to rupture the cellular wall/membrane and release intracellular components. Processing unit #1 yield was defined as 95% for the three strains considered and product recuperation in the subsequent stream was quantified according to the proximal composition. The resulting stream was conducted into processing unit #2, resulting into two separate fractions. Particulate component recovery to Fraction #2 was considered, as a first approach, to be 100% for microalgal biomass, ash, and released lipids. The secondary stream was conducted into the following subsystem (SS3).
2.2.3. Aqueous Fraction Processing (SS3)
The aqueous stream obtained from SS2 was introduced into processing unit #3 for the recovery of water and non-consumed nutrients. Recovery was defined as 90% of the original stream and then recirculated into SS1 for a new production cycle. Soluble biomass-derived components were obtained entirely from Fraction #1. The model developed in SuperPro Designer is represented in Figure 2.
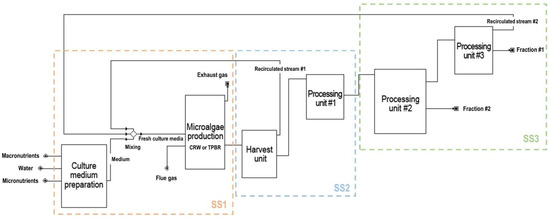
Figure 2.
Simplified flowchart of the model developed in SuperPro Designer for biomass production and downstream processing of the three studied microalgae strains. The microalgae production system (CRW or TPBR) varies according to the species being produced.
2.3. Economic Assessment
In order to estimate the production cost per kg of biomass, operating costs (OPEX) were calculated according to data provided by A4F and freely available price information at a value added tax (VAT) of 23%. Labor costs were calculated on the basis of two operators per shift, three shifts per day, each lasting for 8 h. The value of labor cost per hour was considered as EUR 6.52, according to the average salary values for the Portuguese context, unless otherwise denoted. Human resources costs for the 270 tonAFDW·year−1 production case were supplied by A4F. Three hundred and thirty-five operating days were considered for the calculations. The reference year for the price of raw materials and utilities was set as 2019, to avoid the bias of the COVID-19 pandemic and the Ukraine/Russia conflict and their associated inflation rates. The harmonized index of consumer prices (HICP) was obtained from Eurostat [25]. Capital expenditure (CAPEX), revenues, cash flow and net-present value (NPV) were not considered at this preliminary stage. Thus, the OPEX parcels depending on CAPEX (e.g., CAPEX depreciation, maintenance, overhead, and fixed and general costs) were not taken into account in these early estimates.
2.4. Life-Cycle Assessment
For the LCA, the functional unit (FU) was defined as 1 kg of ash-free dry-weight (AFDW) biomass produced and processed in the system. Following ISO 14040:2006, life-cycle impact assessment (LCIA) was performed from cradle to gate using SimaPro software (v9.4.0.1) with the Ecoinvent database (v3.8) and by applying the environmental footprint impact assessment method (EF 3.0). The environmental footprint (EF) is an initiative of the European Commission, establishing a common methodological approach for quantifying the environmental performance of any good or service throughout its life cycle [26]. The product environmental footprint (PEF) concept builds on existing approaches and international standards, its purpose being to reduce the environmental impacts of goods and services considering supply-chain activities (from the extraction of raw materials, through production and use, to final waste management). The PEF method enables researchers to conduct studies that are more reproducible, comparable, and verifiable, in comparison to existing alternative methods [27].
The life-cycle inventory used for the assessment was obtained from operational data collected at the industrial site and from mass and energy balances made available via the SuperPro Designer model. The sub-systems described in Section 2.2 were considered for the assessment.
A Monte Carlo simulation was also performed to reflect the effects of uncertain factors more accurately. Various distributions, such as range, triangular, normal, and lognormal distributions, have been considered for uncertainty analyses of inventory data. The Monte Carlo simulation approach takes a random value from the result of the data quality index in an uncertainty distribution for each uncertain input data and computes LCA results for this set of sampled values. Standard uncertainty factors from SimaPro software were considered and one thousand iterations were performed to obtain the uncertainty distribution.
3. Results and Discussion
The main objective of the present study was to devise a mathematical model which could provide the basis for the economic and environmental assessment of the production of various microalgal strains. This model was based upon an already-operating industrial-scale facility and the resulting production data. Theoretically, we intended this model to transcribe, as accurately as possible, the mass and energy balances of the production of a given microalgae, as well as its associated impacts, when supplied with operational and productivity data.
3.1. Mass and Energy Balances
As described in the previous section, three strains were considered to evaluate the accuracy of the model: Nannochloropsis sp., Dunaliella sp., and Spirulina sp., hereon described as S#1, S#2, and S#3, respectively. The first requirement when developing the model was to ensure that it would give coherent results even when describing microalgal production undertaken on different production systems (CRW vs. TPBR). The application of the model to the selected strains denoted key variations in microalgal productivity, i.e., the same mathematical model yielded a total production value for S#2 that was approximately 12.5% of the production value attained for S#1, and 13.8% of the production value attained for S#3. This variation is a result of the microorganisms’ behavior under the operation conditions optimized for carotenoid production. Table 3 summarizes the mass flows and electricity usage (per day and per kg of AFDW biomass) for specific streams that were obtained through simulation, for each strain that was evaluated at the actual production scale.

Table 3.
Mass and energy balances for each strain production, obtained from the SuperPro Designer model, at the actual production scale.
Yearly production quantities of 5.63, 0.70, and 5.09 tonsAFDW·year−1 were quantified for S#1, S#2, and S#3, respectively. These values were associated with a yearly CO2 consumption of 11.15, 1.31, and 10.02 tons and a concurrent atmospheric oxygen production of 12.58, 1.41, and 11.85 tons for S#1, S#2, and S#3. Particularly in terms of CO2 consumption, S#1 and S#3 showed similar daily amounts, whereas the carbon dioxide needed for S#2 was almost eight times lower, in proportion with the generated microalgal biomass. Nonetheless, when looking at the consumption per kgAFDW, all microalgae showed similar carbon dioxide consumption values, with a slightly higher value for S#1 and S#3. Regarding water, the daily consumption is roughly identical for S#1 and S#3, and was ca. two times lower for S#2, but when normalized per kgAFDW, S#2 consumed ca. three times more water per biomass produced than the remaining microalgae, which was due to the lower biomass productivity of S#2.
Regarding the electricity consumption in the different defined sub-systems, it was clear that SS1 was the main contributor (per day or per biomass produced), with the consumption being slightly higher for S#1 in the microalgae production process on a daily basis, although S#2 presented the highest electricity consumption per AFDW biomass produced. SS2 and SS3 represented a small portion of the electricity consumption for all strains.
Mass and energy balances for 10 and 270 tonAFDW·year−1 were extrapolated using the developed process model (results available in Supplementary Materials).
3.2. Economic Assessment
An OPEX calculation was performed, focusing on the costs of chemical inputs, water, electricity, and labor involved, while disregarding maintenance and CAPEX depreciation costs, allowing one to obtain preliminary production costs for each microalgae strain according to the assessed system. Table 4 summarizes the yearly operational costs and biomass production costs for Scenario 4. Labor costs were estimated considering the minimum salary for the Portuguese territory with a total of three shifts, consisting of two people per shift working at a total of 335 days per year. Nutrient costs were attained from commercial sources, which are freely available online.

Table 4.
S#1, S#2, and S#3 yearly operating costs according to Scenario 4 (water recirculation and use of flue gas) for both assessed scales, 2019 prices.
The analysis of the production costs of the three strains indicated, as expected, a strong contribution of the raw materials, apart from the labor, that was particularly visible in the costs associated with the production of S#3 due to the use of a more costly nutritive medium (particularly, dipotassium phosphate, representing 50% of the raw materials cost). Higher productivity values per year compensated for this effect, whereas the opposite was observed for S#2 production, which showed the highest production cost among the assessed strains. Quantified production costs remained in the expected range for this type of system, being even lower than the value of 43.3 EUR·kg−1 reported by Rosas et al. [29]. Furthermore, the production process reported in this work led to a much lower production cost for S#1 as compared to the value of 53.32 EUR·kgDW−1 reported by Vásquez-Romero et al. for a production scale of 27.61 ton·year−1 [30]. However, it is important to highlight that the operating costs calculated in this study do not include the capital depreciation costs (associated to CAPEX). The freshwater cost was much higher for S#2, due to lower productivity, as well as the electricity costs, as expected. As no differences were considered in terms of labor (the same number of workers, shifts, and cost per worker), this cost was equal for all strains for each scale.
3.3. Environmental Assessment
LCA was performed according to the methodology described in Section 2. As previously stated, four distinct scenarios were considered. Scenario 1 considers the production of microalgae under the addition of food-grade (purified) CO2, while disregarding the possibility of undertaking water recirculation. Table 5 summarizes the environmental impacts, in distinct categories, for each strain in all the defined scenarios. As expected, Scenario 1 was revealed to be the worst-case scenario as it implies the use of a higher amount of water and chemical inputs, particularly compounds such as sodium chloride (S#2).

Table 5.
Environmental impacts for each strain for the considered scenarios. Impact categories: CC—climate change; OD—ozone depletion; IR—ionizing radiation; POF—photochemical ozone formation; PM—particulate matter; HTNC—human toxicity, non-cancer; HTC—human toxicity cancer; A—acidification; FE—freshwater eutrophication; ME—marine eutrophication; TE—terrestrial eutrophication, FEC—freshwater ecotoxicity; LU—land use; WU—water use; FRU—fossil resource use.
From the perspective of a global overview, S#2 production exhibited higher impact for all categories in comparison to the other strains for all the assessed scenarios. This was mainly due to the lower productivity of this strain, thus leading to the use of higher amounts of raw materials and energy to produce the same weight of biomass. Looking deeper into some specific impact categories and taking climate change (CC) into consideration as a first step for the environmental analysis, quantification led to values of 13.23 and 16.13 kgCO2eq·kgAFDW−1 for S#1 and S#3, respectively, for Scenario 1. S#2 production led to CC values ca. two times higher due to the necessary input of NaCl at the microalgae production level for the generation of the saline environment, as in the Ecoinvent database NaCl is considered to be obtained from a salt mine. This effect can be considerably reduced by using marine salt, seawater, or a salt-rich industrial effluent stream in the culture media such as, for example, dairy production effluents, as referred to by Russo et al. [31]. Concerning freshwater ecotoxicity, disregarding S#2, one can observe that S#3 production had an impact approximately five times higher than that of S#1, which was related to the different culture media used in each case and thus the impact of nutrient production (as an input to the LCA) was divergent for those strains. One other impact category that is relevant to mention is the use of fossil resources, which was slightly higher for S#3 (once again disregarding S#2) due to the higher nutrient input required for the strain’s production.
Current practice at the MULTI-STR3AM demonstration plant accounts for water recirculation and replacement of the industrial water and NaCl input with a salt-rich industrial effluent stream (brine wastewater from a local salt production facility). For Scenarios 2, 3, and 4 the effect of using water recirculation and CO2 from a flue gas source was clearly identifiable, with Scenario 4 being the optimal scenario for all the assessed strains. In Table 6, the environmental impact distribution per sub-system is summarized for Scenario 1. From the three considered subsystems, SS1 was the main cause of impacts for all strains, attaining 93.0%, 92.8%, and 96.0% of the calculated CC impact for S#1, S#2, and S#3, respectively. Further analysis of the subsystem identified electricity, carbon and nitrogen sources, and sodium chloride as the main contributors for this value. The first was a direct consequence of the use of the Portuguese electricity mix for the calculation, a mix which still involves a major contribution from fossil fuels for its production (ranging from 40% to 50%), which is slowly being phased out according to national and European legislation. It is an easily optimizable factor, however, taking into consideration the steady increase in the renewable energy fraction at a rate of 2.6% for the past 7 years [32]. The replacement of fossil CO2 with a locally available renewable carbon source is strongly encouraged, as one of the strongest points behind the cultivation and use of microalgae remains its capacity to convert atmospheric carbon and light into chemical energy. The analysis also indicated particularly high values of impact categories for all analyzed strains related to fossil fuel use and those categories related to water usage, such as freshwater ecotoxicity and water use. From the type of production undertaken, it underlines a strong correlation between water usage and corresponding environmental impacts. The necessity of water recirculation was proven to be as important, if not moreso, than the use of CO2 coming from an industrial side-stream, as it not only strongly diminished water consumption, but also had a considerable impact on the reduction of the required concentration of media components in the fresh stream. This assumption was assessed accordingly in Scenario 3. The effect was particularly notable in the case of S#2 production, reducing water use from 55.5 to 9.2 m3deprived·kgAFDW-1 and culminating in a CC value of 13.6 kgCO2eq·kgAFDW-1 due to the drastic reduction in the necessary nutrient input. The same effect was noted in regard to S#1 and S#3 production, where the CC values decreased by 34 and 23%, respectively. Scenario 4 consisted of the replacement of industrial CO2 use with a locally available CO2-rich flue gas. The impact of this measure was not as dramatic, but it did contribute in terms of the circular economy by treating a gaseous stream from another industry through microalgae cultivation, reducing the associated greenhouse gas (GHG) emissions.

Table 6.
Environmental impact distribution (%) per sub-system (SS) in Scenario 1 for S#1, S#2, and S#3. Impact categories: CC—climate change; OD—ozone depletion; IR—ionizing radiation; POF—photochemical ozone formation; PM—particulate matter; HTNC—human toxicity, non-cancer; HTC—human toxicity cancer; A—acidification; FE—freshwater eutrophication; ME—marine eutrophication; TE—terrestrial eutrophication; FEC—freshwater ecotoxicity; LU—land use; WU—water use; FRU—fossil resource use.
The considered scenarios had a clear influence on some specific environmental impact categories. Figure 3 depicts the variations in climate change and freshwater toxicity categories for each strain in comparison to the worst-case scenario (Scenario 1). Regarding climate change, one can clearly identify the effect of the CO2 origin on S#1 production, since in Scenarios 2 and 4, in which flue gas was used as the carbon dioxide source, the impact was 35% and 59% lower than in Scenario 1, respectively. When there was water recirculation (Scenarios 3 and 4), the effect on the impact on freshwater toxicity was also visible for all the assessed strains. In the case of S#2, the impact of water recirculation was even more dramatic, reaching a reduction of 66% and 55% for CC and FW, respectively, even considering a lower biomass productivity value.
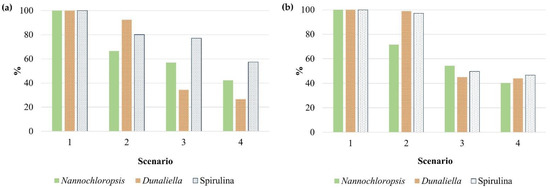
Figure 3.
Variations in (a) climate change and (b) freshwater toxicity impact categories in all scenarios, for the three assessed strains, in comparison to the base-case Scenario 1.
In Figure 4, the environmental impacts of six selected categories (climate change, freshwater toxicity, water use, ionizing radiation, land use, and fossil resource use) are represented for the optimized Scenario 4. The quantified CC values were 5.6, 10.6, and 9.2 kgCO2eq·kgAFDW−1, for S#1, S#2, and S#3, respectively. These values were primarily a result of the operation of subsystem SS1, with the highest contributor being electricity. This highlights the potential for improvement in environmental performance if the proportion of renewable energy in the electricity mix is increased. Taking S#1 as an example, a replacement such as the one described here could lead to a decrease in CC of 84.7%, culminating in a total value of 0.85 kgCO2eq·kgAFDW−1. This result is still higher than the one presented by Moradiya and Marathe [33], who reported a negative value of 1.28 kgCO2eq.kgalgae−1. That study was based upon laboratory results, which might explain the disparity between the results obtained. It is still indicative of the potential for further optimization of the Nannochloropsis sp. production process. The low productivity of S#2, in comparison with the remaining strains, worked to its detriment, as it requires far greater resource inputs and resulted in a direct increase in all environmental impact categories. Mimicking the marine environment through the addition of NaCl to industrial water should be compared with an additional scenario in which its replacement with marine water (pre-treated through sterilization or filtration) or a salt-rich industrial effluent stream is considered. Goswami et al. [34] underlined that the use of wastewater would be particularly appropriate for Dunaliella sp. growth as it seems capable of reducing phosphate and nitrogen compounds, as well as assorted metal ions. It is important to underline that the mathematical process carried out in this study was only focused on the analysis of the production and harvesting stages. This fact, the lack of commonality of the reported functional units, and the limited number of LCA analysis of the studied strains, as reported by Gaber et al. [35] and Thevarajah et al. [21], make it difficult to compare the values herein with those of similar studies. Such studies often consider additional processing steps for the obtention of value-added products or other energy sources, although they are comparable at the cultivation stage [36].
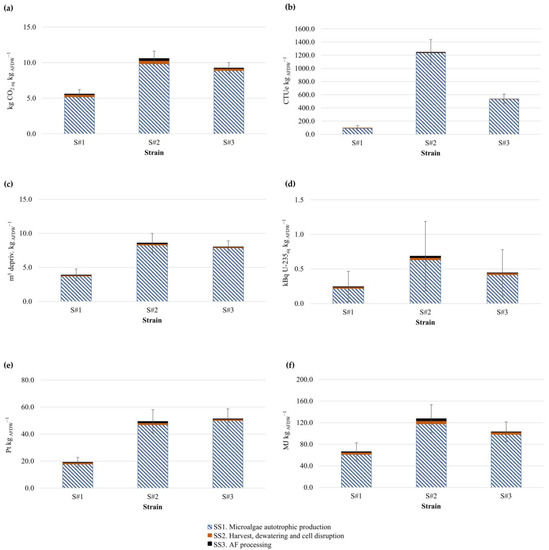
Figure 4.
Environmental impact comparison between the three produced microalgal strains (S#1, S#2, and S#3) for selected environmental categories in Scenario 4: (a) climate change; (b) freshwater toxicity; (c) water use; (d) ionizing radiation; (e) land use; (f) fossil resource use. Uncertainty values for the characterized LCIA profiles for each strain (the error bars represent the uncertainty based on confidence intervals of 95%).
It is evident that, for all the represented impact categories, SS1 (microalgae production) was the main contributor regardless of the strain being processed. SS2 (harvesting and dewatering) and SS3 (aqueous fraction processing) were virtually negligible, though it must be noted that this later subsystem was higher for S#2, in comparison to the other strains, in all the represented impact categories.
Regarding the uncertainty analysis of the life-cycle inventory (LCI) data quality, one can observe from Figure 4 that ionizing radiation (d) was the impact category with highest level of uncertainty among the results presented, particularly for S#2, showing error bars that could lead to the total inversion of the characterized impact for each strain. Climate change presented the lowest uncertainty among the assessed impact categories. LCI data quality could be significantly improved later on in the MULTI-STR3AM project as soon as validated data from the 10 tonAFDW·year−1 scale are available.
3.4. Multi-Strain Biorefinery
Concerning the biorefinery to be developed under the MULTI-STR3AM project, the cultivation of microalgal biomass will also be performed under heterotrophic conditions (for different strains than the ones here reported). It must be highlighted that the alteration of the produced strains and cultivation mode is expected to lead to different results, not only in the production stage but also in the downstream processing for added-value fraction recovery and purification. The integrated concept (multi-strain production being processed in the same biorefinery) will reduce the operating and capital costs of the whole plant and have a lower impact in terms of the environmental impact categories identified as bottlenecks in this work. Thus, further work is necessary to complement the reported data and validate the economic, environmental, and socio-economic sustainability of the multi-strain, multi-product, and multi-fraction concept.
4. Conclusions
Although sustainability studies are becoming more abundant, there is a lack of published research focused on large-scale microalgae cultivation. In the current study, we developed a mathematical model which allowed the estimation of microalgal production/processing and the associated environmental impacts and costs. Its conceptual design and validation were undertaken on the basis of real industrial-scale production data obtained using three different microalgae: Nannochloropsis sp. (S#1), Dunaliella sp. (S#2), and Spirulina sp. (S#3), and the model proved to be easily adaptable to each considered strain while delivering credible results. Regarding production costs, S#1 was revealed to be the most economic option, whereas S#2 the most expensive strain to produce/process mainly due to the lower productivity of the latter, which was most probably compensated for on the biorefinery side by the extraction of added-value products such as β-carotene and glycerol. Preliminary environmental analysis of the optimal scenario (i.e., scenario 4, with the application of water recirculation to the bioreactor and the use of flue gas as a carbon source) led to CC quantifications of 5.6, 10.6, and 9.2 kgCO2eq·kgAFDW−1 for S#1, S#2, and S#3, respectively. The principal contributors to this effect were identified as electricity (for all analyzed strains) and sodium chloride (for S#2). In order to improve environmental performance, we suggested that the use of NaCl and industrial water for the induction of a saline environment could be replaced with a marine water source or a salt-rich industrial effluent stream. Further improvement might be achieved via an increase in the renewable energy fraction in the electricity mix used.
The process model developed here is adaptable to various multi-strain microalgae production systems (autotrophic or heterotrophic) and can easily integrate the biorefining units to be developed during the project in order to obtain the multi-product fractions. Additional stages can be introduced according to the characteristics of the produced microalgae, such as the necessity of additional pretreatment stages, new product stream recovery, etc.
At this preliminary assessment stage, in which the end-product biomass fractions were not yet specified, and thus nor were the current products in the various markets that these fractions were able to replace, it was only possible to determine the environmental impacts of the microalgal biomass fractions produced, but it was not yet possible to determine whether their impact is higher or lower than the current impact of the replaced products. Nonetheless, this work is fundamental in exposing the bottlenecks of the production system and will undoubtedly serve as a stepping-stone for future integration studies, contributing to guiding microalgae production towards a more sustainable future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9030255/s1, Table S1: Mass and energy balances for a biomass production of 10 and 270 tonAFDW·year−1, obtained from the SuperPro Designer model.
Author Contributions
Conceptualization: T.F.L. and J.O.; methodology: T.F.L. and J.O.; data analysis and interpretation: T.F.L., J.O., C.T.M., L.C. and C.R.; formal analysis: C.T.M., L.C., C.R., A.R. and F.G.; writing—original draft preparation: T.F.L. and J.O.; writing—review and editing: C.T.M., L.C., C.R., A.R. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out under the MULTI-STR3AM project, which has received funding from Bio-based Industries Joint Undertaking under the European Union’s Horizon 2020 research and innovation programme under grant agreement No 887227. The work received also funding from the project Algaref (n. 45375), cofinanced by Programa Operacional Regional de Lisboa, Portugal 2020 and the European Union, through the European Regional Development Fund (ERDF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge A4F for providing the experimental data used in process simulation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a Sustainable Bioeconomy: An Overview of World Biomass Production and Utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Ferreira, A.; Gouveia, L. Microalgal Biorefineries. In Handbook of Microalgae Based Processes and Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 771–798. [Google Scholar]
- Porcelli, R.; Dotto, F.; Pezzolesi, L.; Marazza, D.; Greggio, N.; Righi, S. Comparative Life Cycle Assessment of Microalgae Cultivation for Non-Energy Purposes Using Different Carbon Dioxide Sources. Sci. Total Environ. 2020, 721, 137714. [Google Scholar] [CrossRef]
- Schade, S.; Meier, T. Distinct Microalgae Species for Food—Part 1: A Methodological (Top-down) Approach for the Life Cycle Assessment of Microalgae Cultivation in Tubular Photobioreactors. J. Appl. Phycol. 2020, 32, 2977–2995. [Google Scholar] [CrossRef]
- Schade, S.; Stangl, G.I.; Meier, T. Distinct Microalgae Species for Food—Part 2: Comparative Life Cycle Assessment of Microalgae and Fish for Eicosapentaenoic Acid (EPA), Docosahexaenoic Acid (DHA), and Protein. J. Appl. Phycol. 2020, 32, 2997–3013. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Front. Mar. Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Deprá, M.C.; Severo, I.A.; dos Santos, A.M.; Zepka, L.Q.; Jacob-Lopes, E. Environmental Impacts on Commercial Microalgae-Based Products: Sustainability Metrics and Indicators. Algal Res. 2020, 51, 102056. [Google Scholar] [CrossRef]
- Paladino, O.; Neviani, M. Scale-up of Photo-Bioreactors for Microalgae Cultivation by π-Theorem. Biochem. Eng. J. 2020, 153, 107398. [Google Scholar] [CrossRef]
- Subhash, G.V.; Rajvanshi, M.; Kumar, G.R.K.; Sagaram, U.S.; Prasad, V.; Govindachary, S.; Dasgupta, S. Challenges in Microalgal Biofuel Production: A Perspective on Techno Economic Feasibility under Biorefinery Stratagem. Bioresour. Technol. 2022, 343, 126155. [Google Scholar] [CrossRef]
- Dong, T.; Knoshaug, E.P.; Davis, R.; Laurens, L.M.L.; van Wychen, S.; Pienkos, P.T.; Nagle, N. Combined Algal Processing: A Novel Integrated Biorefinery Process to Produce Algal Biofuels and Bioproducts. Algal Res. 2016, 19, 316–323. [Google Scholar] [CrossRef]
- Moncada, J.; Tamayo, J.A.; Cardona, C.A. Integrating First, Second, and Third Generation Biorefineries: Incorporating Microalgae into the Sugarcane Biorefinery. Chem. Eng. Sci. 2014, 118, 126–140. [Google Scholar] [CrossRef]
- Moncada, J.; Cardona, C.A.; Rincón, L.E. Design and Analysis of a Second and Third Generation Biorefinery: The Case of Castorbean and Microalgae. Bioresour. Technol. 2015, 198, 836–843. [Google Scholar] [CrossRef]
- Posada, J.A.; Brentner, L.B.; Ramirez, A.; Patel, M.K. Conceptual Design of Sustainable Integrated Microalgae Biorefineries: Parametric Analysis of Energy Use, Greenhouse Gas Emissions and Techno-Economics. Algal Res. 2016, 17, 113–131. [Google Scholar] [CrossRef]
- Rodríguez, R.; Espada, J.J.; Moreno, J.; Vicente, G.; Bautista, L.F.; Morales, V.; Sánchez-Bayo, A.; Dufour, J. Environmental Analysis of Spirulina Cultivation and Biogas Production Using Experimental and Simulation Approach. Renew. Energy 2017, 129, 724–732. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Li, S.; Kitamura, Y. Evaluation of Hydrolysis-Esterification Biodiesel Production from Wet Microalgae. Bioresour. Technol. 2016, 214, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.F.; Cabanas, C.; Silva, A.; Fonseca, D.; Santos, E.; Guerra, L.T.; Sheahan, C.; Reis, A.; Gírio, F. Process Simulation and Techno-Economic Assessment for Direct Production of Advanced Bioethanol Using a Genetically Modified Synechocystis sp. Bioresour. Technol. Rep. 2019, 6, 113–122. [Google Scholar] [CrossRef]
- Penloglou, G.; Chatzidoukas, C.; Kiparissides, C. A Microalgae-Based Biorefinery Plant for the Production of Valuable Biochemicals: Design and Economics. In Computer Aided Chemical Engineering; Zdravko, K., Miloš, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 1731–1736. [Google Scholar]
- Arashiro, L.T.; Josa, I.; Ferrer, I.; van Hulle, S.W.H.; Rousseau, D.P.L.; Garfí, M. Life Cycle Assessment of Microalgae Systems for Wastewater Treatment and Bioproducts Recovery: Natural Pigments, Biofertilizer and Biogas. Sci. Total Environ. 2022, 847, 157615. [Google Scholar] [CrossRef]
- Ramos, A.A.; Polle, J.; Tran, D.; Cushman, J.C.; Jin, E.-S.; Varela, J.C. The Unicellular Green Alga Dunaliella Salina Teod. as a Model for Abiotic Stress Tolerance: Genetic Advances and Future Perspectives. Algae 2011, 26, 3–20. [Google Scholar] [CrossRef]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and Technological Advancements in the Possible Food Applications of Spirulina and Their Health Benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef]
- Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; Nimarshana, P.H.V.; Nagarajan, D.; Chang, J.S.; Ariyadasa, T.U. Large-Scale Production of Spirulina-Based Proteins and c-Phycocyanin: A Biorefinery Approach. Biochem. Eng. J. 2022, 185, 108541. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of Marine Planktonic Diatoms: I. Cyclotella Nana Hustedt, and Detonula Confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Johnson, M.K.; Johnson, E.J.; MacElroy, R.D.; Speer, H.L.; Bruff, B.S. Effects of Salts on the Halophilic Alga Dunaliella Viridis. J. Bacteriol. 1968, 95, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, C. Contribution a l’etude d’une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques Sur La Croissance et La Photosynthese de Spirulina Mixima. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Eurostat Harmonized Index of Consumer Prices (HIPC), Consulted December 2022. Available online: www.Eurostat.com (accessed on 9 December 2022).
- Directorate-General for Environment. Commission Recommendation of the Use of the Environmental Footprint Methods; Directorate-General for Environment: Brussels, Belgium, 2021. [Google Scholar]
- Zampori, L.; Pant, R. Suggestions for Updating the Product Environmental Footprint (PEF) Method. In Publications Office of the European Union; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Simar, S.I. De Águas e Esgotos Tarifa 2016, Consulted 2022. Available online: http://www.simar-louresodivelas.pt/clientes_pag/tarifa_2016.aspx (accessed on 9 December 2022).
- Rosas, V.T.; Poersch, L.H.; Romano, L.A.; Tesser, M.B. Feasibility of the Use of Spirulina in Aquaculture Diets. Rev. Aquac. 2019, 11, 1367–1378. [Google Scholar] [CrossRef]
- Vázquez-Romero, B.; Perales, J.A.; Pereira, H.; Barbosa, M.; Ruiz, J. Techno-Economic Assessment of Microalgae Production, Harvesting and Drying for Food, Feed, Cosmetics, and Agriculture. Sci. Total Environ. 2022, 837, 155742. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Verardo, V.; Martín-García, B.; di Pierro, P.; Sorrentino, A.; Baselice, M.; Oliviero, M.; Sacchi, R.; Masi, P. Formulation of New Media from Dairy and Brewery Wastes for a Sustainable Production of DHA-Rich Oil by Aurantiochytrium Mangrovei. Mar. Drugs 2021, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Pordata Renewable Energy Fraction Data, Consulted December 2022. Available online: https://www.pordata.pt/ (accessed on 1 December 2022).
- Moradiya, K.K.; Marathe, K.V. Life Cycle Assessment (LCA) of Marine Microalgae Cultivation and Harvesting Process for the Indian Context. Sustain. Energy Technol. Assess. 2023, 56, 103063. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Verma, P. Microalgae Dunaliella as Biofuel Feedstock and β-Carotene Production: An Influential Step towards Environmental Sustainability. Energy Convers. Manag. X 2022, 13, 100154. [Google Scholar] [CrossRef]
- Gaber, K.; Rösch, C.; Biondi, N. Life Cycle Assessment of Total Fatty Acid (TFA) Production from Microalgae Nannochloropsis Oceanica at Different Sites and Under Different Sustainability Scenarios. Bioenergy Res. 2022, 15, 1595–1615. [Google Scholar] [CrossRef]
- Ubando, A.T.; Ng, E.A.S.; Chen, W.-H.; Culaba, A.B.; Kwon, E.E. Life Cycle Assessment of Microalgal Biorefinery: A State-of-the-Art Review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).