Bacteriocinogenic Enterococcus casseliflavus Isolated from Fresh Guava Fruit (Psidium guajava): Characterization of Bacteriocin ST192Gu and Some Aspects of Its Mode of Action on Listeria spp. and Enterococcus spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation, Differentiation, and Identification of Bacteriocin Producer/s
2.1.1. Isolation of the Bacteriocin Producer/s and Evaluation of Antimicrobial Activity
2.1.2. Differentiation and Identification of Bacteriocin Producer/s
2.2. Safety Evaluation of the Selected Isolate
2.3. Confirmation of Protein Nature and Stability to pH, Temperature, and to Chemicals of Potential Practical Relevance
2.4. Spectrum of Inhibitory Activity
2.5. Adsorption of Produced Bacteriocin to the Producer Cells
2.6. Bacterial Growth, Changes in pH, and Production of Bacteriocin
2.7. Mode of Action
2.7.1. Partial Purification
2.7.2. Effect of Bacteriocin-Containing CFS on the Exponential Growth of Test Microorganisms
2.7.3. Determination of Cell Lysis by Measuring the Extracellular Levels of β-Galactosidase and DNA-Leakage
2.7.4. Determination of Cell Lysis of Target Microorganisms in the Presence of Bacteriocin ST192Gu
2.7.5. Determination of the Reduction of Viability of the Target Microorganisms in the Presence of Bacteriocin ST192Gu
2.7.6. Cell Growth and Bacteriocin Production in Mixed Cultures
2.7.7. Combined Application of Ciprofloxacin and Bacteriocin ST192Gu on Growth of L. ivanovii subsp. ivanovii ATCC 19119
2.8. Determination of Approximate Molecular Weight of the Bacteriocin ST192Gu by SDS-PAGE
3. Results and Discussion
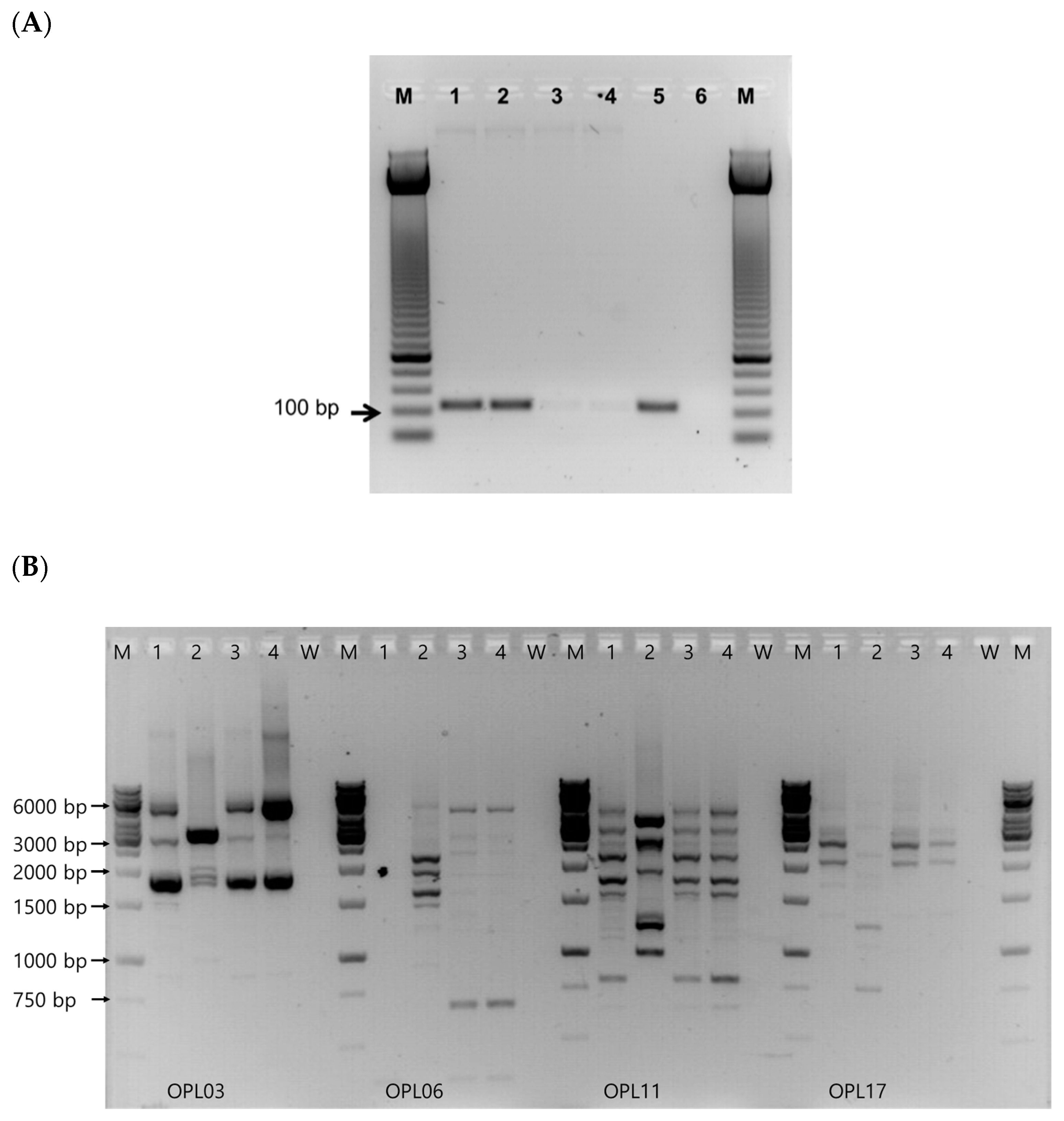
3.1. Isolation, Differentiation, and Identification of Bacteriocinogenic Enterococcus casseliflavus
3.2. Safety Evaluation of Enterococcus casseliflavus ST182Gu
3.3. Establishing the Protein Nature and pH, Temperature, and Chemical Stability of Bacteriocin ST192Gu
3.4. Spectrum of Inhibitory Activity of Bacteriocin ST192Gu
3.5. Adsorption of Bacteriocin ST192Gu to the Producer Cells
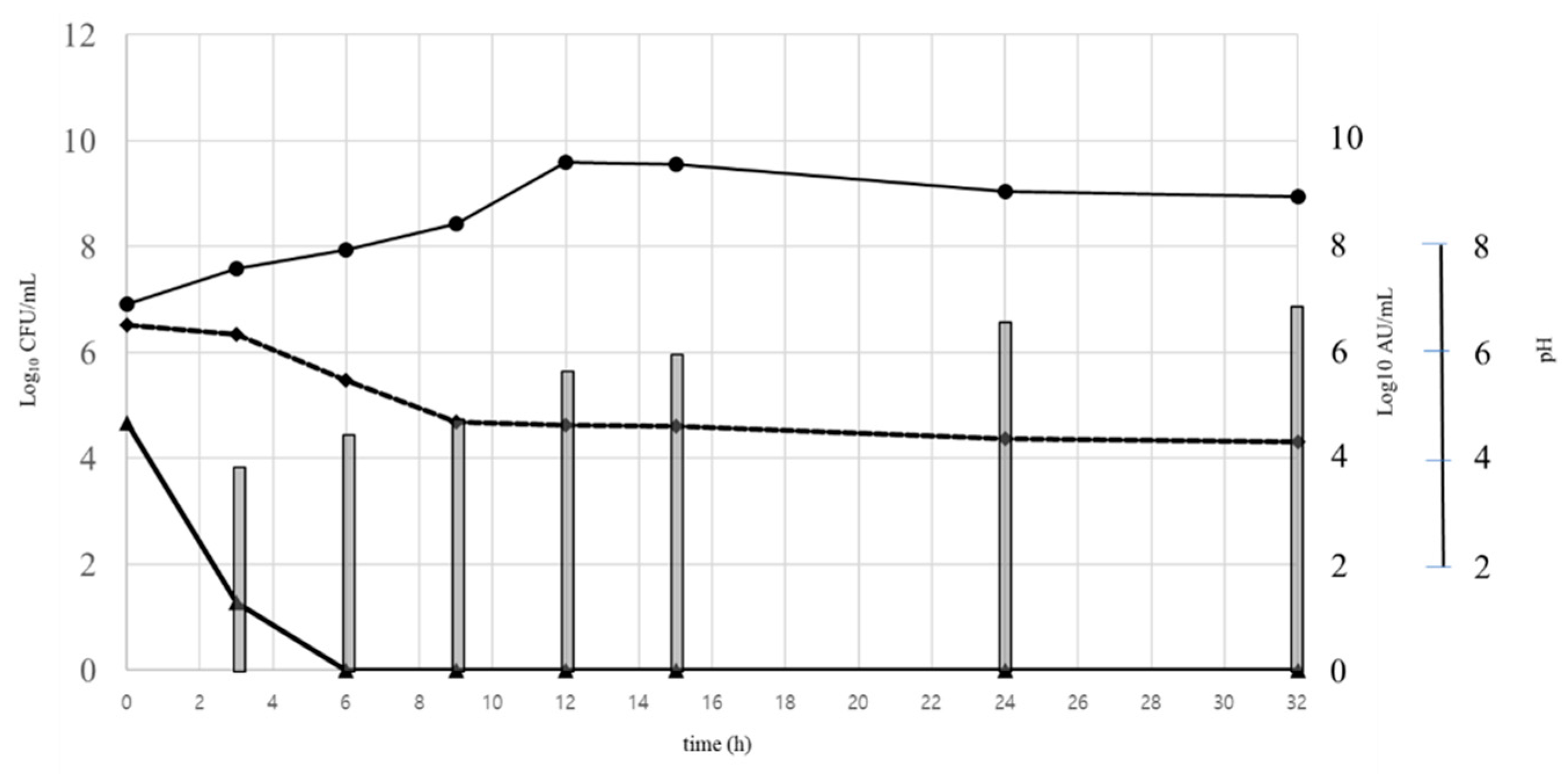
3.6. Dynamics of Bacterial Growth, Changes in pH, and Production of Bacteriocin
3.7. Mode of Action
3.7.1. Partial Purification of Bacteriocin ST192Gu
3.7.2. Effect of Bacteriocin-Containing CFS on Exponentially Growing Test Bacteria
3.7.3. Determination of Cell Lysis by Measuring the Extracellular Levels of β-Galactosidase and DNA
3.7.4. Determination of Cell Lysis of Target Microorganisms in the Presence of Bacteriocin
3.7.5. Determination of the Reduction of Viable Cells of Target Microorganisms in the Presence of the Bacteriocin
3.7.6. Cell Growth and Bacteriocin Production in Mixed Culture
3.7.7. Combined Application of Ciprofloxacin and Bacteriocin on Growth of L. ivanovii subsp. ivanovii ATCC 19119
3.8. Determination of Approximate Molecular Weight of Bacteriocin ST192Gu by SDS-PAGE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Telhig, S.; Ben Said, L.; Zirah, S.; Fliss, I.; Rebuffat, S. Bacteriocins to thwart bacterial resistance in Gram-negative bacteria. Front. Microbiol. 2020, 11, 586433. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241, Erratum in: Front. Microbiol. 2014, 5, 683. [Google Scholar] [CrossRef] [PubMed]
- Favaro, L.; Penna, A.L.B.; Todorov, S.D. Bacteriocinogenic LAB from cheeses—Application in biopreservation? Trends Food Sci. Technol. 2015, 41, 37–48. [Google Scholar] [CrossRef]
- Haider, T.; Pandey, V.; Behera, C.; Kumar, P.; Gupta, P.N.; Soni, V. Nisin and nisin-loaded nanoparticles: A cytotoxicity investigation. Drug. Dev. Ind. Pharm. 2022, 48, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Blake, T.; Mathur, H.; O’Connor, P.M.; Cotter, P.D.; Paul Ross, R.; Hill, C. Bioengineering nisin to overcome the nisin resistance protein. Mol. Microbiol. 2019, 111, 717–731. [Google Scholar] [CrossRef]
- Todorov, S.D.; Dicks, L.M. Bacteriocin production by Pediococcus pentosaceus isolated from marula (Scerocarya birrea). Int. J. Food Microbiol. 2009, 132, 117–126. [Google Scholar] [CrossRef]
- Todorov, S.D.; Prévost, H.; Lebois, M.; Dousset, X.; LeBlanc, J.G.; Franco, B.D.G.M. Bacteriocinogenic Lactobacillus plantarum ST16Pa isolated from papaya (Carica papaya)—from isolation to application: Characterization of a bacteriocin. Food Res. Int. 2011, 44, 1351–1363. [Google Scholar] [CrossRef]
- Soltani, S.; Zirah, S.; Rebuffat, S.; Couture, F.; Boutin, Y.; Biron, E.; Subirade, M.; Fliss, I. Gastrointestinal stability and cytotoxicity of bacteriocins from Gram-positive and Gram-negative bacteria: A comparative in vitro study. Front. Microbiol. 2022, 12, 780355. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, S. Bacteriocins as potential anticancer agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef]
- dos Santos, K.M.O.; de Matos, C.R.; Salles, H.O.; Franco, B.D.G.D.M.; Arellano, K.; Holzapfel, W.H.; Todorov, S.D. Exploring beneficial/virulence properties of two dairy-related strains of Streptococcus infantarius subsp. infantarius. Prob. Antimicrob. Prot. 2020, 12, 1524–1541. [Google Scholar] [CrossRef]
- De Vos, P.; Garrity, G.M.; Jones, D.; Kreig, N.R.; Ludwig, W.; Rainey, F.A.; Schleifel, K.-H.; Whitman, W.B. Bergey’s manual of systematic bacteriology. In The Firmicutes, 2nd ed.; Springer: Dordrecht, The Netherlands, 2009; Volume 3. [Google Scholar]
- de Moraes, G.M.D.; de Abreu, L.R.; do Egito, A.S.; Salles, H.O.; da Silva, L.M.F.; Nero, L.A.; Todorov, S.D.; dos Santos, K.M.O. Functional properties of Lactobacillus mucosae strains isolated from Brazilian goat milk. Prob. Antimicrob. Prot. 2016, 9, 235–245. [Google Scholar] [CrossRef]
- Ke, D.; Picard, F.J.; Martineau, F.; Ménard, C.; Roy, P.H.; Onellette, M.; Bergeron, M.G. Development of a PCR assay for rapid detection of Enterococci. J. Clin. Microbiol. 1999, 37, 3497–3503. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Holzapfel, W.H.; Todorov, S.D. Characterization of partially purified bacteriocins produced by Enterococcus faecium strains isolated from soybean paste active against Listeria spp. and Vancomycin-resistant enterococci. Microorganisms 2021, 9, 1085. [Google Scholar] [CrossRef]
- Yang, R.; Johnson, M.C.; Ray, B.I.B.E.K. Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl. Environ. Microbiol. 1992, 58, 3355–3359. [Google Scholar] [CrossRef]
- Metivier, A.; Pilet, M.-F.; Dousset, X.; Sorokine, O.; Anglade, P.; Zagorec, M.; Piard, J.-C.; Marlon, D.; Cenatiempo, Y.; Fremaux, C. Divercin V41, a new bacteriocin with two disulphide bonds produced by Carnobacterium divergens V41: Primary structure and genomic organization. Microbiology 1998, 144, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Favaro, F.; Basaglia, M.; Casella, S.; Hue, I.; Dousset, X.; Franco, B.D.G.M.; Todorov, S.D. Bacteriocinogenic potential and safety evaluation of non starter Enterococcus faecium strains isolated from home made white brine cheese. Food Microbiol. 2014, 38, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Wachsman, M.; Tomé, E.; Dousset, X.; Destro, M.T.; Dicks, L.M.T.; Franco, B.D.G.M.; Vaz-Velho, M.; Drider, D. Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol. 2010, 27, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Guava (Psidium Guajava)- Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas; Syed, Z.H., Bazila, N., Tahiya, Q., Tabasum, F., Tashooq, A.B., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Holzapfel, W.H.; Todorov, S.D. The overview of natural by-products of beneficial lactic acid bacteria as promising antimicrobial agents. Appl. Food Biotechnol. 2002, 9, 127–143. [Google Scholar]
- Fugaban, J.I.I.; Bucheli, J.E.V.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Franco, B.D.G.M.; Ivanova, I.V.; Holzapfel, W.H.; Todorov, S.D. Antimicrobial properties of Pediococcus acidilactici and Pediococcus pentosaceus isolated from silage. J. Appl. Microbiol. 2022, 132, 311–330. [Google Scholar] [CrossRef]
- Micallef, S.A.; Goldstein, R.E.; George, A.; Ewing, L.; Tall, B.D.; Boyer, M.S.; Joseph, S.W.; Sapkota, A.R. Diversity, distribution and antibiotic resistance of Enterococcus spp. recovered from tomatoes, leaves, water and soil on U.S. Mid-Atlantic farms. Food Microbiol. 2013, 36, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Vasilakopoulou, A.; Vourli, S.; Siafakas, N.; Kavatha, D.; Tziolos, N.; Pournaras, S. Enterococcus casseliflavus bacteraemia in a patient with chronic renal disease. Infect. Dis. Rep. 2020, 12, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Valledor, S.J.D.; Dioso, C.M.; Bucheli, J.E.V.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Kim, B.; Holzapfel, W.H.; Todorov, S.D. Characterization and safety evaluation of two beneficial, enterocin-producing Enterococcus faecium strains isolated from kimchi, a Korean fermented cabbage. Food Microbiol. 2022, 102, 103886. [Google Scholar] [CrossRef] [PubMed]
- Floch, M.H.; Madsen, K.K.; Jenkins, D.J.; Guandalini, S.; Katz, J.A.; Onderdonk, A.; Walker, W.A.; Fedorak, R.N.; Camilleri, M. Recommendations for probiotic use. J. Clin. Gastroenterol. 2006, 40, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, A. What is wrong with enterococcal probiotics? Probiotics Antimicrob. Proteins 2020, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lewus, C.B.; Kaiser, A.; Montville, T.J. Inhibition of food-borne bacteria pathogens by bacteriocins from lactic acid bacteria isolated from meat. Appl. Environ. Microbiol. 1991, 57, 1683–1688. [Google Scholar] [CrossRef]
- Todorov, S.D. Diversity of bacteriocinogenic lactic acid bacteria isolated from boza, a cereal-based fermented beverage from Bulgaria. Food Control 2010, 21, 1011–1021. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Vesterlund, S. Antimicrobial components from lactic acid bacteria. In Lactic Acid Bacteria Microbiological and Functional Aspects, 3rd ed.; Revised and Expanded; Taylor & Francis Group: London, UK, 2004; pp. 375–395. [Google Scholar]
- Heng, N.C.K.; Wescombe, P.A.; Burton, J.P.; Jack, R.W.; Tagg, J.R. The diversity of bacteriocins in gram-positive bacteria. In Bacteriocins; Riley, M.A., Chavan, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 45–92. [Google Scholar] [CrossRef]
- Wu, Y.; Pang, X.; Wu, Y.; Liu, X.; Zhang, X. Enterocins: Classification, synthesis, antibacterial mechanisms and food applications. Molecules 2022, 27, 2258. [Google Scholar] [CrossRef]
- Cintas, L.M.; Casaus, P.; Holo, H.; Hernandez, P.E.; Nes, I.F.; Håvarstein, L.S. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J. Bacteriol. 1998, 180, 1988–1994. [Google Scholar] [CrossRef]
- De Kwaadsteniet, M.; Todorov, S.D.; Knoetze, H.; Dicks, L.M.T. Characterization of a 3 944 Dalton bacteriocin, produced by Enterococcus mundtii ST15, with activity against Gram-positive and Gram-negative bacteria. Int. J. Food Microbiol. 2005, 105, 433–444. [Google Scholar] [CrossRef]
- Todorov, S.D.; Wachsman, M.B.; Knoetze, H.; Meincken, M.; Dicks, L.M.T. An antibacterial and antiviral peptide produced by Enterococcus mundtii ST4V isolated from soybeans. Int. J. Antimicrob. Agents 2005, 25, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Danova, S.T.; Van Reenen, C.A.; Meincken, M.; Dinkova, G.; Ivanova, I.V.; Dicks, L.M.T. Characterization of bacteriocin HV219, produced by Lactococcus lactis subsp. lactis HV219 isolated from human vaginal secretions. J. Basic Microbiol. 2006, 46, 226–238. [Google Scholar] [PubMed]
- Todorov, S.D.; Franco, B.D.G.M.; Tagg, J.R. Bacteriocins of Gram-positive bacteria having activity spectra extending beyond closely related species. Benef. Microbs. 2019, 10, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Umu, Ö.C.O.; Rudi, K.; Diep, D.B. Modulation of the gut microbiota by prebiotic fibres and bacteriocins. Microb. Ecol. Health. Dis. 2017, 28, 1348886. [Google Scholar] [CrossRef] [PubMed]
- Parente, E.; Ricciardi, A. Production, recovery and purification of bacteriocins from lactic acid bacteria. Appl. Microbiol. Biotechnol. 1999, 52, 628–638. [Google Scholar] [CrossRef]
- Yamashita, H.; Tomita, H.; Inoue, T.; Ike, Y. Genetic organization and mode of action of a novel bacteriocin, bacteriocin 51: Determinant of VanA-type vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 2011, 55, 4352–4360. [Google Scholar] [CrossRef] [PubMed]
- Ennahar, S.; Sashihara, T.; Sonomoto, K.; Ishizaki, A. Class IIa bacteriocins: Biosynthesis, structure and activity. FEMS Microbiol. Rev. 2000, 24, 85–106. [Google Scholar] [CrossRef]
- Todorov, S.; Onno, B.; Sorokine, O.; Chobert, J.; Ivanova, I.; Dousset, X. Detection and characterization of a novel antibacterial substance produced by Lactobacillus plantarum ST31 isolated from sourdough. Int. J. Food Microbiol. 1999, 48, 167–177. [Google Scholar] [CrossRef]
- Bhugaloo-Vial, P.; Grajek, W.; Dousset, X.; Boyaval, P. Continuous bacteriocin production with high cell density bioreactors. Enzym. Microb. Technol. 1997, 21, 450–457. [Google Scholar] [CrossRef]
- Song, D.; Zhu, M.; Gu, Q. Purification and characterization of plantaricin ZJ5, a new bacteriocin produced by Lactobacillus plantarum ZJ5. PLoS ONE 2014, 9, e105549. [Google Scholar] [CrossRef] [PubMed]
- Surovtsev, V.; Borzenkov, V.; Levchuk, V. Purification of bacteriocins by chromatographic methods. Appl. Biochem. Microbiol. 2015, 51, 881–886. [Google Scholar] [CrossRef]
- Bastos, M. do C.; Coelho, M.L.; Santos, O.C. Resistance to bacteriocins produced by Gram-positive bacteria. Microbiol. 2015, 161 Pt 4, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Dicks, L.M.T. Screening for bacteriocin producer lactic acid bacteria from boza, a traditional cereal beverage from Bulgaria. Characterization of produced bacteriocins. Process Biochem. 2006, 41, 11–19. [Google Scholar] [CrossRef]
- Yıldırım, Z.; Avşar, Y.K.; Yıldırım, M. Factors affecting the adsorption of buchnericin LB, a bacteriocin produced by Lactocobacillus buchneri. Microbiol. Res. 2002, 157, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.K.; Johnson, M.C.; Ray, B.; Kalchayanand, N. Mode of action of pediocin AcH from Pediococcus acidilactici H on sensitive bacterial strains. J. Appl. Bacteriol. 1991, 70, 25–33. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Minahk, C.J.; Dupuy, F.; Morero, R.D. Enhancement of antibiotic activity by sub-lethal concentrations of enterocin CRL35. J. Antimicrob. Chemother. 2004, 53, 240–246. [Google Scholar] [CrossRef]
- Satish, K.R.; Kanmani, P.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Purification and characterization of enterocin MC13 produced by a potential aquaculture probiont Enterococcus faecium MC13 isolated from the gut of Mugil cephalus. Can. J. Microbiol. 2011, 57, 993–1001. [Google Scholar] [CrossRef]
- Krämer, J.; Henning, B. Mode of Action of Two Streptococcus faecium Bacteriocins. Antimicrob. Agents Chemoth. 1975, 7, 117–120. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Togawa, Y.; Shimosaka, M.; Okazaki, M. Purification and characterization of a novel bacteriocin produced by Enterococcus faecalis strain RJ-11. Appl. Environ. Microbiol. 2003, 69, 5746–5753. [Google Scholar] [CrossRef] [PubMed]
- Tulini, F.L.; Gomes, B.C.; de Martins, E.C.P. Partial purification and characterization of a bacteriocin produced by Enterococcus faecium 130 isolated from mozzarella cheese. Food Sci. Technol. 2011, 31, 155–159. [Google Scholar] [CrossRef]
- Aymerich, T.; Holo, H.; Havarstein, L.S.; Hugas, M.; Garriga, M.; Nes, I. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 1996, 62, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- du Toit, M.; Franz, C.M.; Dicks, L.M.; Holzapfel, W.H. Preliminary characterization of bacteriocins produced by Enterococcus faecium and Enterococcus faecalis isolated from pig faeces. J. Appl. Microbiol. 2000, 88, 482–494. [Google Scholar] [CrossRef] [PubMed]




| Target Strains | Growth Medium | Inhibition # |
|---|---|---|
| Acinetobacter baumannii | BHI | 0/2 |
| Bacteroides fragilis | BHI | 0/1 |
| Escherichia coli | BHI | 0/4 |
| Enterobacter cloacae | BHI | 0/1 |
| E. faecalis | MRS | 13/14 |
| E. faecium | MRS | 9/9 |
| Klebsiella pneumoniae | BHI | 0/2 |
| Lactobacillus acidophilus | MRS | 2/2 |
| Lb. curvatus | MRS | 0/4 |
| Lb. delbrueckii | MRS | 0/6 |
| Lb. fermentum | MRS | 0/4 |
| Lb. paracasei | MRS | 1/6 |
| Lb. plantarum | MRS | 0/10 |
| Lb. rhamnosus | MRS | 0/5 |
| Lb. salivarius | MRS | 0/2 |
| Lb. sakei | MRS | 0/3 |
| Lactococcus lactis subsp. lactis | MRS | 1/1 |
| Leuconostoc lactis | BHI | 2/2 |
| Listeria innocua | BHI | 6/6 |
| L. ivanovii subsp. ivanovii | BHI | 1/1 |
| L. monocytogenes | BHI | 27/27 |
| Pediococcus acidilactici | MRS | 0/2 |
| Pediococcus pentosaceus | MRS | 1/6 |
| Staphylococcus aureus | BHI | 2/10 |
| Staph. uberis | BHI | 1/1 |
| Streptococcus agalactiae | BHI | 0/3 |
| Str. caprinus | BHI | 2/2 |
| Str. gallolyticus subsp. macedonicus | MRS | 0/1 |
| Str. infantarius subsp. infantarius | MRS | 2/2 |
| Str. pneumoniae | BHI | 0/5 |
| Bacillus subtilis | BHI | 0/5 |
| Bacillus sp. | BHI | 0/8 |
| Antibiotics | Inhibition (mm) |
|---|---|
| amoxacilin/clavulonic acid (30 μg per disk) | 30 |
| ampicillin/sulbactam (20 μg per disk) | 32 |
| bacitracin (10 units per disk) | 09 |
| cefepime (30 μg per disk) | 15 |
| cefotaxim (30 μg per disk) | 30 |
| cefriaxon (30 μg per disk) | 34 |
| ceftiofur (30 μg per disk) | 35 |
| cefuroxim (30 μg per disk) | 31 |
| chloramphenicol (30 μg per disk) | 32 |
| ciprofloxacin (5 μg per disk) | 13 |
| clindamycin (2 μg per disk) | 21 |
| enrofloxacin (5 μg per disk) | 11 |
| erytromycin (15 μg per disk) | 23 |
| florfenicol (30 μg per disk) | 12 |
| gentamicin (10 μg per disk) | 12 |
| imipenem (10 μg per disk) | 21 |
| levofloxacine (5 μg per disk) | 12 |
| neomycin N (10 μg per disk) | 10 |
| penicillin (10 units per disk) | 23 |
| tetracycline (30 μg per disk) | 24 |
| vancomycin (30 μg per disk) | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorov, S.D.; Holzapfel, W.H.; Tagg, J.R. Bacteriocinogenic Enterococcus casseliflavus Isolated from Fresh Guava Fruit (Psidium guajava): Characterization of Bacteriocin ST192Gu and Some Aspects of Its Mode of Action on Listeria spp. and Enterococcus spp. Fermentation 2023, 9, 226. https://doi.org/10.3390/fermentation9030226
Todorov SD, Holzapfel WH, Tagg JR. Bacteriocinogenic Enterococcus casseliflavus Isolated from Fresh Guava Fruit (Psidium guajava): Characterization of Bacteriocin ST192Gu and Some Aspects of Its Mode of Action on Listeria spp. and Enterococcus spp. Fermentation. 2023; 9(3):226. https://doi.org/10.3390/fermentation9030226
Chicago/Turabian StyleTodorov, Svetoslav Dimitrov, Wilhelm Heinrich Holzapfel, and John Robert Tagg. 2023. "Bacteriocinogenic Enterococcus casseliflavus Isolated from Fresh Guava Fruit (Psidium guajava): Characterization of Bacteriocin ST192Gu and Some Aspects of Its Mode of Action on Listeria spp. and Enterococcus spp." Fermentation 9, no. 3: 226. https://doi.org/10.3390/fermentation9030226
APA StyleTodorov, S. D., Holzapfel, W. H., & Tagg, J. R. (2023). Bacteriocinogenic Enterococcus casseliflavus Isolated from Fresh Guava Fruit (Psidium guajava): Characterization of Bacteriocin ST192Gu and Some Aspects of Its Mode of Action on Listeria spp. and Enterococcus spp. Fermentation, 9(3), 226. https://doi.org/10.3390/fermentation9030226






