High-Grain Diet Feeding Altered Blood Metabolites, Rumen Microbiome, and Metabolomics of Yaks
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sample Collection
2.3. Blood Metabolites and Rumen Fermentation Parameters Analyses
2.4. DNA Extraction, Sequencing, and Bioinformatic Analysis
2.5. Metabolic Profile Analysis
2.6. Statistical Analysis
3. Results
3.1. Blood Metabolites
3.2. Rumen Fermentation Parameters
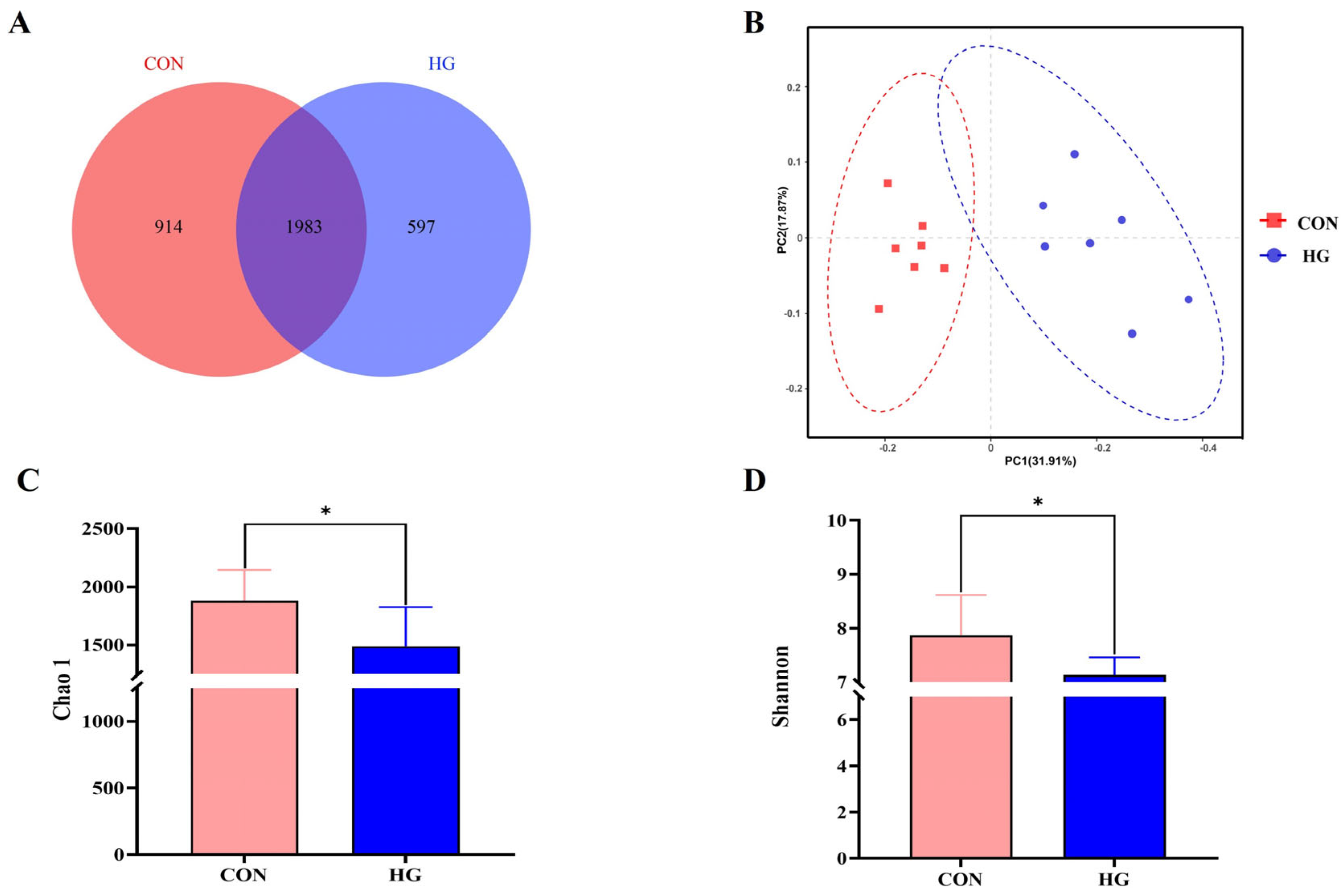
3.3. Rumen Bacterial Diversity Analysis
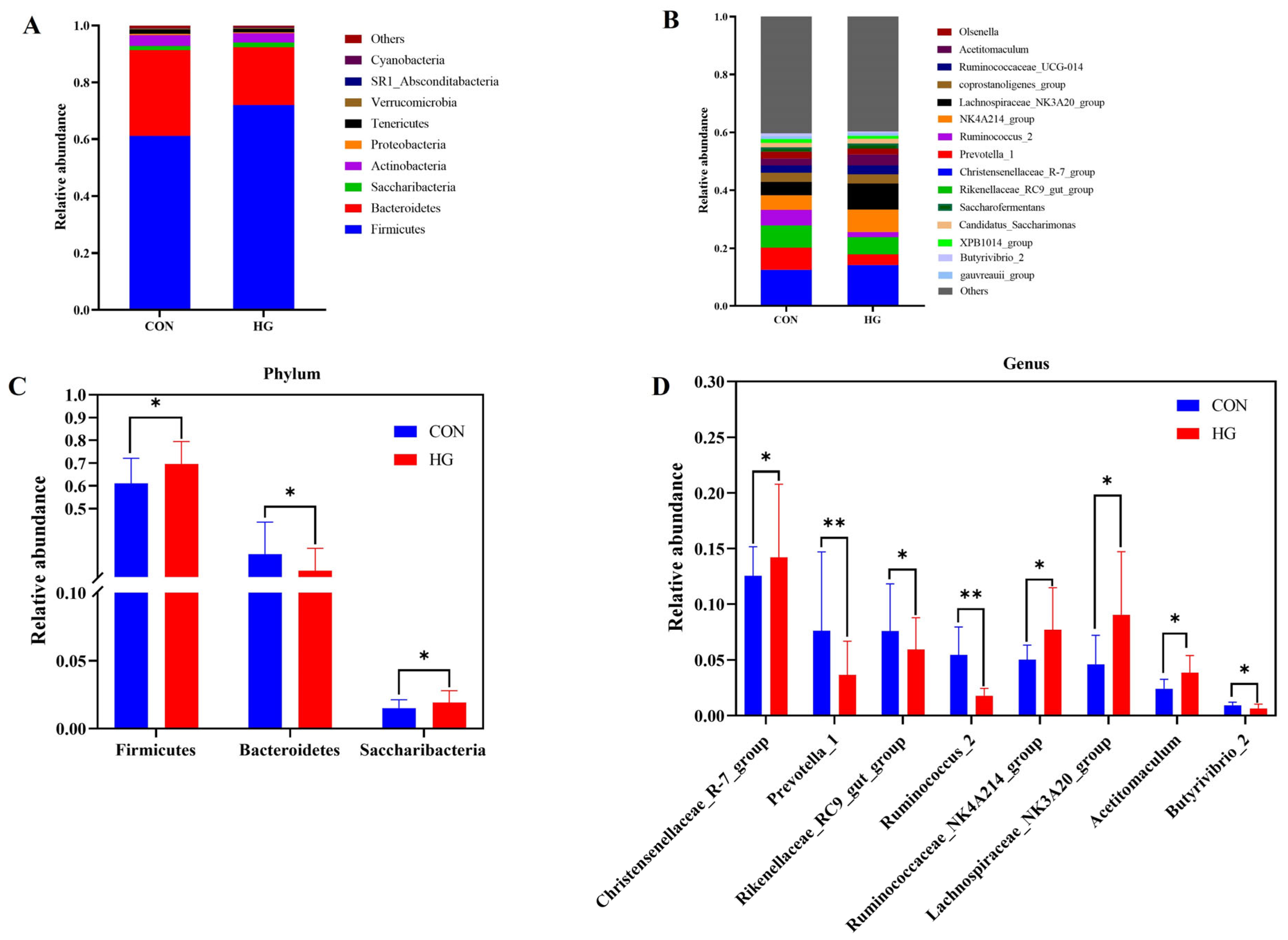
3.4. Differences in Bacterial Community Composition between Two Groups
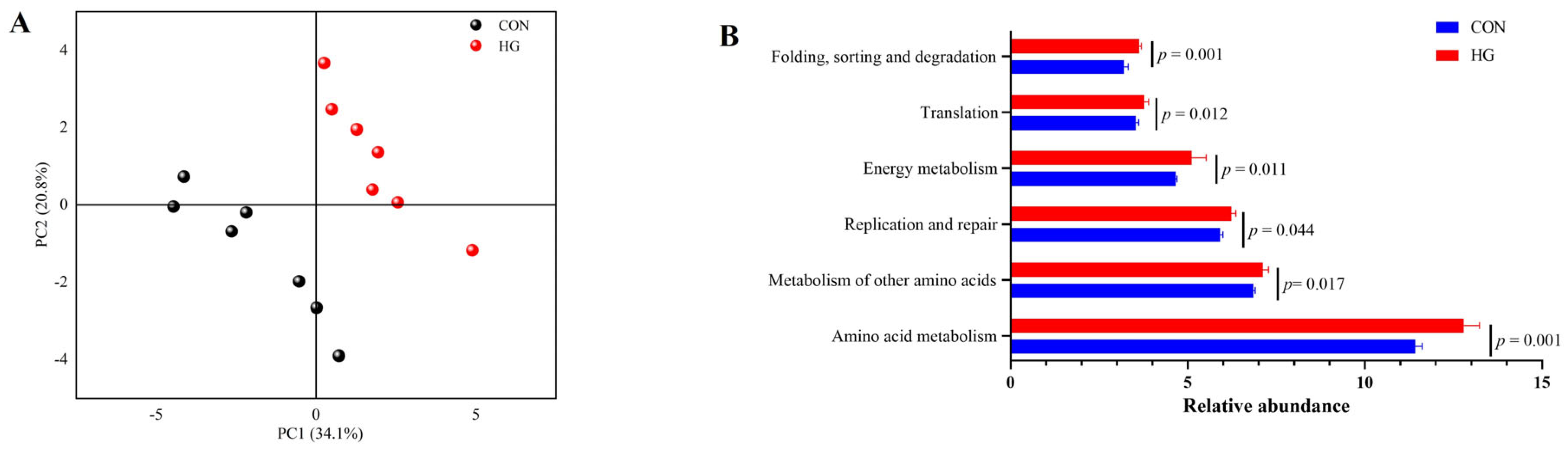
3.5. Predicted Functional of the Rumen Bacterial Community
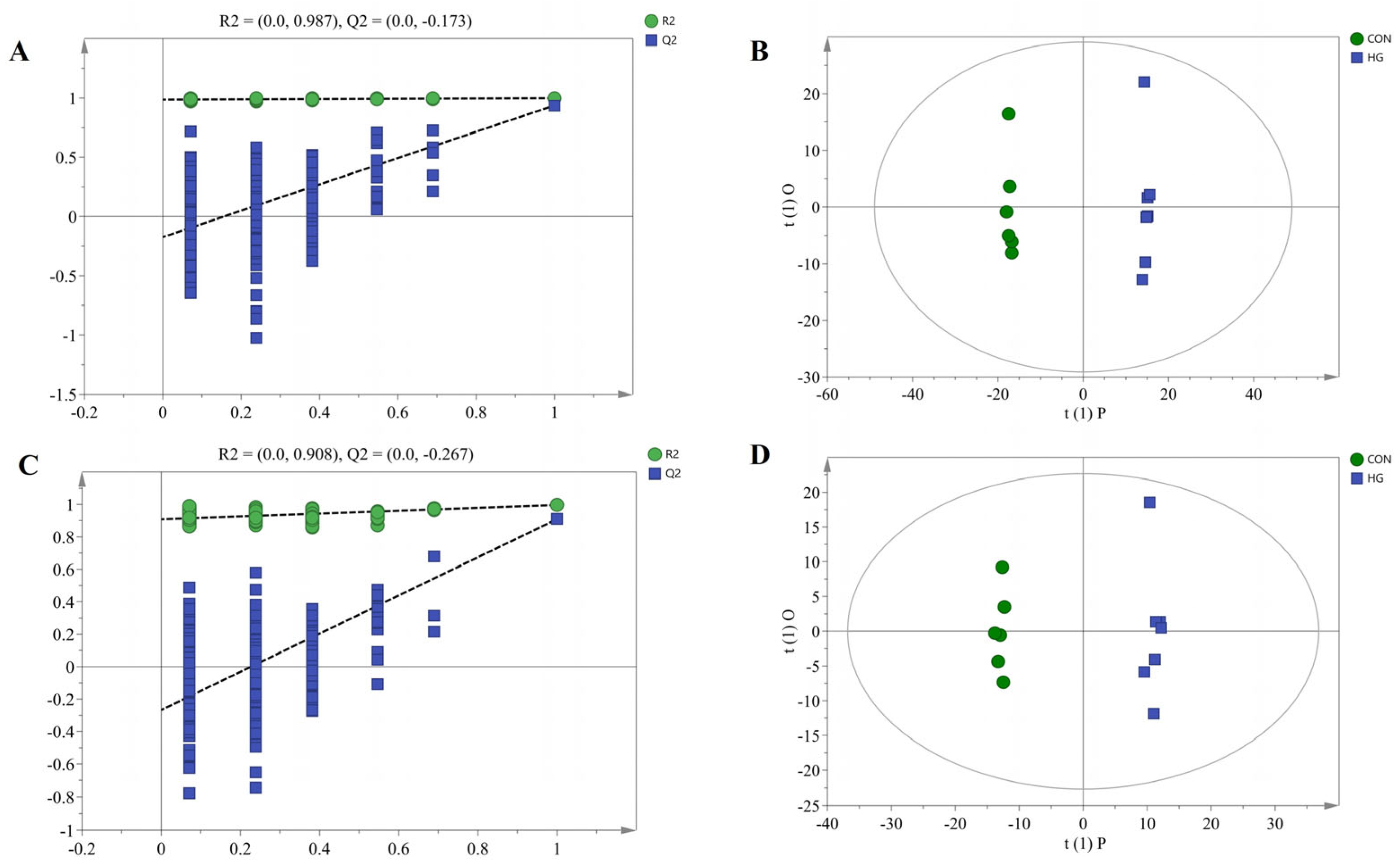
3.6. Rumen Metabolomics Profiles and Metabolic Pathway Analyses
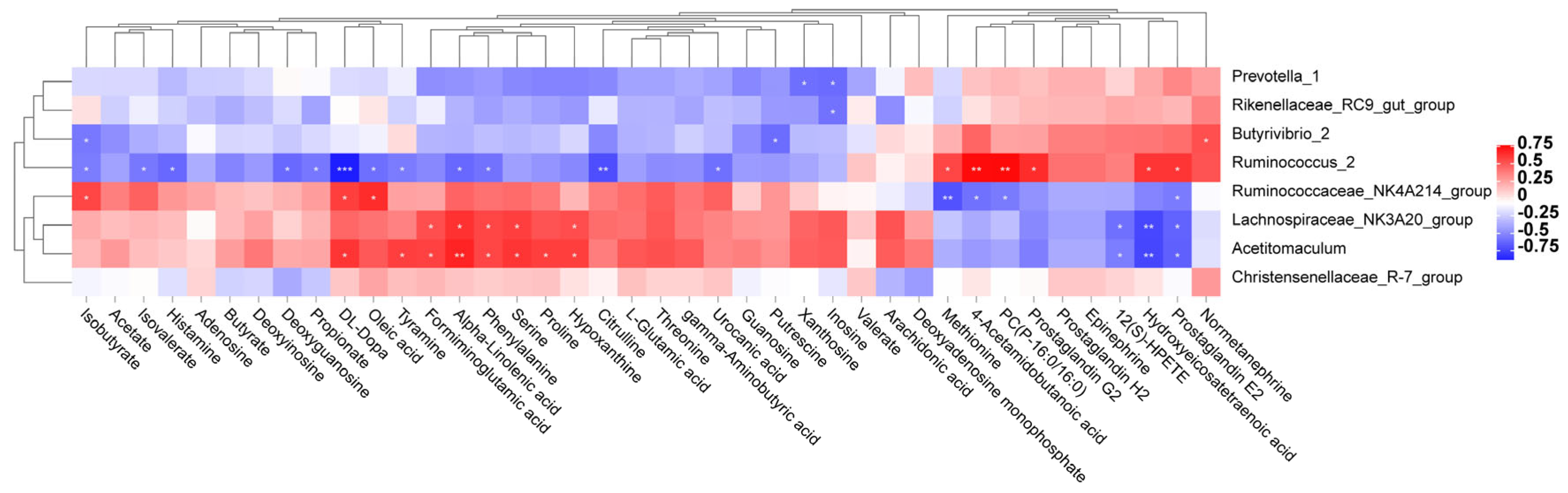
3.7. Correlation between Metabolites and Rumen Bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, L.; Xu, S.; Liu, H.; Xu, T.; Hu, L.; Zhao, N.; Han, X.; Zhang, X. Yak rumen microbial diversity at different forage growth stages of an alpine meadow on the Qinghai-Tibet plateau. PeerJ 2019, 7, e7645. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Si, H.; Yan, X.; Liu, C.; Ding, L.; Long, R.; Li, Z.; Qiu, Q. Bacterial communities in the solid, liquid, dorsal, and ventral epithelium fractions of yak (bos grunniens) rumen. MicrobiologyOpen 2019, 9, e963. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Adamowski, J.F.; Deo, R.C.; Xu, X.; Gong, Y.; Feng, Q. Grassland degradation on the Qinghai-Tibetan plateau: Reevaluation of causative factors. Rangel. Ecol. Manag. 2019, 72, 988–995. [Google Scholar] [CrossRef]
- Miao, F.; Guo, Z.; Xue, R.; Wang, X.; Shen, Y. Effects of grazing and precipitation on herbage biomass, herbage nutritive value, and yak performance in an alpine meadow on the Qinghai–Tibetan plateau. PLoS ONE 2015, 10, e127275. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Pang, K.; Liu, S.; Wang, X.; Yang, Y.; Chai, S.; Wang, S. Effects of concentrate supplementation on growth performance, rumen fermentation, and bacterial community composition in grazing yaks during the warm season. Animals 2022, 12, 1398. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; Sun, B.; Zhang, S.; Zhang, S.; Yang, Y.; Lei, Y.; Chang, L.; Xie, P.; Suo, H. Multi-omics analysis reveals a dependent relationship between rumen bacteria and diet of grass- and grain-fed yaks. Front. Microbiol. 2021, 12, 642952. [Google Scholar] [CrossRef]
- Ogata, T.; Makino, H.; Ishizuka, N.; Iwamoto, E.; Masaki, T.; Ikuta, K.; Kim, Y.; Sato, S. Long-term high-grain diet altered the ruminal ph, fermentation, and composition and functions of the rumen bacterial community, leading to enhanced lactic acid production in Japanese black beef cattle during fattening. PLoS ONE 2019, 14, e225448. [Google Scholar] [CrossRef]
- Allen, M.S. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 90, 17–38. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; Mcbride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Zhang, K.; Chang, G.; Xu, T.; Xu, L.; Guo, J.; Jin, D.; Shen, X. Lipopolysaccharide derived from the digestive tract activates inflammatory gene expression and inhibits casein synthesis in the mammary glands of lactating dairy cows. Oncotarget 2016, 7, 9652–9665. [Google Scholar] [CrossRef]
- Dong, H.; Wang, S.; Jia, Y.; Ni, Y.; Zhang, Y.; Zhuang, S.; Shen, X.; Zhao, R. Long-term effects of subacute ruminal acidosis (sara) on milk quality and hepatic gene expression in lactating goats fed a high-concentrate diet. PLoS ONE 2013, 8, e82850. [Google Scholar] [CrossRef]
- Zebeli, Q.; Metzler-Zebeli, B.U. Interplay between rumen digestive disorders and diet-induced inflammation in dairy cattle. Res. Vet. Sci. 2012, 93, 1099–1108. [Google Scholar] [CrossRef]
- Hua, C.; Tian, J.; Tian, P.; Cong, R.; Luo, Y.; Geng, Y.; Tao, S.; Ni, Y.; Zhao, R. Feeding a high concentration diet induces unhealthy alterations in the composition and metabolism of ruminal microbiota and host response in a goat model. Front. Microbiol. 2017, 8, 138. [Google Scholar] [CrossRef]
- Zhang, R.; Ye, H.; Liu, J.; Mao, S. High-grain diets altered rumen fermentation and epithelial bacterial community and resulted in rumen epithelial injuries of goats. Appl. Microbiol. Biotechnol. 2017, 101, 6981–6992. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, T.; Wang, X.; Geng, Y.; Zhao, N.; Hu, L.; Liu, H.; Kang, S.; Xu, S. Effect of Dietary Protein Levels on Dynamic Changes and Interactions of Ruminal Microbiota and Metabolites in Yaks on the Qinghai-Tibetan Plateau. Front. Microbiol. 2021, 12, 684340. [Google Scholar] [CrossRef]
- Zhang, Q.; Degen, A.; Hao, L.; Huang, Y.; Niu, J.; Wang, X.; Chai, S.; Liu, S. An increase in dietary lipid content from different forms of double-low rapeseed reduces enteric methane emission in datong yaks on the Qinghai-Tibetan plateau. Anim. Sci. J. 2020, 91, e13489. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one fastq preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Kuczynski, J.; Stombaugh, J.; Walters, W.A.; González, A.; Caporaso, J.G.; Knight, R. Using Qiime to analyze 16s rrna gene sequences from microbial communities. Curr. Protoc. Bioinform. 2011, 36, 814–821. [Google Scholar] [CrossRef]
- Bacci, G.; Bani, A.; Bazzicalupo, M.; Ceccherini, M.T.; Galardini, M.; Nannipieri, P.; Pietramellara, G.; Mengoni, A. Evaluation of the performances of ribosomal database project (rdp) classifier for taxonomic assignment of 16s rrna metabarcoding sequences generated from illumina-solexangs. J. Genom. 2015, 3, 36–39. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; Mcdonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C. Predictive functional profiling of microbial communities using 16s rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. Fiehnlib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, W.; Jiang, L.; Mao, S. Comparative metabolome analysis of ruminal changes in holstein dairy cows fed low- or high-concentrate diets. Metabolomics 2017, 13, 74–88. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Guan, L.L. Gut microbiome and omics: A new definition to ruminant production and health. Anim. Front. 2016, 6, 8–12. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef] [PubMed]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- Shen, J.; Han, X.; Zheng, L.; Liu, S.; Jin, C.; Liu, T.; Cao, Y.; Lei, X.; Yao, J. High rumen-degradable starch diet promotes hepatic lipolysis and disrupts enterohepatic circulation of bile acids in dairy goats. J. Nutr. 2020, 150, 2755–2763. [Google Scholar] [CrossRef]
- Deng, Q.; Liu, G.; Liu, L.; Zhang, Y.; Yin, L.; Shi, X.; Wang, J.; Yuan, X.; Sun, G.; Li, Y.; et al. Bhba influences bovine hepatic lipid metabolism via ampk signaling pathway. J. Cell. Biochem. 2015, 116, 1070–1079. [Google Scholar] [CrossRef]
- Wells, J.E.; Russell, J.B. Why do many ruminal bacteria die and lyse so quickly? J. Dairy Sci. 1996, 79, 1487–1495. [Google Scholar] [CrossRef]
- Li, S.; Khafipour, E.; Krause, D.O.; Kroeker, A.; Rodriguez-Lecompte, J.C.; Gozho, G.N.; Plaizier, J.C. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J. Dairy Sci. 2012, 95, 294–303. [Google Scholar] [CrossRef]
- Dong, G.; Liu, S.; Wu, Y.; Lei, C.; Zhou, J.; Zhang, S. Diet-induced bacterial immunogens in the gastrointestinal tract of dairy cows: Impacts on immunity and metabolism. Acta Vet. Scand. 2011, 53, 48. [Google Scholar] [CrossRef]
- Fairfield, A.M.; Plaizier, J.C.; Duffield, T.F.; Lindinger, M.I.; Bagg, R.; Dick, P.; Mcbride, B.W. Effects of prepartum administration of a monensin controlled release capsule on rumen pH, feed intake, and milk production of transition dairy cows. J. Dairy Sci. 2007, 90, 937–945. [Google Scholar] [CrossRef]
- Emmanuel, D.G.V.; Dunn, S.M.; Ametaj, B.N. Feeding high proportions of barley grain stimulates an inflammatory response in dairy cows. J. Dairy Sci. 2008, 91, 606–614. [Google Scholar] [CrossRef]
- Murata, H.; Shimada, N.; Yoshioka, M. Current research on acute phase proteins in veterinary diagnosis: An overview. Vet. J. 2004, 168, 28–40. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, X.; Zhao, P.; Gao, M.; Han, H.; Hu, H. Effects of increasing non-fiber carbohydrate to neutral detergent fiber ratio on rumen fermentation and microbiota in goats. J. Integr. Agron. 2013, 12, 319–326. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Mao, S.Y.; Zhu, W.Y. Rumen chemical and bacterial changes during stepwise adaptation to a high-concentrate diet in goats. Animal 2010, 4, 210–217. [Google Scholar] [CrossRef]
- Bevans, D.W.; Beauchemin, K.A.; Schwartzkopf-Genswein, K.S.; McKinnon, J.J.; McAllister, T.A. Effect of rapid or gradual grain adaptation on subacute acidosis and feed intake by feedlot cattle1,2. J. Anim. Sci. 2005, 83, 1116–1132. [Google Scholar] [CrossRef]
- Nagata, R.; Kim, Y.-H.; Ohkubo, A.; Kushibiki, S.; Ichijo, T.; Sato, S. Effects of repeated subacute ruminal acidosis challenges on the adaptation of the rumen bacterial community in Holstein bulls. J. Dairy Sci. 2018, 101, 4424–4436. [Google Scholar] [CrossRef]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.N.; Drochner, W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Dai, D.; Wu, H.; Chai, S.; Liu, S.; Meng, Q.; Zhou, Z. Dietary concentrate-to-forage ratio affects rumen bacterial community composition and metabolome of yaks. Front. Nutr. 2022, 9, 927206. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.Y.; Zhang, R.Y.; Wang, D.S.; Zhu, W.Y. Impact of subacute ruminal acidosis (sara) adaptation on rumen microbiota in dairy cattle using pyrosequencing. Anaerobe 2013, 24, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.C.; Li, S.; Danscher, A.M.; Derakshani, H.; Andersen, P.H.; Khafipour, E. Changes in microbiota in rumen digesta and feces due to a grain-based subacute ruminal acidosis (sara) challenge. Microb. Ecol. 2017, 74, 485–495. [Google Scholar] [CrossRef]
- Burrin, D.G.; Britton, R.A. Response to Monensin in Cattle during Subacute Acidosis. J. Anim. Sci. 1986, 63, 888–893. [Google Scholar] [CrossRef]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Genet. 2013, 11, 497–504. [Google Scholar] [CrossRef]
- Albertsen, M.; Hugenholtz, P.; Skarshewski, A.; Nielsen, K.L.; Tyson, G.W.; Nielsen, P.H. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat. Biotechnol. 2013, 31, 533–538. [Google Scholar] [CrossRef]
- Perea, K.; Perz, K.; Olivo, S.K.; Williams, A.; Lachman, M.; Ishaq, S.L.; Thomson, J.; Yeoman, C.J. Feed efficiency phenotypes in lambs involve changes in ruminal, colonic, and small-intestine-located microbiota. J. Anim. Sci. 2017, 95, 2585–2592. [Google Scholar] [CrossRef]
- Fan, Q.; Wanapat, M.; Yan, T.; Hou, F. Altitude influences microbial diversity and herbage fermentation in the rumen of yaks. BMC Microbiol. 2020, 20, 370. [Google Scholar] [CrossRef]
- Lopes, D.R.G.; La Reau, A.J.; Duarte, M.S.; Detmann, E.; Bento, C.B.P. The Bacterial and Fungal Microbiota of Nelore Steers Is Dynamic Across the Gastrointestinal Tract and Its Fecal-Associated Microbiota Is Correlated to Feed Efficiency. Front. Microbiol. 2019, 10, 1263. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Hao, L.; Degen, A.; Wang, D.; Ma, Y.; Niu, J.; Cheng, Y.; Liu, S. Effect of the ratio of dietary metabolizable energy to nitrogen content on production performance, serum metabolites, rumen fermentation parameters, and bacterial diversity in yaks. Front. Microbiol. 2022, 13, 1013980. [Google Scholar] [CrossRef]
- Kumar, S.; Treloar, B.P.; Teh, K.H.; Mckenzie, C.M.; Henderson, G.; Attwood, G.T.; Waters, S.M.; Patchett, M.L.; Janssen, P.H. Sharpea and kandleria are lactic acid producing rumen bacteria that do not change their fermentation products when co-cultured with a methanogen. Anaerobe 2018, 54, 31–38. [Google Scholar] [CrossRef]
- Neubauer, V.; Petri, R.; Humer, E.; Kröger, I.; Mann, E.; Reisinger, N.; Wagner, M.; Zebeli, Q. High-grain diets supplemented with phytogenic compounds or autolyzed yeast modulate ruminal bacterial community and fermentation in dry cows. J. Dairy Sci. 2018, 101, 2335–2349. [Google Scholar] [CrossRef]
- Tajima, K.; Aminov, R.I.; Nagamine, T.; Matsui, H.; Nakamura, M.; Benno, Y. Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl. Environ. Microbiol. 2001, 67, 2766–2774. [Google Scholar] [CrossRef]
- Thoetkiattikul, H.; Mhuantong, W.; Laothanachareon, T.; Tangphatsornruang, S.; Pattarajinda, V.; Eurwilaichitr, L.; Champreda, V. Comparative analysis of microbial profiles in cow rumen fed with different dietary fiber by tagged 16s RRNA gene pyrosequencing. Curr. Microbiol. 2013, 67, 130–137. [Google Scholar] [CrossRef]
- Zened, A.; Combes, S.; Cauquil, L.; Mariette, J.; Klopp, C.; Bouchez, O.; Troegeler-Meynadier, A.; Enjalbert, F. Microbial ecology of the rumen evaluated by 454 gs flx pyrosequencing is affected by starch and oil supplementation of diets. FEMS Microbiol. Ecol. 2013, 83, 504–514. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Y.; Wang, Y.; Wang, D.; Hou, Y.; Yao, D.; Tian, J.; Jin, Y. Rumen bacteria and meat fatty acid composition of sunit sheep reared under different feeding regimens in China. J. Sci. Food Agron. 2021, 101, 1100–1110. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Kittelmann, S.; Miri, V.H.; Zeth, M. Effect of DNA extraction methods and sampling techniques on the ap-parent structure of cow and sheep rumen microbial communities. PLoS ONE 2013, 11, e74787. [Google Scholar]
- Ji, S.K.; Zhang, H.; Yan, H.; Azarfar, A.; Shi, H.T.; Alugongo, G.; Li, S.L.; Cao, Z.J.; Wang, Y.J. Comparison of rumen bacteria distribution in original rumen digesta, rumen liquid and solid fractions in lactating Holstein cows. J. Anim. Sci. Biotechnol. 2017, 1, 16. [Google Scholar] [CrossRef]
- Chen, Y.B.; Lan, D.L.; Tang, C.; Yang, X.N.; Li, J. Effect of DNA Extraction Methods on the Apparent Structure of Yak Rumen Microbial Communities as Revealed by 16S rDNA Sequencing. Pol. J. Microbiol. 2015, 64, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, J.D.; Bogert, B.; Edwards, J.E.; Boekhorst, J.; Van, G.S.; Saccenti, E.; Plugge, C.M.; Smidt, H. The Effect of DNA Extraction Methods on Observed Microbial Communities from Fibrous and Liquid Rumen Fractions of Dairy Cows. Front. Microbiol. 2018, 9, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dong, G.; Wang, Z.; Liu, J.; Chen, J.; Zhang, Z. Treatment of corn with lactic acid or hydrochloric acid modulates the rumen and plasma metabolic profiles as well as inflammatory responses in beef steers. BMC Vet. Res. 2018, 14, 408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, F.; Ma, X.; Li, F.; Wang, Z. Effects of barley starch level in diet on fermentation and microflora in rumen of hu sheep. Animals 2022, 12, 1941. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.C.; Purvis, H.T.; Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; Desilva, U. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef]
- Kajikawa, H.; Mitsumori, M.; Ohmomo, S. Stimulatory and inhibitory effects of protein amino acids on growth rate and efficiency of mixed ruminal bacteria. J. Dairy Sci. 2002, 85, 2015–2022. [Google Scholar] [CrossRef]
- Krishna Rao, R. Role of glutamine in protection of intestinal epithelial tight junctions. J. Epithel. Biol. Pharmacol. 2012, 5, 47–54. [Google Scholar] [CrossRef]
- Zhang, T.; Mu, Y.; Zhang, R.; Xue, Y.; Guo, C.; Qi, W.; Zhang, J.; Mao, S. Responsive changes of rumen microbiome and metabolome in dairy cows with different susceptibility to subacute ruminal acidosis. Anim. Nutr. 2022, 8, 331–340. [Google Scholar] [CrossRef]
- Phuntsok, T.; Froetschel, M.A.; Amos, H.E.; Zheng, M.; Huang, Y.W. Biogenic amines in silage, apparent postruminal passage, and the relationship between biogenic amines and digestive function and intake by steers. J. Dairy Sci. 1998, 81, 2193–2203. [Google Scholar] [CrossRef]
- Bailey, S.R.; Marr, C.M.; Elliott, J. Identification and quantification of amines in the equine caecum. Res. Vet. Sci. 2003, 74, 113–118. [Google Scholar] [CrossRef]
- Mao, S.; Huo, W.; Liu, J.; Zhang, R.; Zhu, W. In vitro effects of sodium bicarbonate buffer on rumen fermentation, levels of lipopolysaccharide and biogenic amine, and composition of rumen microbiota. J. Sci. Food Agron. 2017, 97, 1276–1285. [Google Scholar] [CrossRef]
- Andersen, P.H.; Hesselholt, M.; Jarloev, N.K.V.O. Endotoxin and arachidonic acid metabolites in portal, hepatic and arterial blood of cattle with acute ruminal acidosis. Acta Vet. Scand. 1994, 35, 223–234. [Google Scholar] [CrossRef]

| Item | Diet | |
|---|---|---|
| Hay | High-Grain | |
| Diet ingredient, % DM | ||
| Oat hay | 75.46 | 35.00 |
| Alfalfa | 19.53 | 0.00 |
| Corn | 0.00 | 39.75 |
| Wheat | 0.00 | 30.25 |
| CaHPO4 | 1.50 | 1.50 |
| NaCL | 1.00 | 1.00 |
| Premix | 2.50 | 2.50 |
| Nutrient levels | ||
| Crude protein, % DM | 10.49 | 10.73 |
| Crude fat, % DM | 3.55 | 3.59 |
| Neutral detergent fiber, % DM | 53.32 | 29.23 |
| Acid detergent fiber, % DM | 34.48 | 14.55 |
| Crude ash, % DM | 9.68 | 6.36 |
| Items b | Diet a | SEM c | p-Value | |
|---|---|---|---|---|
| CON | HG | |||
| AST, mmol/L | 32.28 | 39.71 | 1.53 | 0.009 |
| ALT, mmol/L | 80.14 | 84.57 | 3.05 | 0.490 |
| GGT, U/L | 8.11 | 12.20 | 0.49 | <0.001 |
| HPT, ng/mL | 42.83 | 50.88 | 1.93 | 0.031 |
| SAA, ug/mL | 23.26 | 28.15 | 0.78 | <0.001 |
| IL1-β, ng/L | 42.96 | 51.89 | 1.45 | <0.001 |
| IL-6, ng/L | 397.14 | 446.35 | 8.72 | 0.001 |
| TNF-α, ng/L | 169.94 | 195.25 | 4.86 | 0.004 |
| LPS, EU/mL | 0.62 | 1.17 | 0.08 | <0.001 |
| Items b | Diet a | SEM c | p-Value | |
|---|---|---|---|---|
| CON | HG | |||
| Ruminal pH | 6.54 | 5.73 | 0.12 | <0.001 |
| TVFA, mmol/L | 60.01 | 81.36 | 3.28 | <0.001 |
| VFAs, molar % of TVFA Acetate, % | 64.17 | 60.75 | 1.58 | 0.052 |
| Propionate, % | 22.28 | 22.81 | 1.34 | 0.699 |
| Isobutyrate, % | 0.76 | 1.00 | 0.10 | 0.026 |
| Butyrate, % | 10.61 | 12.56 | 0.50 | 0.002 |
| Isovalerate, % | 0.81 | 1.28 | 0.13 | 0.003 |
| Valerate, % | 1.38 | 1.13 | 0.19 | 0.200 |
| Acetate: propionate ratio | 2.94 | 2.68 | 0.46 | 0.301 |
| Metabolites | VIP a | FC b | p-Value | Type c | Metabolic Classes |
|---|---|---|---|---|---|
| L-Glutamic acid | 1.259 | 0.598 | 0.002 | Down | Amino acids, peptides, and analogues |
| Formiminoglutamic acid | 1.203 | 3.143 | 0.004 | Up | |
| Phenylalanine | 1.143 | 1.883 | 0.008 | Up | |
| Serine | 1.179 | 2.702 | 0.005 | Up | |
| Methionine | 1.331 | 0.267 | <0.001 | Down | |
| Threonine | 1.1735 | 2.730 | 0.006 | Up | |
| Proline | 1.142 | 2.242 | 0.007 | Up | |
| Citrulline | 1.142 | 1.873 | 0.0087 | Up | |
| Gamma-Aminobutyric acid | 1.170 | 2.088 | 0.006 | Up | |
| 4-Acetamidobutanoic acid | 1.378 | 0.583 | 0.007 | Down | |
| DL-Dopa | 1.402 | 1.999 | <0.001 | Up | |
| Alpha-Linolenic acid | 1.359 | 3.167 | <0.001 | Up | Lipids and lipid-like molecules |
| Arachidonic acid | 1.407 | 0.301 | <0.001 | Down | |
| Urocanic acid | 1.363 | 2.935 | <0.001 | Up | |
| PC(P-16:0/16:0) | 1.537 | 0.480 | <0.001 | Down | |
| 20-Hydroxyeicosatetraenoic acid | 1.516 | 0.311 | <0.001 | Down | |
| Oleic acid | 1.622 | 4.021 | <0.001 | Up | |
| Prostaglandin H2 | 1.323 | 3.597 | <0.001 | Up | |
| Prostaglandin E2 | 1.435 | 2.841 | <0.001 | Up | |
| 12(S)-HPETE | 1.116 | 2.232 | 0.011 | Up | |
| Deoxyadenosine monophosphate | 1.013 | 2.394 | 0.028 | Up | Nucleosides, nucleotides, and analogues |
| Deoxyinosine | 1.130 | 2.399 | <0.001 | Up | |
| Deoxyguanosine | 1.415 | 2.278 | <0.001 | Up | |
| Adenosine | 1.332 | 1.933 | 0.001 | Up | |
| Xanthosine | 1.050 | 7.064 | 0.014 | Up | |
| Inosine | 1.161 | 2.863 | <0.001 | Up | |
| Guanosine | 1.188 | 2.571 | 0.007 | Up | |
| Histamine | 1.945 | 1.674 | 0.035 | Up | Amines |
| Tyramine | 1.775 | 8.30 | 0.010 | Up | |
| Putrescine | 1.401 | 1.448 | 0.039 | Up | |
| Prostaglandin G2 | 1.146 | 2.481 | <0.001 | Up | Others |
| Hypoxanthine | 1.285 | 1.604 | 0.001 | Up | |
| Normetanephrine | 1.282 | 0.409 | 0.002 | Down | |
| Epinephrine | 1.259 | 0.654 | 0.007 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, D.; Wang, S.; Wang, X.; Gao, C.; Chai, S.; Xu, X. High-Grain Diet Feeding Altered Blood Metabolites, Rumen Microbiome, and Metabolomics of Yaks. Fermentation 2023, 9, 215. https://doi.org/10.3390/fermentation9030215
Dai D, Wang S, Wang X, Gao C, Chai S, Xu X. High-Grain Diet Feeding Altered Blood Metabolites, Rumen Microbiome, and Metabolomics of Yaks. Fermentation. 2023; 9(3):215. https://doi.org/10.3390/fermentation9030215
Chicago/Turabian StyleDai, Dongwen, Shuxiang Wang, Xun Wang, Changpeng Gao, Shatuo Chai, and Xiaofeng Xu. 2023. "High-Grain Diet Feeding Altered Blood Metabolites, Rumen Microbiome, and Metabolomics of Yaks" Fermentation 9, no. 3: 215. https://doi.org/10.3390/fermentation9030215
APA StyleDai, D., Wang, S., Wang, X., Gao, C., Chai, S., & Xu, X. (2023). High-Grain Diet Feeding Altered Blood Metabolites, Rumen Microbiome, and Metabolomics of Yaks. Fermentation, 9(3), 215. https://doi.org/10.3390/fermentation9030215






