Abstract
The present study aimed to optimize the production of L-asparaginase from Aspergillus arenarioides EAN603 in submerged fermentation using a radial basis function neural network with a specific genetic algorithm (RBFNN-GA) and response surface methodology (RSM). Independent factors used included temperature (x1), pH (x2), incubation time (x3), and soybean concentration (x4). The coefficient of the predicted model using the Box–Behnken design (BBD) was R2 = 0.9079 (p < 0.05); however, the lack of fit was significant indicating that independent factors are not fitted with the quadratic model. These results were confirmed during the optimization process, which revealed that the standard error (SE) of the predicted model was 11.65 while the coefficient was 0.9799, at which 145.35 and 124.54 IU mL−1 of the actual and predicted enzyme production was recorded at 34 °C, pH 8.5, after 7 days and with 10 g L−1 of organic soybean powder concentrations. Compared to the RBFNN-GA, the results revealed that the investigated factors had benefits and effects on L-asparaginase, with a correlation coefficient of R = 0.935484, and can classify 91.666667% of the test data samples with a better degree of precision; the actual values are higher than the predicted values for the L-asparaginase data.
1. Introduction
L-asparaginase (E.C. 3.5.1.1 L-asparagina amidohydrolase) is a recognized drug for the treatment of acute lymphoblastic leukemia [1]. L-asparaginase acts by catalyzing the substrate of asparagine which is essential for the survival of leukemia cells to ammonium and L-aspartic acid. L-asparaginase has appeared as an excellent antineoplastic agent with anti-carcinogenic activity and has a high potential in medical applications [2]. Several substrates have been used to produce L-asparagine from fungi. Soybean (Glycine max) is among the substrates with high contents of protein (~35–40%), lipids (~20%), dietary fiber (~9%), and moisture (~8.5%), which support the fungal growth and induce the enzyme production. The potential of soybean substrate as a perfect substrate for L-asparaginase production has been reported in the literature [3,4,5]. However, the limitations lie in the production cost which depends on the fungal strain and optimization of the solid-state fermentation process to achieve a high production rate. This issue is being overcome by using novel fungal strains and selecting the factors that influence the production process.
The L-asparaginase enzyme has been isolated from A. niger [6], Sarocladium strictum [7], Fusarium sp. [8], Talaromyces pinophilus [9], Trichosporon asahii [10], A. terreus [11], A. tubingensis [12], Lasiodiplodia theobromae [13], and A. oryzae [14]. A. arenarioides EAN603 is a new strain among six new fungal strains, including Purpureocillium lilacinum EAN601, Parengyodontium album EAN602, Penicillium pedernalense EAN604, A. iizukae EAN605, and Paraconiothyrium brasiliense EAN202 obtained from peat soil [15]. A. arenarioides EAN603 exhibited unique characteristics and the secondary metabolite products of this fungal strain in a pumpkin peel medium were used for synthesizing Cu–Zn bio nanocomposites [16].
The enhancement of L-asparaginase production from a fungal strain was perfected in previous studies using response surface methodology (RSM) [4]. They optimized the production of L-asparaginase from Streptomyces brollosae in solid-state fermentation using the Plackett–Burman experimental design with 16 independent variables. The study revealed that the actual production of L-asparaginase was 145.57 U gds−1 compared to 149.97 U gds−1 predicted by the regression model, with a coefficient of 97.06% and standard error (SE) less than 5% between the actual and prediction models. However, the optimization process with 16 factors needs huge numbers of the experimental run which increase. Recently, Sharma and Mishra [17] investigated Aspergillus niger with de-oiled cake as the sole substrate. The optimization process was conducted with four factors including moisture content, autoclaving time, pH, and temperature using RSM and an artificial neural network (ANN). The best operating parameters for high production (34.65 ± 2.18 IU g−1d−1) were obtained with moisture content after 30.3 min at pH 6.2 and 30 °C. The artificial neural network (ANN) model showed superior prediction with a low mean squared error (MSE) of 0.072, and R2 0.99. However, different independent factors might affect positively or negatively on the optimization process.
The hybrid radial basis function neural network (RBFNN) is established with the metaheuristic technique called the genetic algorithm (GA). The RBFNN is a feed-forward neural network and it is the best network compared to other networks, such as the Hopfield neural network (HNN) [18] and multilayer perceptron neural network (MLP) [19]. The RBFNN has been widely utilized in many fields due to its simpler network structure, better approximation capabilities, and faster learning speeds [20]. The GA is the robust evolutionary paradigm that has attracted much research in maximization and optimization problems [21]. The GA combines the idea of reproduction, cross-over, and mutation [22]. Therefore, the GA can be implemented in the RBFNN to become the best mathematical model and one of the good models to be used in prediction or classification. This study provides the best model called the neural network of the radial basis function with a specific genetic algorithm (RBFNNGA) to improve the production of L-asparaginase.
In the present study, the optimization of L-asparaginase enzyme from a new fungal strain A. arenarioides EAN603 in the submerged fermentation process was optimized using RSM to evaluate the effects of multiple factors and their interactions on one or more response variables. The application of an algorithm, such as those provided in MATLAB or other software, might be not accurate to provide the prediction results compared to the actual results, which are supposed to be verified in the laboratory and confirm the fitness and efficiency. Moreover, a statistical approach was employed through the RBFNN-GA hybrid artificial neural network (ANN) with a specific genetic algorithm model used to find significant variables influencing the production, and this emphasizes the novelty of the current work. The efficiency and accuracy of the predicted models of RSM and RBFNN-GA were validated in the laboratory, while the difference between predicted results and actual results was evaluated based on the determination of errors using RMSE, standard error of prediction (SEP), mean absolute percentage error (MAPE), mean absolute error (MAE), Akaike information criterion (AIC), correlation coefficient (R), and accuracy.
2. Materials and Methods
2.1. Fungal Strains and Cultural Conditions
A total of six fungal strains including P. lilacinum EAN601, P. album EAN602, A. arenarioides EAN603, P. pedernalense EAN604, A. iizukae EAN605, and P. brasiliense EAN202 recovered from the peat soil sample were cultured on a modified Czapek Dox (MCD) agar medium [15]. The fungal strain spores were suspended in 20% (v/v) glycerol and kept at −20 °C.
2.2. Semi-Quantitative Screening for L-asparaginase Production
The screening for the production of L-asparaginase was performed using a plate assay with MCD that has 1 mL L−1 (v/v) of phenol red (0.25%, w/v dissolved in 96% ethanol), and L-asparagine was the only nitrogen source; the pH of the medium was adjusted to pH 6.2 [23]. The cultured medium was incubated for one week at 30 °C, while the change in color from yellowish to pink was examined daily. The positive fungal colonies were sounded in the pink zone. The colony and color change into pink due to L-asparaginase activity zone diameter was measured for each fungal strain. The confirmation of L-asparagine production by the fungal strains was carried out using bromothymol blue (BTB) [24,25]. In brief, 10 mL of 0.04% (w/v) BTB stock solution was added to 90 mL DW, and 1 mL of the dilution was supplemented in 1 L of the MCD medium. The fungal strains were cultured in agar media plates and incubated for one week at 30 °C. The L-asparaginase activity index was calculated according to Equation (1).
2.3. Production of L-asparaginase in Submerged Fermentation
The production by the most potent fungal strains (P. lilacinum EAN601, A. arenarioides EAN603, P. pedernalense EAN604, A. iizukae EAN605, and P. brasiliense EAN202), which showed positive results in the rapid plate assay, was carried out in a submerged fermentation medium (SmF).
A fixed volume (2 mL) of spore suspension in a 5-day-old MCD broth medium (containing 109 spores / mL for each fungal strain) was transferred to 250 Erlenmeyer flasks having 100 mL of MCD broth containing 1% (w/v) L-asparagine and incubated for one week at 28 °C and 120 rpm on a rotary shaker. The culture supernatant was separated using filter paper and used to determine the enzyme production.
The enzyme production was determined based on measuring ammonia released by Nesslerization as described by Hatamzadeh et al. [26]. A fixed volume (100 µL) of culture supernatant was mixed with 200 µL of asparagine substrate (0.04 M), 100 µL of (1 M, Tris HCl (pH 7)), and 100 µL of sterile distilled water (SDW) and incubated at 30 ± 2 °C for one hour. The enzymatic reaction was stopped by adding 100 µL of trichloroacetic acid (1.5 M, TCA), while the blank was prepared in the same procedure without asparagine substrate. To determine the enzyme activity in the mixture, 100 µL of the mixture was mixed with 300 µL of Nessler’s reagent and 750 µL of sterile distilled water. Pure L-asparaginase was used as a positive control. The absorbance of the mixture was determined at A450 nm using a UV–visible spectrophotometer after the incubation period of 20 min at 28 ± 2 °C. The enzyme concentrations were expressed as a unit of L-asparaginase required to release 1 µmol of ammonia per minute at 30 ± 2 °C [26].
where 0.6 is the initial enzyme mixture (mL), 0.1 is the enzyme mixture in the final reaction (mL), 60 is the reaction time (min), and 0.2 is the enzyme (mL).
The standard curve used for the L-asparaginase activity was prepared by dissolving 47.15 mg (NH₄) ₂SO₄ in 0.25 L DW. Different dilutions were prepared from the stock solution, and a fixed volume (25 µL) from each solution was mixed with 3 mL of DW and 1 mL of Nessler reagent. The absorbance was determined at A450 nm with a UV–Vis DR6000 spectrophotometer (Hach, Ames, IA, USA).
2.4. Optimizing L-asparaginase Production by A. arenarioides EAN603 Using a Box–Behnken Design
The Box–Behnken design (BBD) was used to determine the interactions between and among all the variables and their combined effect on asparaginase production by A. arenarioides EAN603, which was selected as the most potent fungal strains that produce large amounts of the enzyme during the screening process. Four independent factors were involved in the optimization process, including temperature () (28–34 °C), pH () (5.5 to 8.5), incubation time () (5 to 7), and substrate concentration, and soybean () (2.5 to 10) with three ranges for each factor (low (−1), medium (0), and high (1) levels). For each factor, two experimental runs were used as a control conducted at a value less than and greater than the range. The production process was carried out at each run suggested by the BBD in 50 mL Erlenmeyer flasks (SmF process) with 2.5 g of organic soybean powder, and 15 mL of phosphate buffer (0.01 M) was added to ensure the stability of the pH medium, while the pH was adjusted to 8.5. The sterilized production medium was inoculated with 2 mL of spore suspension inoculum, while the production process was carried out at 28 °C for 5 days. After each experimental run, the fungal biomass was separated by a filtration process using Whatman No. 1, and the supernatant with the crude enzyme was mixed with 90 mL of sodium phosphate buffer (0.1 M) (pH 7) and agitated for 1 h at 25 ± 2 °C in a rotary shaker (150 rpm). A total of 2 mL of the mixture was transferred to the Eppendorf tube and centrifuged at 10,000× g for 10 min to obtain the crude enzyme and determine the enzyme concentrations as described in Section 2.3.
2.5. Radial Basis Function Neural Network with a Specific Genetic Algorithm (RBFNNGA)
The study methodology of the hybrid RBFNNGA model in L-asparaginase data was carried out as presented in Figure 1. The training data set of L-asparaginase y included temperature (), pH (), incubation time (), and substrate concentration (), and divided the data to 60% training data set and 40% testing data set in the RBFNNGA. This is the robust model for predicting output value (dependent variable) given a set of input variables (also known as independent variables). The center of the hidden neuron in the RBFNNGA was calculated using Equation (3):
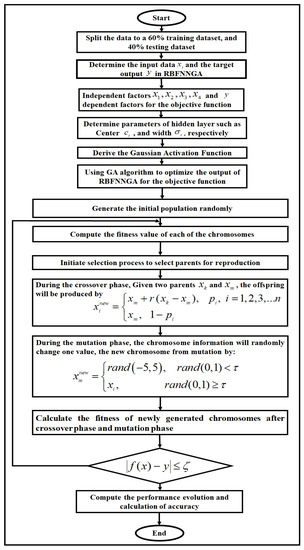
Figure 1.
The RBFNN-GA flowchart.
The width of each hidden neuron was calculated using Equation (4):
The activation function of RBFNN values was calculated using Equation (5):
The initial output was obtained using Equation (6):
The GA algorithm was used to optimize the output of the RBFNN.
The population of chromosomes was initialized as follows:
The value of the objective function of each chromosome produced in the step of initialization was evaluated according to Equation (8):
The fitness value of each of the chromosomes was evaluated using the following Equation:
The fittest chromosomes that have a higher probability of surviving to the next generation were selected according to Equation (10):
During the crossover phase, the information about the parent’s chromosomes was exchanged to produce new chromosomes. In this step, the number of cross-populations will be determined according to the crossover rate.
Mutation used the parameter of mutation rate to determine the number of chromosomes that have the mutation in the population. During the mutation phase, the chromosome information will randomly change one value from the chromosome value, which leads to improving the solution (output) of the RBFNNGA.
All performance evolution and calculation of the best error and result were obtained. The following complete flowchart in Figure 1 shows the methodology of the RBFNNGA in this study.
In L-asparaginase data, the network is required to find the best output by solving the linear equation that consists of L-asparaginase data. In this case, the RBFNNGA is used to improve the solution of the output. The solution (best output) in the RBFNNGA generated by the GA is called a chromosome. These chromosomes in the GA will be undergoing mutation and crossover until the chromosome’s fitness is optimal (an error is diminished). Throughout this phase of crossover, the information of the parent’s chromosome will be exchanged to produce new chromosomes with higher fitness and lower error. During the mutation phase, the information of the chromosome will randomly change one value of the output to improve the solution (best output) of the RBFNNGA. The GA after that will be undergoing 10,000 iterations (i.e., generations) to enhance chromosomes until the SRBFNN output is less or equivalent to the target output.
The experiments were performed with Microsoft Windows 10 Professional 64-bit, with a 500 GB hard drive specification, 4096 MB of RAM, and a 3.40 GHz processor. The simulations are performed on the Microsoft Visual Dev C++ Express program. The effectiveness of the training model was estimated using various time counts. In this study, seven different metrics, RMSE, MAPE, SEP, MAE, AIC, R, and accuracy, were used to evaluate the performance of our model for training and testing L-asparaginase data. The model’s capacity to test the L-asparaginase data set depends on the accuracy and the correlation coefficient (R).
The RBFNNGA was used to optimize L-asparaginase production.
2.6. Purification and Characterization
L-asparaginase was purified from the fungal supernatant by mixing with (NH₄)₂SO₄. A new production medium (5 L w/v) was inoculated with the A. arenarioides EAN603 spore suspension and incubated at the best operating parameters recorded during the optimization study (Section 2.4). The fungal biomass was removed from the culture medium by a filtration process, the culture supernatant was centrifuged at 10,000 rpm for 10 min at 4 °C, and the enzyme activity was detected according to the method described in Section 2.3. The supernatant obtained after the centrifugation was divided into 5 fractions (100 mL per fraction). A fixed weight of (NH4)2SO4 20 (20), 40 (40), 60 (60), and 80 g (80%) was added separately. The mixture of supernatant and (NH4)2SO4 was placed on hot plate magnetic stirrers for 10 min for homogenizing at 100 rpm for dissolving (NH4)2SO4. The centrifugation process was performed again for each fraction, and the crude enzyme concentrations in the precipitation and supernatant for each fraction were detected according to the method described in Section 2.5 at 4 °C to avoid the denaturation of the enzyme. The precipitation with high enzyme concentrations was suspended in 50 mL of a Tris-HCl buffer (50 mM) overnight, dialyzed against tap water overnight, and then transferred to a container has sucrose to remove the water content [27,28]. The final precipitant was dissolved in a 50 mL sodium phosphate buffer (0.1 M) (pH 7) and utilized for studying the characteristics of the crude enzyme. The total protein concentration in the cell-free extract (supernatant) and crude enzyme in the sodium phosphate buffer was determined by the NanoDrop 2000 C spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at A280 nm consuming bovine serum albumin (BSA) 10 mg/mL as the standard. In summary, 2 μL of enzyme samples were pipetted directly onto the Nanodrop measurement pedestal [29].
The characteristics of the crude L-asparaginase enzyme as a response to pH and temperature were determined by using an asparagine substrate (0.04 M) as described in Section 2.3. pH values used were 3, 4, 5, 6, 7, 8, 9, and 10. The buffers used were glycine-NaOH (9.0–10), Tris-HCl (8.0–9.0), sodium phosphate (6.0–8.0), and citrate phosphate (pH 3.0–7.0), while the incubation period was conducted for 20 min at 30 °C. The temperature used to study the enzyme stability was 10, 20, 30, 40, 50, and 60 °C for 20 min at pH 7.
3. Results and Discussion
3.1. L-asparaginase Production by Fungal Strains
Aspergillus arenarioides EAN603 exhibited the highest color change into pink due to the L-asparaginase activity zone around the grown colony (37.2 mm), followed by A. iizukae EAN605 (33.5 mm) with a zone index of 2.1, while growth in P. lilacinum EAN601 (22 mm colony diameter) was compared to P. brasiliense EAN202 (19 mm) and A. arenarioides EAN603 (18 mm) (Table 1).

Table 1.
L-asparaginase activity diameter, colony diameter, and zone index of L-asparaginase produced from six fungal strains in the study.
These findings indicated that fungal growth is not correlated to enzyme production. The semi-quantitative method is a primary screening used to detect the presence or absence of the enzyme from the microorganism. However, the process should be followed by the production of the enzyme in the liquid medium, and the enzyme concentration by spectrophotometric methods with a specified substrate. The semiquantitative has several limitations including the low relationship between the cleared zone diameters and the enzyme activities and the difficulty to detect by the eye. Therefore, the rapid plate assay method was used to confirm the production and concentration of the enzyme in the broth medium. Among the five fungal strains used in the secondary screening process, A. arenarioides EAN603 produced the highest enzyme 168.2 IUmL−1 compared to the positive control (pure enzyme) 90.6 IUmL−1, followed by P. lilacinum EAN601, where 45.5 IUmL−1 of L-asparaginase (Table 2). Doriya and Kumar [12] revealed that four fungal strains produced L-asparaginase according to semiquantitative methods with phenol red and BTB; the maximum enzyme production was 33.59 U mL−1 and 1.57 of the zone index. In comparison, the fungal strains used in the present study showed more potential to produce compared to that reported by Doriya and Kumar [12]. However, Aspergillus carneus, Penicillium camembertii, and Cladosporium tenuissimum exhibited high enzyme production. C. tenuissimum in a glucose-containing medium produced 5558 U mL−1 [30]. The differences might be related to the culture medium and fungal strains which have different enzyme production.

Table 2.
The concentration of released ammonium in the final solution (mM) and crude enzyme (IUmL−1).
3.2. Optimal Conditions for Producing L-asparaginase by A. arenarioides EAN603
Four independent factors were involved in the optimization process including temperature () (28–34 °C), pH () (5.5 to 8.5), incubation time () (5 to 7 days), substrate concentration, and soybean () (2.5 to 10). Maximum production was observed at 28 °C, pH 8.5, after 7 days and with 10 g L−1 of soybean as a production substrate; the predicted and actual production was 134.19 vs. 142.64 IU mL−1 (Run 26) followed by Run 17 at 28 °C, pH 8.5, after 5 days and with 10 g/L of soybean as a production substrate; predicted and actual production was 131.17 vs. 124.54 IU mL−1. These findings indicated that the main factors contributing in enzyme production are the incubation period (Table S1).
In a view of a single effect of each independent factor, it was noted that the factors exhibited a linear and secondary significant role in the production process (p < 0.05), except for pH which occurred only a linear effect, and temperature which exhibited more influence as a secondary factor (Table S2).
The linear effects of temperature have a negative relation with enzyme production and increasing temperature contributes to the reduction in enzyme production, while increasing substrate concentration is associated with reducing enzyme production as a secondary factor. The interaction analysis revealed that the pH and incubation period had a significant negative interaction (p < 0.05), the increase in the incubation period might lead to a change in the production medium pH and then the enzyme production. The increasing of pH might be associated with high degradation of the substrate and increased enzyme reduction; both factors exhibited a synergistic effect on enzyme production (p < 0.05). Similar findings were noted between the incubation period and the production substrates where the fungi needed more time to degrade the substrate and produce the enzyme; this might be related to the production stage of L-asparaginase production as an inducible enzyme product.
The RSM of enzyme production as a function of independent factors is depicted in Figure 2. It was observed that pH had a greater influence on enzyme production than temperature (Figure 2A), while time and temperature had a similar trend in their effects on enzyme production, with a negative impact at a temperature between 30–32 °C (Figure 2B). The substrate concentration exhibited more influences on the enzyme production compared to temperature (Figure 2C), and a slight difference was recorded between the effect of pH and time (Figure 2D).
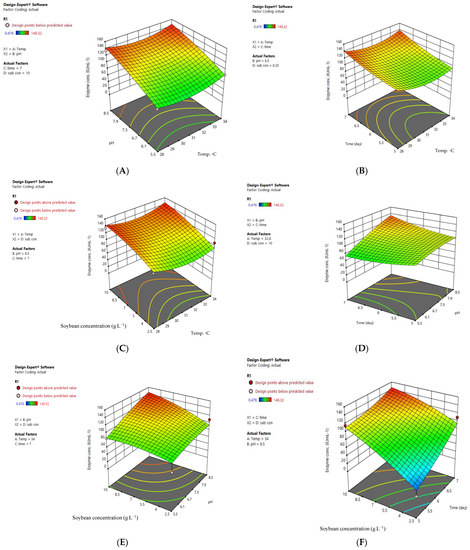
Figure 2.
Interaction between independent factors and their effects on L-asparaginase production by A. arenarioides EAN603. Interaction between pH and Temperature (A); Interaction between time and Temperature (B); Interaction between Soybean concentration and Temperature (C); Interaction between pH and time (D); Interaction between pH and Soybean concentration (E); Interaction between time and Soybean concentration (F).
These findings indicated that the main factor contributing to enzyme production was soybean concentration and pH; enzyme production of the enzyme increased as soybean concentration increased to 10 g L−1 and pH increased to pH 8.5 (Figure 2E). The time and soybean concentration exhibited similar trends; their contribution occurred more at high soybean concentrations (10 g L−1) and after a long incubation period (7 days) (Figure 2F). Shanthipriya et al. [31] revealed that pH 7.0 was optimal for L-asparaginase production from Scytalidium thermophilum, Malbranchea cinnamomea, and Thielavia terrestris (thermophilic caprophilous fungi), while the optimal temperature was between 45 and 50 °C. In our study, the optimum temperature was 28 °C, which is like the soil temperature. Based on the F value, soybean concentrations contribute to enzyme production by 34.39%, while time contributes by 9.23%, and both factor as a primary effect (Figure 3). In contrast, the temperature was effective as a second factor by 19.05%. In comparison with previous studies, Vimal, and Kumar [32] found that the main factor in the production of L-asparaginase from Penicillium lilacinum was pH first (24.55%), substrate 21.28, and temperature 15.28%. These differences belong to different fungal strains used in the production of the enzyme.
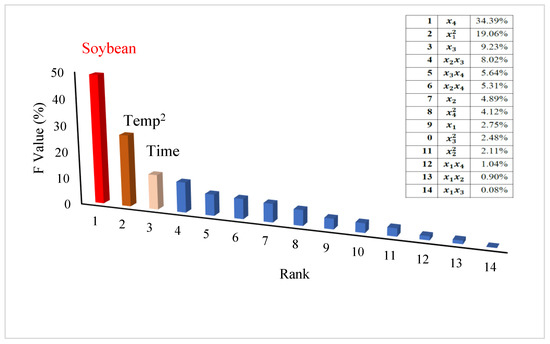
Figure 3.
A Pareto diagram showing the main factors and their interaction effects on L-asparaginase production (in SmF) in ascending order along with the percentage contribution of each parameter.
The quadratic equation for the enzyme production as a response to the independent factor is presented below.
The coefficient of the predicted model by BBD was R2 = 0.9079 (p < 0.05); however, the lack of fit was also significant (p < 0.05). These findings indicated that the effect of the independent factors on enzyme production is not fitted with the quadratic model. These results were confirmed during the optimization process, which revealed that the SE of the predicted model was 11.65 while the coefficient was 0.9799, at which 145.35 and 124.54 IU mL−1 of the actual and predicted enzyme production was obtained at 34 °C, pH 8.5 after 7 days, and with 10 g L−1 of soybean concentrations.
Several studies have used RSM for optimizing enzyme production from different microorganisms. However, the present study claimed that RSM is not the best prediction model for optimizing the production process due to the high SE. The machine learning model might be more accurate and flexible to achieve high enzyme production because these models depend on the training process of the data with high frequencies repeated to achieve the best prediction model.
3.3. Purification and Stability
The results of enzyme purification revealed that the best concentrations of (NH₄)₂SO₄ required to precipitate L-asparaginase were 60%, at which the enzyme concentrations were 138.7 IU mL−1 compared to 55.44 IU mL−1 in the supernatant (Table 3). The concentration of L-asparaginase in the cell-free extract was 249 IU mL−1, while there was 172 IU mL−1 in the partially purified crude enzyme (Table 4). The crude enzyme exhibited high stability at pH 8 and 30 °C (Figure S1).

Table 3.
Enzyme concentration in supernatant and precipitate after adding (NH₄)₂SO₄.

Table 4.
Enzyme activity during the purification of L-asparaginase from A. arenarioides EAN603 in SmF.
3.4. Prediction Models Using Machine Learning
The results of the hybrid model RBFNNGA are summarized in Figure 4A–E and Table 5. The model stated above was utilized to create a logic rule that examines the link between the candidate’s features and helps to properly predict the production of L-asparaginase over time. The model helps not only to properly predict the production of L-asparaginase more effectively but also to enhance the production of L-asparaginase. The best model, according to the experimental findings, is RBFNNGA, which can classify data according to logic mining using the lowest possible values of RMSE, MAE, MAPE, SEP, and AIC. Basically, Figure 4 showed the comparison of the actual value vs. predicted values. In this case, we can see that the forecast is a good fit. The model has the skill and a forecast that looks sensible according to the lowest possible values of RMSE, MAE, MAPE, SEP, and AIC. As shown in Figure 4, increasing the complexity of the model by increasing the number of input neurons led to an increase in time, RMSE, MAE, MAPE, SEP, and AIC. These findings indicate that the RBFNNGA model behaves well on the L-asparaginase data it has already seen. On the other hand, Figure 4 showed that it is clear that the increase in the complexity of the model led to improved predicted values in addition to the evaluations of RMSE, MAE, MAPE, SEP, and AIC evaluations; we can say that the RBFNNGA model is the optimal model to predict L-asparaginase data. Based on the findings in Table 5, the hybrid RBFNNGA model is the optimum model for establishing the logical rule and categorizing the relationship between the candidate’s attributes. This helps to achieve a greater level of accurate anticipating changes for L-asparaginase data. According to the results, the RBFNNGA is the best model in logic mining, as a long-term relationship between the variables provided was confirmed, demonstrating that all the independent factors were crucial in determining the behavior or movement of the L-asparaginase data. This result demonstrated that temperature (x1), pH (x2), incubation time (x3), and substrate concentration (x4) had a benefit and an effect on the L-asparaginase, with a correlation coefficient of R = 0.94, and can classify 91.67% of the test data samples with a better degree of accuracy, as shown in the Figures where the actual values are higher than the forecasted values for the L-asparaginase data.
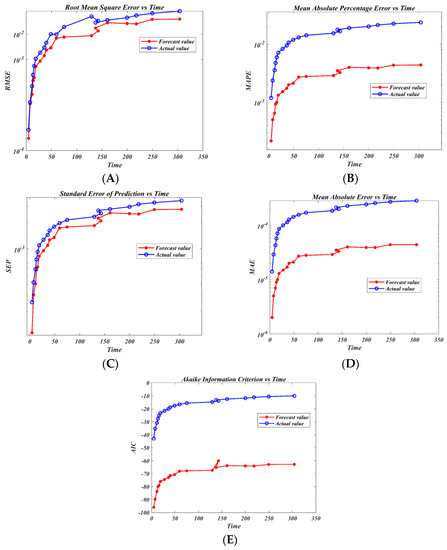
Figure 4.
Predicting L-asparaginase data using a hybrid RBFNNGA model. RMSE valuation (A); MAPE valuation (B); SEP assessment (C); MAE evaluation (D); AIC assessment (E).

Table 5.
Shows the best accuracy, correlation coefficient (R), and the most influencing variables. Best accuracy and correlation coefficient (R).
4. Conclusions
Enhancement of L-asparaginase production from Aspergillus arenarioides EAN603 on submerged fermentation using the RBFNN-GA and response surface methodology was successfully conducted. Independent factors used included temperature (x1), pH (x2), and incubation time (x3), and soybean concentration (x4) was used for optimization using BBD. The findings indicated that the BBD coefficient was R2 = 0.9079 (p < 0.05), but the independent factors were not fitted with the quadratic model since the SE of the predicted model was 11.65. The RBFNN-GA has a correlation coefficient of R = 0.94 and can classify 91.67% of test data samples with a better degree of accuracy; the actual values are higher than the forecast values for the L-asparaginase data. The primary factors driving enzyme production are soybean concentration and incubation time.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9030200/s1, Figure S1: Enzyme stability as a response for pH (A); and Temperature (B); Table S1: Comparison between the experimental and prediction data for producing L-asparaginase by A. arenarioides EAN603 in SmF process with soybean as a production substrate. Table S2. Analysis of the variance (ANOVA) of the quadratic model for producing L-asparaginase by A. arenarioides EAN603 in SmF process with soybean.
Author Contributions
Conceptualization, A.A.-G. and M.M.A.-s.; methodology, E.A.N., M.M.A.-s. and S.A.A.; software, A.A.-G. and S.A.A.; validation, R.M.S.R.M., M.S., N.M.Z. and K.G.T.; formal analysis, A.A.-G.; investigation, E.A.N. and M.M.A.-s.; resources, M.S. and H.A.E.E.; data curation, A.A.-G.; writing—original draft preparation, A.A.-G. and S.A.A.; writing—review and editing, E.A.N., R.A., M.M.A.-s. and S.A.A.; visualization, AAG.; supervision, R.M.S.R.M., N.M.Z., K.G.T. and H.A.E.E.; project administration, A.A.-G.; funding acquisition, H.A.E.E. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support from Universiti Teknologi Malaysia (UTM) through industrial grants (R.J130000.7609.4C359 and R.J130000.7609.4C465) in supporting this research. Communication of this research was made possible through monetary assistance from Universiti Tun Hussein Onn Malaysia and the UTHM Publisher’s Office via Publication Fund E15216.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qeshmi, F.I.; Homaei, A.; Fernandes, P.; Javadpour, S. Marine microbial L-asparaginase: Biochemistry, molecular approaches and applications in tumor therapy and in food industry. Microbiol. Res. 2018, 208, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Cachumba, J.J.M.; Antunes, F.A.F.; Peres, G.F.D.; Brumano, L.P.; Santos, J.C.D.; Da Silva, S.S. Current applications and different approaches for microbial L-asparaginase production. Braz. J. Microbiol. 2016, 47, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Baskar, G.; Renganathan, S. Production of L-asparaginase from natural substrates by Aspergillus terreus MTCC 1782: Effect of substrate, supplementary nitrogen source and L-asparagine. Int. J. Chem. React. Eng. 2009, 7. [Google Scholar] [CrossRef]
- El-Naggar, N.; Moawad, H.; Abdelwahed, N.A. Optimization of fermentation conditions for enhancing extracellular production of L-asparaginase, an anti-leukemic agent, by newly isolated Streptomyces brollosae NEAE-115 using solid state fermentation. Ann. Microbiol. 2017, 67, 1–15. [Google Scholar] [CrossRef]
- Meghavarnam, A.K.; Janakiraman, S. Solid state fermentation: An effective fermentation strategy for the production of L-asparaginase by Fusarium culmorum (ASP-87). Biocatal. Agric. Biotechnol. 2017, 11, 124–130. [Google Scholar] [CrossRef]
- Vala, A.K.; Sachaniya, B.; Dudhagara, D.; Panseriya, H.Z.; Gosai, H.; Rawal, R.; Dave, B.P. Characterization of L-asparaginase from marine-derived Aspergillus niger AKV-MKBU, its antiproliferative activity and bench scale production using industrial waste. Int. J. Biol. Macromol. 2018, 108, 41–46. [Google Scholar] [CrossRef]
- Fan, B.; Dewapriya, P.; Li, F.; Grauso, L.; Blümel, M.; Mangoni, A.; Tasdemir, D. Pyrenosetin D, a new pentacyclic decalinoyltetramic acid derivative from the algicolous fungus pyrenochaetopsis sp. FVE-087. Mar. Drugs 2020, 18, 281. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.A.; Awad, M.F.; El-Shenawy, F.S.; El-Bondkly, A.M.A. Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J. Biol. Sci. 2021, 28, 2540–2548. [Google Scholar] [CrossRef]
- Krishnapura, P.R.; Belur, P.D. Partial purification and characterization of L-asparaginase from an endophytic Talaromyces pinophilus isolated from the rhizomes of Curcuma amada. J. Mol. Catal. B Enzym. 2016, 124, 83–91. [Google Scholar] [CrossRef]
- Baskar, G.; Sree, N.S. Synthesis, characterization and anticancer activity of β-cyclodextrin-Asparaginase nanobiocomposite on prostate and lymphoma cancer cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101417. [Google Scholar] [CrossRef]
- Paul, V.; Tiwary, B.N. An investigation on the acrylamide mitigation potential of l-asparaginase from Aspergillus terreus BV-C strain. Biocatal. Agric. Biotechnol. 2020, 27, 101677. [Google Scholar] [CrossRef]
- Doriya, K.; Kumar, D.S. Solid state fermentation of mixed substrate for l-asparaginase production using tray and in-house designed rotary bioreactor. Biochem. Eng. J. 2018, 138, 188–196. [Google Scholar] [CrossRef]
- Moubasher, H.A.; Balbool, B.A.; Helmy, Y.A.; Alsuhaibani, A.M.; Atta, A.A.; Sheir, D.H.; Abdel-Azeem, A.M. Insights into Asparaginase from Endophytic Fungus Lasiodiplodia theobromae: Purification, Characterization and Antileukemic Activity. Int. J. Environ. Res. Public Health 2022, 19, 680. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.C.; Aguilar, J.G.D.S.; Orrillo Lindo, S.M.D.R.; de Castro, R.J.S.; Sato, H.H. L-asparaginase from Aspergillus oryzae spp.: Effects of production process and biochemical parameters. Prep. Biochem. Biotechnol. 2022, 52, 253–263. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, T.; Mohamed, B.R.; Almoheer, R.; Al-Shaorgani, N. First Report of Six New Environmental Fungal Strains in Malaysia on a New Culture Medium (EVA Medium): Morpho-logical, Molecular, and Microstructure Characteristics. J. Sustain. 2022. revised. [Google Scholar]
- Al-Gheethi, A.; Noman, E.; Mohamed, R.M.S.R.; Talip, B.; Vo, D.V.N.; Algaifi, H.A. Cephalexin removal by a novel Cu–Zn bionanocomposite biosynthesized in secondary metabolic products of Aspergillus arenarioides EAN603 with pumpkin peels medium: Optimization, kinetic and artificial neural network models. J. Hazard. Mater. 2021, 419, 126500. [Google Scholar] [CrossRef]
- Sharma, D.; Mishra, A. L-asparaginase production in solid-state fermentation using Aspergillus niger: Process modeling by artificial neural network approach. Prep. Biochem. Biotechnol. 2022, 52, 549–560. [Google Scholar] [CrossRef]
- Alzaeemi, S.A.; Sathasivam, S. Artificial immune system in doing 2-satisfiability based reverse analysis method via a radial basis function neural network. Processes 2020, 8, 1295. [Google Scholar] [CrossRef]
- Sathasivam, S.; Alzaeemi, S.A.; Ismail, M.T.; VaniPachala, V. Palm oil price forecasting in Malaysia using 2 satisfiability based reverse analysis method via radial basis function neural network. Solid State Technol. 2020, 63, 334–339. [Google Scholar]
- Li, T.; Liu, X.; Lin, Z.; Morrison, R. Ensemble offshore Wind Turbine Power Curve modelling–An integration of Isolation Forest, fast Radial Basis Function Neural Network, and metaheuristic algorithm. Energy 2022, 239, 122340. [Google Scholar] [CrossRef]
- Katoch, S.; Chauhan, S.S.; Kumar, V. A review on genetic algorithm: Past, present, and future. Multimed. Tools Appl. 2021, 80, 8091–8126. [Google Scholar] [CrossRef]
- Zhang, Q.; Abdullah, A.R.; Chong, C.W.; Ali, M.H. A study on regional gdp forecasting analysis based on radial basis function neural network with genetic algorithm (RBFNN-GA) for shandong economy. Comput. Intell. Neurosci. 2022, 2022, 8235308. [Google Scholar] [CrossRef]
- Gulati, R.; Saxena, R.K.; Gupta, R. A rapid plate assay for screening l-asparaginase producing micro-organisms. Lett. Appl. Microbiol. 1997, 24, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.V.; Saran, S.; Saxena, R.K.; Srivastava, A.K. A rapid, efficient and sensitive plate assay for detection and screening of l-asparaginase-producing microorganisms. FEMS Microbiol. Lett. 2013, 341, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Hatamzadeh, S.; Rahnama, K.; Nasrollahnejad, S.; Fotouhifar, K.B.; Hemmati, K.; White, J.F.; Taliei, F. Isolation and identification of L-asparaginase-producing endophytic fungi from the Asteraceae family plant species of Iran. PeerJ 2020, 8, e8309. [Google Scholar] [CrossRef] [PubMed]
- Noman, E.; Al-Gheethi, A.A.; Talip, B.A.; Mohamed, R.; Kassim, A.H. Oxidative enzymes from newly local strain Aspergillus iizukae EAN605 using pumpkin peels as a production substrate: Optimized production, characterization, application and techno-economic analysis. J. Hazard. Mater. 2020, 386, 121954. [Google Scholar] [CrossRef]
- Burgess, R.R. Protein precipitation techniques. Methods Enzymol. 2009, 463, 331–342. [Google Scholar]
- Zhang, X.N.; Cheng, Q.; Chen, J.; Lam, A.T.; Lu, Y.; Dai, Z.; Pei, H.; Evdokimov, N.M.; Louie, S.G.; Zhang, Y. A ribose-functionalized NAD+ with unexpected high activity and selectivity for protein poly-ADP-ribosylation. Nat. Commun. 2019, 10, 4196. [Google Scholar] [CrossRef]
- Hamed, M.; Osman, A.A.; Ateş, M. Semi-quantitative detection for L-asparaginase producing fungi and the impact of carbon and nitrogen sources on enzyme activity. Biorxiv 2021. [Google Scholar] [CrossRef]
- Shanthipriya, A.; Rao, V.K.; Girisham, S.; Reddy, S.M. Factors influencing L-asparaginase production by three thermophilic caprophilous fungi. Res. Pharm. Sci. 2015, 5, 1242–1248. [Google Scholar]
- Vimal, A.; Kumar, A. Optimized Production of Medically Significant Enzyme L-Asparaginase Under Submerged and Solid-State Fermentation from Agricultural Wastes. Curr. Microbiol. 2022, 79, 394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).