Abstract
The restrictions for halal and vegetarian fermented products apply not only to the food ingredients, but also to the inoculum media. The utilization of a medium for lactic acid bacteria (LAB) leads to some issues from animal-derived proteins sources that may be doubtful for halal and/or vegetarian use. This study aimed to develop a plant-based medium for culturing and maintaining LAB. The result demonstrated that 10 g/L soybean powder in sweet potato extract was suitable for cultivating Lactiplantibacillus plantarum TISTR 2075 with no significant difference (p < 0.05) from MRS (de Man, Rogosa and Sharpe) in the cell number (9.12 log CFU/mL) and specific growth rate (0.04). The feasibility of a plant-based medium to grow and maintain the LAB strains from different origins was evaluated. Compared to MRS, Lpb. plantarum TISTR 2075, Lpb. plantarum MW3, and Lacticaseibacillus casei TISTR 1463 could grow almost as well in a plant-based medium. This medium was also suitable for maintaining the viability of LAB during storage, especially when subjected to slant agar stock culture. It is practical and costs at least 10 times less than MRS. Thus, this study created a low-cost plant-based medium that could be used in laboratories, especially for applications in halal and vegetarian food products.
1. Introduction
Lactic acid bacteria (LAB) are one of the most significant and widely used microbial groups in fermented food production. Through their metabolic activities, LAB can produce many beneficial substances, such as antibacterial compounds and organic acids [1,2,3,4].
LAB are commonly cultured in de Man, Rogosa and Sharpe (MRS) medium. To accommodate LAB growth, MRS is supplemented with a variety of nutrients, including carbon, nitrogen, minerals, and vitamin sources [5]. However, this medium has limitations for starter culture preparation. As MRS consists of various materials, it takes considerable time and effort to prepare the individual components. In terms of cost, notably on a large scale, the use of MRS tends to be high-priced due to its protein and nitrogen sources, such as beef and yeast extract. Another factor to consider is consumer concern about food sources due to their religious beliefs or lifestyle. In the case of halal fermented foods, products made with microorganisms must be devoid of all elements acquired from prohibited sources under Islamic law, on the other side, vegetarian foods must not use animal-derived substances. This comprises not only the product ingredients but also the starter cultures. In most cases, animal-derived substances are utilized as the substrate or the enzyme in the manufacture of peptone, meat, or yeast extract in MRS. The inclusion of such indicated elements renders the medium dubious for use in halal and vegetarian fermented food manufacturing. The source of the animal ingredients could be from any type, including certain forbidden animals or methods under Islamic law, and they are obviously contrary to the concept of a vegetarian diet.
There are some exceptions to the halal status of vegetarian foods and materials. Alcohol and other intoxicants, as well as some plant-based additives, are not considered halal. On the other hand, not all halal foods are suitable for vegetarians. Beef slaughtered in the halal way, for example, cannot be included in vegetarian foods.
Sweet potato (Ipomoea batatas (L.) Lam) is highly nutritious and can promote LAB growth, providing carbohydrates, sugars, vitamins, and minerals [6,7]. Another raw material compatible with bacterial growth is soybean, since it contains many nutrients, including high protein levels and many different amino acids [8,9,10]. Both sweet potato and soybean are rich in many nutrients and are halal; thus, they can be used as basic materials for a culture medium. Furthermore, both come from plants, suitable for vegetarian use. In addition, these materials are produced worldwide, making them conveniently accessible and affordable.
Not only is microbial culture cultivation important, but also is microbial culture preservation, especially in research and industry. Regular subculture is one of the commonly used methods for culture preservation. In this method, the cell culture has to be transferred to fresh, sterile slant agar medium periodically and stored in a refrigerator. Stock cultures can also be made using the freezing method. Studies by Berner and Viernstein [11] and Novik et al. [12] reported that frozen stock culture could be preserved by freezing the cells in media with or without the presence of cryoprotectant. It has been revealed that each medium has a different effect on cell viability.
Several previous studies have developed LAB cultivation media using alternative halal and/or plant-based materials [6,10,13,14]. However, most of the previous studies have used many components and steps in their formulations to obtain comparable bacterial growth to MRS. Some of these experiments used nonvegetarian and/or questionable halal components, as well as high-cost ingredients such as meat peptone and yeast extract [6]. Thus, the aim of this study was to develop a low-cost and easy-to-prepare culture medium based on sweet potato and soybean for culturing and preserving LAB for applications in halal and vegetarian food products. To our knowledge, this present study is the first work exploring the feasibility of using a low-cost plant-based and halal medium for different LAB strains in slant agar and frozen stock cultures.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
Three LAB strains isolated from different fermented products were used in this study. Lactiplantibacillus plantarum TISTR 2075 isolated from fermented vegetables and Lacticaseibacillus casei TISTR 1463 isolated from yogurt were obtained from the Thailand Institute of Scientific and Technology Research (TISTR) Culture Collection. Lactiplantibacillus plantarum MW3 isolated from fermented fish was obtained from the Department of Biotechnology, Khon Kaen University culture collection. Lpb. plantarum TISTR 2075 and Lpb. plantarum MW3 were incubated at 30 °C. Lcb. casei TISTR 1463 was incubated at 37 °C. Each bacterium was activated twice, first by incubating in skim milk medium consisting of 100 g/L halal certified skim milk (Indoprima, Indonesia) and 10 g/L sucrose (Mitr Phol, Thailand) overnight and then for 9 h. For the inoculum, the initial LAB concentration was approximately 5−6 log CFU/mL, which was obtained by transferring the cells into sterile saline solution to achieve an optical density (OD) at 600 nm of 0.4.
2.2. Effects of Soybean Powder Concentration and Sweet Potato Extract on the Growth of Lpb. plantarum TISTR 2075
Fresh sweet potatoes were purchased from a supermarket (Tesco Lotus) in Khon Kaen Province, Thailand. The sweet potato extract preparation was modified from Hayek et al. [6] and Johnson et al. [15]. Whole raw sweet potato was baked in an oven (Binder, Germany) at 160 °C for 1 h. Peeled baked sweet potato was blended with distilled water (DW) at a ratio of 12.5 of DW (mL) to 1 of sweet potato (g dry weight), and then the sweet potato slurry was filtered through a filter cloth. The liquid part was collected as the sweet potato extract.
Peeled split soybeans were purchased from a market (Tesco Lotus) in Khon Kaen Province, Thailand. The soybeans were ground using a blender and then sieved to obtain soybean powder. The sweet potato extract was supplemented with different levels of soybean powder: 0, 10, 20 and 30 g/L. MRS (Himedia, India) was used as the control medium. All media were sterilized at 110 °C for 28 min. Subsequently, 10% (v/v) Lpb. plantarum TISTR 2075 inoculum was inoculated into the media and incubated for 48 h at 30 °C. Samples were taken at 0, 12, 24, 36 and 48 h of incubation for cell counting by dilution and the plate count method.
The determination of the specific growth rate was performed by plotting the logarithmic values of the colony forming units (CFU)/mL every 2 h for 12 h of incubation, which were plotted against the time (h). The specific growth rate was obtained from the exponential equation below:
where µ, N, N0, and t are the specific growth rate, cell number at a certain time, and the difference in the cell number between time zero and time, respectively.
N = N0 eµt
2.3. Growth of LAB Strains in Plant-Based and MRS Media
Three LAB strains were tested for their growth. Lpb. plantarum TIRTR 2075, Lpb. plantarum MW 3, and Lcb. casei TISTR 1463 were grown in plant-based and MRS media. Each strain inoculum (10% (v/v)) was inoculated into each sterile medium and incubated for 24 h. Viable cells were determined at 0 and 24 h by serial dilution in saline solution and plating on MRS agar plates.
2.4. Analysis of the Nutrient Composition of the Culture Media
The nutrient content of the growth media was analyzed. Total carbohydrate was determined using the phenol-sulfuric acid method [16]. The protein content was estimated by the Lowry method [17]. The total sugar was analyzed using a liquid chromatographic method according to the in-house method based on AOAC (2019) 977.20. The nitrogen of the plant-based and MRS media was analyzed using the block digestion method according to the in-house method TE-CH-042 based on AOAC (2019) 981.10 [18].
2.5. Viability of LAB Strains from Frozen Stock Culture
For the determination of cell viability, frozen stock cultures of Lpb. plantarum TISTR 2075, Lpb. plantarum MW 3, and Lcb. casei TISTR 1463 were made in three different media. MRS, plant-based, and skim milk 10% (w/v) media were used as the cryoprotectants. First, each strain was prepared by growing in the media (MRS, plant-based, and skim milk) at a suitable temperature for two times, first for 24 h and the second for 9 h. The obtained cells were used to make the frozen stock cultures by mixing the cells with MRS, plant-based, and skim milk separately. The frozen stock cultures were stored at −20 °C. Viable cells were determined before storage, and then every 2 weeks over 3 months by thawing the frozen stock culture at room temperature, and then serially diluting it in saline solution for plate counting on MRS agar. The viability (%) was determined as the number of viable cells after storage divided by the number of viable cells before storage.
2.6. Viability of LAB Strains on Slant Agar Stock Culture
For the determination of cell viability, slant agar stock cultures of Lpb. plantarum TISTR 2075, Lpb. plantarum MW 3, and Lcb. casei TISTR 1463 were prepared in both MRS and plant-based media. MRS and plant-based slant agar media were made by adding agar powder to the broth medium. The cultures of LAB were transferred by streaking the cells using an inoculating loop on the surface of the agar medium, incubated until growth was evident, and then refrigerated at 4 °C. Viable cells were quantified before storage and weekly for 1 month. The cells from the slant agar media were transferred into sterile saline solution to adjust the OD at every sampling time and serially diluted in saline solution for plating on MRS agar plates. The viability (%) was determined as the number of viable cells after storage divided by the number of viable cells before storage.
2.7. Statistics
Experiments were performed in triplicate. Data were assessed using one-way analysis of variance (one-way ANOVA) in IBM SPSS Statistic Version 23 software. Statistical significance was accepted at p < 0.05.
3. Results and Discussion
3.1. Effects of Soybean Powder and Sweet Potato Extract on the Growth of Lpb. plantarum TISTR 2075
To develop a plant-based medium for culturing LAB, the growth of Lpb. plantarum TISTR 2075 in various combinations of soybean powder and sweet potato extract were examined and compared to those from MRS. At 24 and 36 h of incubation, the cell number in sweet potato extract combined with 10 g/L soybean powder was not significantly different from that in MRS (Table 1). The growth of Lpb. plantarum TISTR 2075 in sweet potato extract mixed with 10 g/L soybean powder and sweet potato extract mixed with 20 g/L soybean powder media was also not remarkably different. Table 2 shows the specific growth rate of Lpb. plantarum TISTR 2075 based on the cell population data during the 12 h incubation. The specific growth rates in MRS and sweet potato extract mixed with soybean powder (0 and 10 g/L) were not substantially different (p < 0.05).

Table 1.
Cell populations of Lpb. plantarum TISTR 2075 in MRS, sweet potato extract (SPE) mixed with 0, 10, 20, and 30 g/L of soybean powder media during 48 h of incubation.

Table 2.
Specific growth rate of Lpb. plantarum TISTR 2075 in MRS and sweet potato extract (SPE) mixed with 0, 10, 20, and 30 g/L of soybean powder media.
Lactobacillus strains were previously shown to grow well in sweet potato media supplemented with protease peptone, beef extract, and yeast extract as nitrogen sources [6]. Due to its high nutrient content, sweet potatoes have been employed in a variety of food sectors as well as for human consumption. The sweet potato nutrient content varies according to variety, climate, and soil type [19,20]. Sweet potato is a rich source of carbohydrates, mainly starch and sugars such as maltose, fructose, sucrose, and glucose [21]. Protein, various amino acids, vitamins A, B, C, riboflavin, magnesium, calcium, potassium, and phosphorus are all present [22].
Soybean has been used as a protein source in culture media for microorganisms such as Escherichia coli, Pseudomonas sp., Bacillus sp., Staphylococcus sp., and Klebsiella sp. [23]. This study demonstrated that the supplementation of sweet potato extract medium with 10 g/L soybean powder as a complex nitrogen source resulted in a higher cell number than that in only sweet potato extract medium (soybean powder 0 g/L). Based on the cell populations (at 24 and 36 h) and specific growth rate (12 h), higher concentrations of soybean powder (>10 g/L) could not increase the growth of Lpb. plantarum TISTR 2075. According to other findings, additional amounts of nitrogen sources beyond the essential requirements might not improve the growth rates and bacterial population of Lactobacillus strains [6,24]. The result clearly demonstrated that 10 g/L soybean powder in sweet potato extract was suitable for cultivating Lpb. plantarum TISTR 2075 with no significant difference (p < 0.05) from MRS in the cell number (9.12 log CFU/mL) and specific growth rate (0.04). Hence, sweet potato extract combined with 10 g/L soybean powder was chosen as the optimum medium and used in the subsequent experiments. This medium will be referred to as a plant-based medium from now on.
3.2. Growth of LAB Strains in Plant-Based and MRS Media
Three LAB strains from different origins were tested for their ability to grow in both plant-based and MRS media. Lpb. plantarum TISTR 2075, Lpb. plantarum MW3, and Lcb. casei TISTR 1463 grew well in both MRS and plant-based media, with no significant difference in the cell number of Lpb. plantarum MW3 and Lcb. casei TISTR 1463 after 24 h of incubation (Figure 1). On the other hand, the cell number of Lpb. plantarum TISTR 2075 was higher in MRS than in plant-based media (Figure 1).
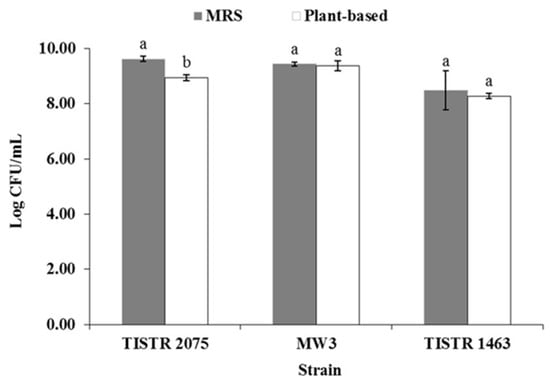
Figure 1.
Growth of LAB in MRS and plant-based media after 24 h of incubation. Each color is the average of three replicates. Different superscripts for each strain indicate significant difference (p < 0.05).
Many researchers have found that some species of Lactobacilli and some strains of Lcb. casei are capable of utilizing carbon sources in the form of glucose, fructose, lactose, sucrose, and maltose [24,25,26,27,28]. The total carbohydrate, protein (soluble), nitrogen, and sugar contents of the media are shown in Table 3 and Table 4. Each medium contained different levels and types of nutrients. Although the results showed that the total carbohydrate content of the plant-based medium was higher than that in MRS (Table 3), Table 4 shows that MRS contained dextrose (glucose), which is the preferred monosaccharide for Lpb. plantarum [29]. On the other hand, the plant-based medium contained sucrose and maltose (Table 4). These forms of carbon sources would take longer to be utilized by the cells.

Table 3.
Total carbohydrate and protein of media.

Table 4.
Nitrogen and total sugar of media.
The MRS medium had higher total protein and nitrogen than the plant-based medium (Table 3 and Table 4). In addition, both the sugar/soluble nitrogen and C/N ratios in the plant-based medium were higher than those in the MRS medium. These results supported the reason why Lpb. plantarum TISTR 2075 grew better in MRS than in plant-based medium. Differences in the biomass and growth characteristics between LAB strains in the same medium do occur, probably due to the strain effect [30,31,32]. Compared to MRS, Lpb. plantarum TISTR 2075, Lpb. plantarum MW3, and Lcb. casei TISTR 1463 could grow as well in the plant-based medium.
3.3. Viability of LAB Strains from Frozen Stock Culture Using Different Media
The feasibility of the plant-based medium to maintain the LAB was evaluated. As shown in Figure 2a, there was no significant difference in viability during six weeks of Lpb. plantarum TISTR 2075 storage using MRS, plant-based, or skim milk 10% (w/v) media. MRS and skim milk 10% (w/v) had the same viability over three months of storage, whereas the plant-based medium had lower viability in the eighth week and it remained at this level until the end of storage. At the end of storage, no significant difference was observed in the viability using skim milk 10% (w/v) (97%), MRS (95%), or plant-based (92%) media. For Lpb. plantarum MW3 (Figure 2b), 10% skim milk (w/v) was the most suitable medium for making the frozen stock culture. The viability in this medium was maintained the same from the beginning until the end of three months of storage. The viability of Lpb. plantarum MW3 using MRS started to drop in the tenth week, whereas when using a plant-based medium, it declined in the second, fourth, tenth, and twelfth weeks. At the end of the third month of storage, a significant difference was found in the viability in 10% skim milk (w/v) (97%), followed by MRS (88%) and plant-based (52%) media.
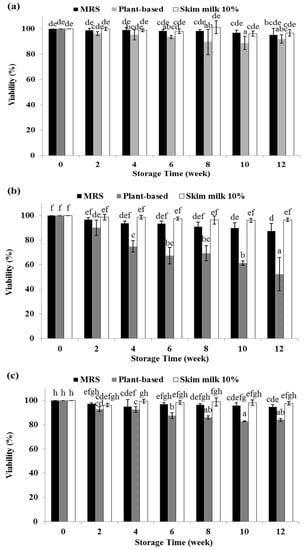
Figure 2.
Viability of Lpb. plantarum TISTR 2075 (a); Lpb. plantarum MW3 (b); Lcb. casei TISTR 1463 (c) after frozen storage for three months in different media (frozen stock culture). Each color is the average of three replicates. Different superscripts indicate significant difference (p < 0.05).
As shown in Figure 2c, the viability of Lcb. casei TISTR 1463 in 10% skim milk (w/v) remained the same over three months of storage. The viability of Lcb. casei TISTR 1463 in MRS declined in the second week but then remained the same until the last week of storage. On the other hand, the first decline in viability using a plant-based medium was observed in the second week of storage. A second decline in viability was found in the sixth week and then it remained the same until the end of storage. In the last week of storage, there was a significant difference in viability among all of the media used. Skim milk 10% (w/v) gave the highest viability (98%), followed by MRS (95%) and plant-based (84%) media.
Skim milk has been widely used as a cryoprotectant for preserving cells. Previous studies have shown that skim milk is a better cryoprotectant with or without combination with another protectant compared to other media or solutions, such as glycerol, MRS, and sucrose [11,12], and similar findings were observed in this study. For all strains used in this study, the highest viability of the frozen stock culture was obtained in 10% skim milk (w/v). Skim milk might affect the fatty acid content of the cell membrane, altering the membrane fluidity [33,34]. The calcium contained in skim milk might also contribute to the stability of the cellular enzymes [35].
A complex medium, for example, MRS, could also be used as a cryoprotectant since this medium contains various compounds that have cryoprotective effects, such as glucose, peptone, and Tween 80 [12]. The sugar alone could have a cryoprotective effect on bacterial cells. According to Berner and Viernstein [11], disaccharides (lactose, sucrose and maltose) resulted in better viabilities than monosaccharides (glucose and fructose). This might explain why the plant-based medium was still able to protect against cell death during storage due to the maltose and sucrose contents in that medium, as shown in Table 4. It has also been reported that 10% sucrose and maltose solutions individually supported viability at approximately 40% after storage for 1 week prior to freeze-thawing. On the other hand, the plant-based medium contained only 0.91 g/100 g of sucrose, 1.88 g/100 g of maltose, 4.58 g/L of protein, and 0.05 g/100 g of nitrogen (Table 3 and Table 4). With those concentrations and types of compounds, a plant-based medium might possibly result in lower viability after storage compared to MRS and skim milk since it contains fewer cryoprotective compounds, mainly from carbon and nitrogen sources (sweet potato and soybean). Therefore, the addition of plant-based halal protectant agents should be explored to improve cell viability when storing cells in plant-based media. However, a plant-based medium resulted in higher viability after three months than sugar solution alone after one week of storage in the research by Berner and Viernstein [11]. This could be possible since it was made from sweet potato and soybean, which contain not only sugar but also protein, vitamins, and amino acids, which might have a protective effect on the cells during freezing.
The decrease in cell viability during frozen storage could have occurred because of the ice crystals that formed. The ice might cause dehydration of the bacterium cells, and because the solutes had accumulated in high concentrations, these would denature biomolecules. In addition, ice might rupture the cell membrane. The results from the current study suggested that the plant-based medium was also suitable for maintaining the viability of LAB during storage for three months. However, the addition of halal cryoprotectant especially for Lpb. plantarum MW3 frozen stock culture is highly suggested.
3.4. Viability of LAB Strains on Slant Agar Stock Culture Using Different Media
The practicality of this medium was examined for maintaining the viability of LAB for 4 weeks when subjected to slant agar stock culture. Figure 3 depicts the viability of Lpb. plantarum TISTR 2075, Lpb. plantarum MW3, and Lcb. casei TISTR 1463 on MRS and plant-based slant agar media. No significant differences in the viability of Lpb. plantarum TISTR 2075 at any time point during one month of storage using MRS and plant-based media were observed (Figure 3a). In both media, there was a slight difference in cell viability after one week of storage, but the cell viability remained the same until the third week. The viability of the cells declined more in the fourth week of storage. In the first until the fourth week of storage, the viability of cells in MRS slant agar ranged from 82 to 94%. Meanwhile, the viability of cells in plant-based slant agar ranged from 79 to 95%.
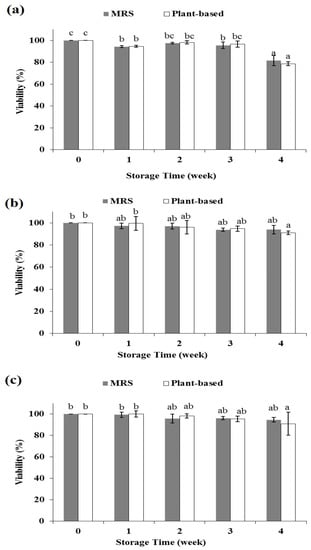
Figure 3.
Viability of Lpb. plantarum TISTR 2075 (a); Lpb. plantarum MW3 (b); Lcb. casei TISTR 1463 (c) after cold storage for one month in different media (slant agar stock culture). Each color is the average of three replicates. Different superscripts indicate significant difference (p < 0.05).
As shown in Figure 3b, during the first until the fourth week of storage, the viability of Lpb. plantarum MW3 in MRS slant agar was not significantly different from week to week (94–97%). On the other hand, the viability of cells from plant-based agar medium was the same until the third week of storage, and then it slightly decreased in the fourth week of storage (91–99%). Nevertheless, at the end of storage, the viability of Lpb. plantarum MW3 stored in plant-based agar medium was not significantly different (91%) from that stored in MRS slant agar medium (94%).
Viable cell counting revealed that the viability of Lcb. casei TISTR 1463 (Figure 3c) at all time points during one month of storage were all similar, with no significant differences in either MRS or plant-based slant agar. Although the viability of cells in the plant-based medium slightly decreased compared to that of before storage within the fourth storage week, there was no significant difference in viability between storage on MRS (95%) or plant-based slant agar media (91%).
According to these results, the viabilities of the three different LAB strains on slant agar stock cultures during one month of storage were not significantly different between MRS or plant-based media. Despite the fact that the total sugar and nitrogen in the plant-based medium were lower than those in the MRS medium (Table 4), these results showed that the plant-based medium could support keeping the cultures viable just as well as MRS. This could be because the metabolic activities of the microbes will slow down considerably, but not entirely halt during cold storage. The cell will only utilize a small amount of nutrients. Thus, this study created a practical plant-based medium for culturing and maintaining LAB.
The cost of the plant-based medium was evaluated and compared with MRS, since MRS is utilized as a common culture medium to grow LAB. As shown in Table 5, preparation by using MRS broth powder directly or weighing out the individual components based on the formula to form MRS broth may result in different expenses. The cost of 1 L of MRS by weighing each ingredient is approximately USD 10, while that of using commercial MRS powder is USD 13, depending on the Himedia brand. The prices for making MRS broth medium can be quite varied, not only depending on the brand, but also on additional costs per purchase. On the other hand, the plant-based medium cost approximately USD 0.6. Consequently, the expense is less than 1.0 dollars to prepare 1 L of plant-based medium in this study. The cost of plant-based media is strikingly less than that of commercial MRS media. Thus, this study created a low-cost plant-based medium that could be used in laboratories and in the food industry.

Table 5.
Cost calculation of plant-based and MRS media 2.
4. Conclusions
In this study, a plant-based medium was developed using sweet potato extract combined with 10 g/L soybean powder. This medium was obtained not only through a simple preparation but also minimal ingredients, which was the plant-based materials without any additional component. Compared to the MRS medium, the three strains of LAB could grow almost as well in a plant-based medium. This medium was also suitable for maintaining the viability of LAB during storage, especially when subjected to slant agar stock culture, but the addition of halal cryoprotectant for frozen stock culture is highly suggested. Thus, this study created a low-cost plant-based medium for culturing and maintaining LAB cells isolated from three different origins. This medium could be used in the food industry and in laboratories, especially for applications in halal and vegetarian food products.
Author Contributions
B.T.A., Conceptualization, Methodology, Investigation, Validation, Writing—original draft. N.C., Methodology, Investigation, Formal analysis, Writing—Reviewing & Editing. J.A., Supervision, Conceptualization, Methodology, Formal analysis, Project administration, Resources, Validation, Visualization, Writing—Reviewing and Editing, Funding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science, Research, and Innovation Fund (NSRF) through Fundamental Fund, Khon Kaen University, Thailand. Authors acknowledge KKU Scholarship for ASEAN and GMS Countries Personnel for kindly supporting the research fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Wichai Soemphol for providing the Lpb. plantarum MW3.
Conflicts of Interest
The authors declare that they have no competing interest with respect to the work described in this manuscript.
References
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takishima, T.; Kometani, T.; Yokogoshi, H. Psychological stress-reducing effect of chocolate enriched with gamma-aminobutyric acid (GABA) in humans: Assessment of stress using heart rate variability and salivary chromogranin A. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S5), 106–113. [Google Scholar] [CrossRef] [PubMed]
- Suwanmanon, K.; Hsieh, P.-C. Effect of γ-aminobutyric acid and nattokinase-enriched fermented beans on the blood pressure of spontaneously hypertensive and normotensive wistar-kyoto rats. J. Food Drug Anal. 2014, 22, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Woraprayote, W.; Malila, Y.; Sorapukdee, S.; Swetwiwathana, A.; Benjakul, S.; Visessanguan, W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016, 120, 118–132. [Google Scholar] [CrossRef] [PubMed]
- De MAN, J.C.; Rogosa, M.; Sharpe, M.E. A Medium for the cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Hayek, S.A.; Shahbazi, A.; Awaisheh, S.S.; Shah, N.P.; Ibrahim, S.A. Sweet potatoes as a basic component in developing a medium for the cultivation of Lactobacilli. Biosci. Biotechnol. Biochem. 2013, 77, 2248–2254. [Google Scholar] [CrossRef]
- Mohanraj, R.; Sivasankar, S. Sweet potato (Ipomoea batatas [L.] Lam)—A valuable medicinal food: A review. J. Med. Food 2014, 17, 733–741. [Google Scholar] [CrossRef]
- Goldflus, F.; Ceccantini, M.; Santos, W. Amino acid content of soybean samples collected in different Brazilian states—Harvest 2003/2004. Braz. J. Poult. Sci. 2006, 8, 105–111. [Google Scholar] [CrossRef]
- O’Keefe, S.F.; Bianchi, L.; Sharman, J. Soybean Nutrition. SM J. Nutr. Metab. 2015, 1, 1006. [Google Scholar]
- Utami, T.; Cindarbhumi, A.; Khuangga, M.C.; Rahayu, E.S.; Cahyanto, M.N.; Nurfiyani, S.; Zulaichah, E. Preparation of indigenous lactic acid bacteria starter cultures for large scale production of fermented milk. Digit. Press Life Sci. 2020, 2, 00010. [Google Scholar] [CrossRef]
- Berner, D.; Viernstein, H. Effect of protective agents on the viability of Lactococcus lactis subjected to freeze-thawing and freeze-drying. Sci. Pharm. 2006, 74, 137–149. [Google Scholar] [CrossRef]
- Novik, G.; Sidarenka, A.; Rakhuba, D.; Kolomiets, E. Cryopreservation of bifidobacteria and bacteriophages in belarusia collection 0f non-pathogenic microorganisms. J. Cult. Colect. 2009, 6, 76–84. [Google Scholar]
- Pathak, M.; Martirosyan, D. Optimization of an effective growth medium for culturing probiotic bacteria for applications in strict vegetarian food products. Funct. Foods Health Dis. 2012, 2, 369–378. [Google Scholar] [CrossRef]
- Pratiwi, R.D.; Zanjabila, S.; Fairuza, D.; Aminah, A.; Praharyawan, S.; Fuad, A.M. Evaluation of alternative components in growth media of Lactobacillus brevis for halal probiotic preparation. Ann. Bogor. 2020, 24, 11. [Google Scholar] [CrossRef]
- Johnson, A.S.; Holliday, D.L.; Mubarak-Assad, K. Impact of baking time and temperature on nutrient content and sensory quality of sweet potatoes. J. Culin. Sci. Technol. 2016, 14, 13–21. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Dako, E.; Retta, N.; Desse, G. Comparison of three sweet potato (Ipomoea batatas (L.) Lam) varieties on nutritional and anti-nutritional factors. Glob. J. Sci. Front. Res. 2016, 16, 63–72. [Google Scholar]
- Rose, I.; Vasanthakaalam, H. Comparison of the nutrient composition of four sweet potato varieties cultivated in Rwanda. Am. J. Food Nutr. 2011, 1, 34–38. [Google Scholar] [CrossRef]
- Owusu Mensah, E.; Oduro, I. Cooking treatment effects on sugar profile and sweetness of eleven-released sweet Potato aarieties. J. Food Process. Technol. 2016, 07. [Google Scholar] [CrossRef]
- Ojimelukwe, P.C.; Ukom, A.N.; Kalu, O.O. Contribution of planting space and harvesting period on the nutrient compositions of some OFSP sweet potato varieties grown in Southeast Nigeria Ultisol. J. Nutr. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Uthayasooriyan, M.; Pathmanathan, S.; Ravimannan, N.; Sathyaruban, S. Formulation of alternative culture media for bacterial and fungal growth. Pharm. Lett. 2016, 8, 431–436. [Google Scholar]
- Cheng, F.; Chen, H.; Lei, N.; Zhang, M.; Wan, H. Effects of carbon and nitrogen sources on activity of cell envelope proteinase produced by LP69. Acta Univ. Cibiniensis Ser. E Food Technol. 2019, 23, 11–18. [Google Scholar] [CrossRef]
- Andreevskaya, M.; Johansson, P.; Jääskeläinen, E.; Rämö, T.; Ritari, J.; Paulin, L.; Björkroth, J.; Auvinen, P. Lactobacillus oligofermentans glucose, ribose and xylose transcriptomes show higher similarity between glucose and xylose catabolism-induced responses in the early exponential growth phase. BMC Genom. 2016, 17, 539. [Google Scholar] [CrossRef]
- Chooklin, S.; Kaewsichan, L.; Kaewsrichan, J. Potential use of Lactobacillus casei TISTR 1500 for the bioconversion from palmyra sap and oil palm sap to lactic acid. Electron. J. Biotechnol. 2011, 14, 10. [Google Scholar]
- Mohseni, J.; Fazeli, M.; Lavasani, A.S. Effect of various parameters of carbon and nitrogen sources and environmental conditions on the growth of Lactobacillus casei in the production of lactic acid. J. Med. Res. 2017, 16, 66–73. [Google Scholar]
- Watson, D.; O’Connell Motherway, M.; Schoterman, M.H.C.; van Neerven, R.J.J.; Nauta, A.; van Sinderen, D. selective carbohydrate utilization by Lactobacilli and Bifidobacteria. J. Appl. Microbiol. 2013, 114, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- Gubelt, A.; Blaschke, L.; Hahn, T.; Rupp, S.; Hirth, T.; Zibek, S. Comparison of different lactobacilli regarding substrate utilization and their tolerance towards lignocellulose degradation products. Curr. Microbiol. 2020, 77, 3136–3146. [Google Scholar] [CrossRef]
- Miloud, B.; Halima, Z.-K.; Nour-Eddine, K. Development of a sweet whey-based medium for culture of Lactobacillus. Afr. J. Biotechnol. 2017, 16, 1630–1637. [Google Scholar] [CrossRef]
- Das, A.J.; Das, M.J.; Miyaji, T.; Deka, S.C. Growth and metabolic characterization of four lactic acid bacteria species isolated from rice beer prepared in Assam, India. Access Microbiol. 2019, 1, e000028. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Song, Z.; Shan, C.; Zhu, R.; Liu, F. Development of a simple, low-cost and eurytopic medium based on Pleurotus eryngii for lactic acid bacteria. AMB Express 2016, 6, 65. [Google Scholar] [CrossRef]
- Annous, B.A.; Kozempel, M.F.; Kurantz, M.J. Changes in membrane fatty acid composition of Pediococcus Sp. Strain NRRL B-2354 in response to growth conditions and its effect on thermal resistance. Appl. Environ. Microbiol. 1999, 65, 2857–2862. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Effects of various sugars added to growth and drying media upon thermotolerance and survival throughout storage of freeze-dried Lactobacillus Delbrueckii Ssp. Bulgaricus. Biotechnol. Prog. 2004, 20, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Barach, J.T.; Adams, D.M.; Speck, M.L. Stabilization of a psychrotrophic Pseudomonas protease by calcium against thermal inactivation in milk at ultrahigh temperature. Appl. Environ. Microbiol. 1976, 31, 875–879. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).