Repeated-Batch Ethanol Fermentation from Sweet Sorghum Stem Juice under a Very High Gravity Condition Using a Stirred Tank Bioreactor Coupled with a Column Bioreactor by Immobilized Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Ethanol Production Medium Preparation
2.2. Microorganism and Inoculum Preparation
2.3. Carrier Preparation and Characterization
2.4. Experiments
2.4.1. Effects of Rattan Loading for Use as Carriers in Batch Ethanol Fermentation
2.4.2. Repeated-Batch Ethanol Fermentation Using a CR
2.4.3. Repeated-Batch Ethanol Fermentation Using STR Coupled with CR
2.5. Analytical Methods
3. Results and Discussion
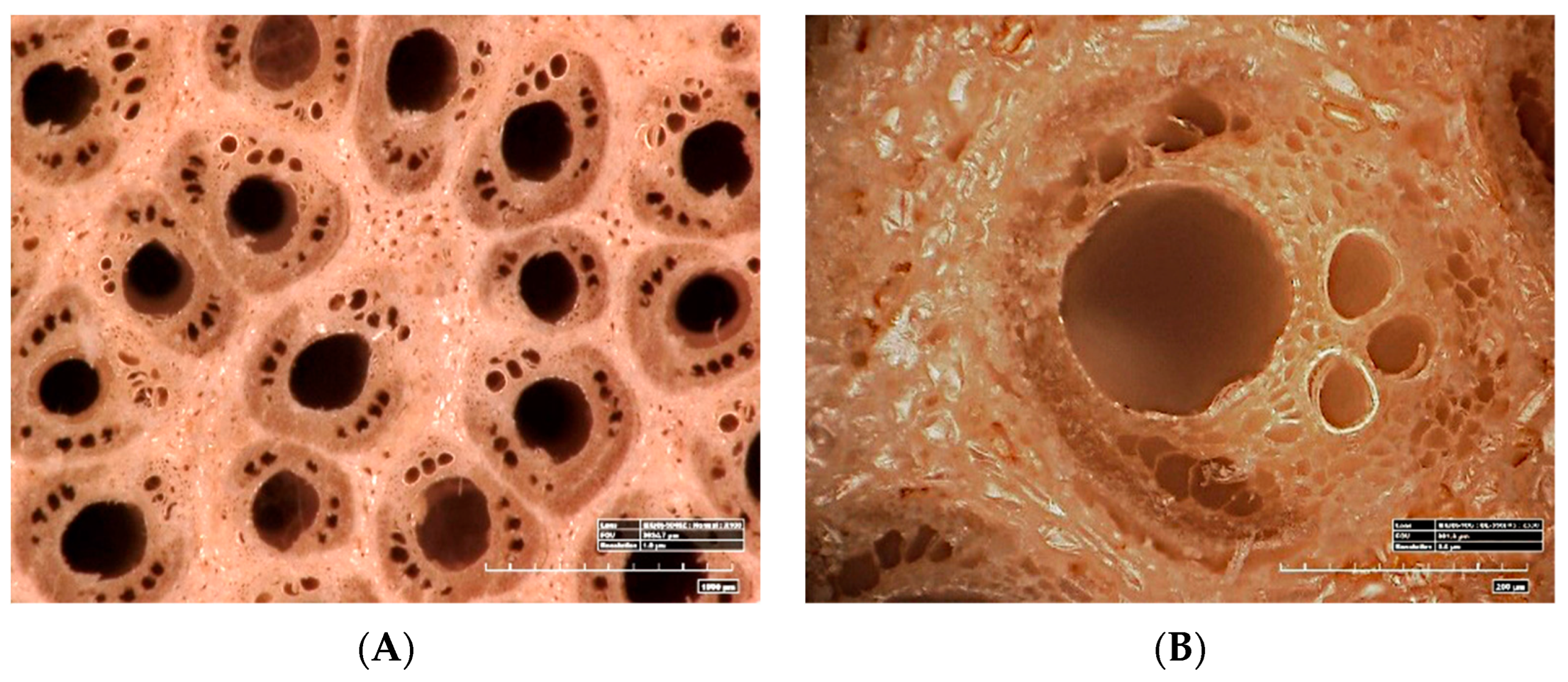
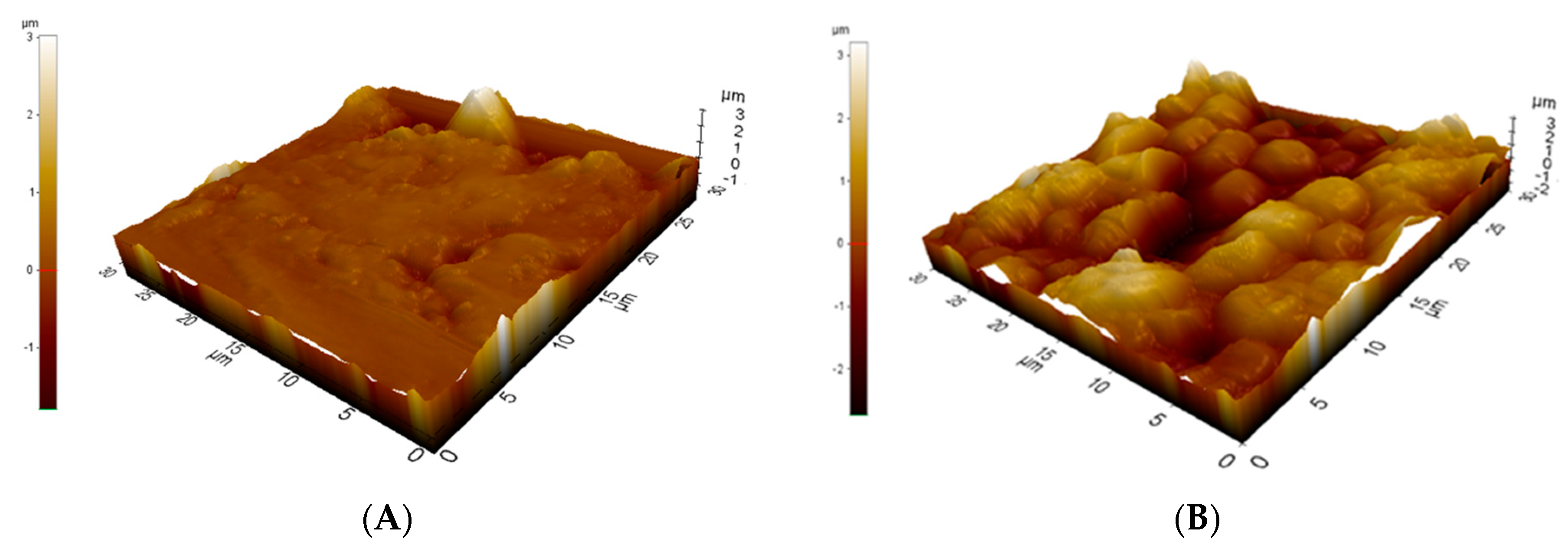
3.1. Appearance and Morphology of the Rattan
3.2. Batch Ethanol Fermentation by Immobilized Cells on Rattan Pieces at Various Carrier Loadings in a CR
3.3. Repeated-Batch Fermentation in a CR by Immobilized Cells
3.4. Repeated-Batch Ethanol Fermentation by Immobilized Cells Using an STR Coupled with a CR
3.4.1. System I (STR Coupled with CR-I)
3.4.2. System II (STR Coupled with CR-F and CR-I)
3.5. Characteristics of Immobilized Cells on Rattan in Repeated-Batch Ethanol Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaya, W.; Jesdapipat, S.; Tripetchkul, S.; Santitaweeroek, Y.; Gheewala, S.H. Challenges and pitfalls in implementing Thailand’s ethanol plan: Integrated policy coherence and gap analysis. Energy Policy 2019, 132, 1050–1063. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Shi, Y.C. Effects of nitrogen source on ethanol production in very high gravity fermentation of corn starch. J. Taiwan Inst. Chem. Eng. 2017, 70, 229–235. [Google Scholar] [CrossRef]
- Thanapornsin, T.; Laopaiboon, P.; Laopaiboon, L. An alternative approach to improve the butanol production efficiency from sweet sorghum stem juice using immobilized cell combined with an in situ gas stripping system. Fermentation 2022, 8, 464. [Google Scholar] [CrossRef]
- Cui, Y.; Liang, Y. Sweet sorghum syrup as a renewable material for microbial lipid production. Biochem. Eng. J. 2015, 93, 229–234. [Google Scholar] [CrossRef]
- Sriputorn, B.; Laopaiboon, P.; Phukoetphim, N.; Polsokchuak, N.; Butkun, K.; Laopaiboon, L. Enhancement of ethanol production efficiency in repeated-batch fermentation from sweet sorghum stem juice: Effect of initial sugar, nitrogen and aeration. Electron. J. Biotechnol. 2020, 46, 55–64. [Google Scholar] [CrossRef]
- Phukoetphim, N.; Chan-U-Tit, P.; Laopaiboon, P.; Laopaiboon, L. Improvement of bioethanol production from sweet sorghum juice under very high gravity fermentation: Effect of nitrogen, osmoprotectant, and aeration. Energies 2019, 12, 3620. [Google Scholar] [CrossRef]
- Rivera, E.C.; Yamakawa, C.K.; Saad, M.B.W.; Atala, D.I.P.; Ambrosio, W.B.; Bonomi, A.; Junior, J.; Rossell, C.E.V. Effect of temperature on sugarcane ethanol fermentation: Kinetic modeling and validation under very-high-gravity fermentation conditions. Biochem. Eng. J. 2017, 119, 42–51. [Google Scholar] [CrossRef]
- Kawa-rygielska, J.; Pietrzak, W. Ethanol fermentation of very high gravity (VHG) maize mashes by Saccharomyces cerevisiae with spent brewer’s yeast supplementation. Biomass Bioenergy 2013, 60, 50–57. [Google Scholar] [CrossRef]
- Kłosowski, G.; Mikulski, D. Complementarity of the raw material composition of Very High Gravity (VHG) mashes as a method to improve efficiency of the alcoholic fermentation process. Process Biochem. 2018, 74, 1–9. [Google Scholar] [CrossRef]
- Appiah-Nkansah, N.B.; Li, J.; Rooney, W.; Wang, D. A review of sweet sorghum as a viable renewable bioenergy crop and its technoeconomic analysis. Renew. Energy 2019, 143, 1121–1132. [Google Scholar] [CrossRef]
- Dashti, M.G.; Abdeshahian, P.; Yusoff, W.M.W.; Kalil, M.S.; Hamid, A.A. Repeated batch fermentation biotechnology for the biosynthesis of lipid and gamma-linolenic acid by Cunninghamella bainieri 2A1. BioMed Res. Int. 2014, 2014, 831783. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Shen, Y.; Zhang, X.; Zan, Z.; Xia, M.; Luo, J.; Wang, M. Efficient repeated batch production of androstenedione using untreated cane molasses by Mycobacterium neoaurum driven by ATP futile cycle. Bioresour. Technol. 2020, 309, 123307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, N.; Zou, Y.; Ying, H.; Chen, Y. Feasibility of ethanol production from expired rice by surface immobilization technology in a new type of packed bed pilot reactor. Renew. Energy 2020, 149, 321–328. [Google Scholar] [CrossRef]
- Armani, M.; Morozova, K.; Scampicchio, M. Immobilization of Saccharomyces cerevisiae on nylon-6 nanofibrous membranes for grape juice fermentation. LWT 2019, 110, 360–364. [Google Scholar] [CrossRef]
- Rouf, A.; Kanojia, V.; Naik, H.R. Cell immobilization: An overview on techniques and its applications in food industry. Int. J. Chem. Stud. 2017, 5, 1817–1824. [Google Scholar]
- Ejaz, U.; Ahmed, A.; Sohail, M. Statistical optimization of immobilization of yeast cells on corncob for pectinase production. Biocatal. Agric. Biotechnol. 2018, 14, 450–456. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Tan, T. An novel immobilization method of Saccharomyces cerevisiae to sorghum bagasse for ethanol production. J. Biotechnol. 2007, 129, 415–420. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, Y.P.; Zhang, L.; Zhu, M.J.; Liang, S.Z.; Huang, Y.N. Study of sugarcane pieces as yeast supports for ethanol production from sugarcane juice and molasses. J. Ind. Microbiol. Biotechnol. 2008, 35, 1605–1613. [Google Scholar] [CrossRef]
- Ariyajaroenwong, P.; Laopaiboon, P.; Laopaiboon, L. Capability of sweet sorghum stalks as supporting materials for yeast immobilization to produce ethanol under various fermentation processes. J. Taiwan Inst. Chem. Eng. 2015, 49, 79–84. [Google Scholar] [CrossRef]
- Ta, L.N.N.; Nguyen, T.H.C.; Le, V.V.M. Immobilization of Saccharomyces Cerevisae cells on water hyacinth stem pieces and application to repeated batch fermentation for ethanol production. Songklanakarin J. Sci. Technol. 2016, 38, 333–341. [Google Scholar]
- Berbegal, C.; Polo, L.; García-Esparza, M.J.; Lizama, V.; Ferrer, S.; Pardo, I. Immobilisation of yeasts on oak chips or cellulose powder for use in bottle-fermented sparkling wine. Food Microbiol. 2019, 78, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Adelabu, B.A.; Kareem, S.O.; Oluwafemi, F.; Abideen Adeogun, I. Bioconversion of corn straw to ethanol by cellulolytic yeasts immobilized in Mucuna urens matrix. J. King Saud Univ. Sci. 2019, 31, 136–141. [Google Scholar] [CrossRef]
- Khongsay, N.; Laopaiboon, L.; Laopaiboon, P. Improvement of continuous ethanol fermentation from sweet sorghum juice by Saccharomyces cerevisiae using stirred tank bioreactor coupling with plug flow bioreactor. Chiang Mai J. Sci. 2015, 42, 282–293. [Google Scholar]
- Antunes, F.A.F.; Chandel, A.K.; Brumano, L.P.; Hilares, R.T.; Peres, G.F.D.; Ayabe, L.E.S.; Sorato, V.S.; Santos, J.R.; Santos, J.C.; Da Silva, S.S. A novel process intensification strategy for second-generation ethanol production from sugarcane bagasse in fluidized bed reactor. Renew. Energy 2018, 124, 189–196. [Google Scholar] [CrossRef]
- Bai, F.W.; Chen, L.J.; Zhang, Z.; Anderson, W.A.; Moo-Young, M. Continuous ethanol production and evaluation of yeast cell lysis and viability loss under very high gravity medium conditions. J. Biotechnol. 2004, 110, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Terán-Hilares, R.; Reséndiz, A.L.; Martínez, R.T.; Silva, S.S.; Santos, J.C. Successive pretreatment and enzymatic saccharification of sugarcane bagasse in a packed bed flow-through column reactor aiming to support biorefineries. Bioresour. Technol. 2016, 203, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Devarapalli, M.; Lewis, R.S.; Atiyeh, H.K. Continuous ethanol production from synthesis gas by Clostridium ragsdalei in a trickle-bed reactor. Fermentation 2017, 3, 23. [Google Scholar] [CrossRef]
- Sriputorn, B.; Laopaiboon, P.; Phukoetphim, N.; Uppatcha, N.; Phuphalai, W.; Laopaiboon, L. Very high gravity ethanol fermentation from sweet sorghum stem juice using a stirred tank bioreactor coupled with a column bioreactor. J. Biotechnol. 2021, 332, 1–10. [Google Scholar] [CrossRef]
- Zoecklein, B.; Fugelsang, K.; Gump, B.; Nury, F. Wine Analysis and Production, 1st ed.; Aspen Publishers Inc.: New York, NY, USA, 1995; p. 639. [Google Scholar] [CrossRef]
- Cherng, J.H.; Chou, S.C.; Chen, C.L.; Wang, Y.W.; Chang, S.J.; Fan, G.Y.; Leung, F.S.; Meng, E. Bacterial cellulose as a potential bio-scaffold for effective re-epithelialization therapy. Pharmaceutics 2021, 13, 1592. [Google Scholar] [CrossRef]
- Kawahara, Y.; Kitajima, S.; Shimaoka, C. Low spinnability of sericin dominates the forcibly spinning speed. J. Text. Eng. 2018, 64, 141–145. [Google Scholar] [CrossRef]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol-sulphuric acid method assisted by multivariate calibration. Chemom. Intell. Lab. Syst. 2005, 79, 84–90. [Google Scholar] [CrossRef]
- Suzana, C.S.M.; Claudia, M.M.; Larissa, M.C.G.F.; Sandra, T.S. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr. J. Biotechnol. 2013, 12, 4412–4418. [Google Scholar] [CrossRef]
- Genisheva, Z.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Evaluating the potential of wine-making residues and corn cobs as support materials for cell immobilization for ethanol production. Ind. Crops Prod. 2011, 34, 979–985. [Google Scholar] [CrossRef]
- Zhu, D.; Li, X.; Liao, X.; Shi, B. Immobilization of Saccharomyces cerevisiae using polyethyleneimine grafted collagen fibre as support and investigations of its fermentation performance. Biotechnol. Biotechnol. Equip. 2018, 32, 109–115. [Google Scholar] [CrossRef]
- María, A.L.; Mussatto, S.I.; Peñuela, M.; Teixeira, J.A. Physicochemical Characterization of the Yeast Cells and Lignocellulosic Waste Used in Cell Immobilization for Ethanol Production. In Intech; IntechOpen Limited: London, UK, 2017; pp. 229–247. [Google Scholar] [CrossRef]
- Karagoz, P.; Bill, R.M.; Ozkan, M. Lignocellulosic ethanol production: Evaluation of new approaches, cell immobilization and reactor configurations. Renew. Energy 2019, 143, 741–752. [Google Scholar] [CrossRef]
- Bos, R.; Van Der Mei, H.C.; Busscher, H.J. Physics-chemistry of initial microbial adhesive interactiions—Its mechanisms and methods for study. FEM Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef]
- Rattan Fiber|Physical, Chemical, and Mechanical Properties|. Available online: https://leartex.com/rattan-fiber/ (accessed on 12 April 2021).
- Mikoliunaite, L.; Makaraviciute, A.; Suchodolskis, A.; Ramanaviciene, A.; Oztekin, Y.; Stirke, A.; Jurkaite, G.; Ukanis, M.; Carac, G.; Cojocaru, P.; et al. Atomic force microscopy study of living baker’s yeast cells. Adv. Sci. Lett. 2011, 4, 368–376. [Google Scholar] [CrossRef]
- Kim, M.H.; Kino-oka, M.; Kawase, M.; Yagi, K.; Taya, M. Response of human epithelial cells to culture surfaces with varied roughnesses prepared by immobilizing dendrimers with/without d-glucose display. J. Biosci. Bioeng. 2007, 103, 192–199. [Google Scholar] [CrossRef]
- Sowatad, A.; Todhanakasem, T. Bioethanol production by repeated batch using immobilized yeast cells on sugarcane bagasse. Waste Biomass Valorizat. 2020, 11, 2009–2016. [Google Scholar] [CrossRef]
- Rattanapan, A.; Limtong, S.; Phisalaphong, M. Ethanol production by repeated batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons. Appl. Energy 2011, 88, 4400–4404. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Zhou, T.; Li, B.; Yao, S.; Li, A.; Wu, J.; Ying, H. Ethanol production by repeated batch and continuous fermentations by Saccharomyces cerevisiae immobilized in a fibrous bed bioreactor. J. Microbiol. Biotechnol. 2013, 23, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Yu, J.; Zhang, X.; Tan, T. Characteristics of an immobilized yeast cell system using very high gravity for the fermentation of ethanol. Appl. Biochem. Biotechnol. 2012, 168, 21–28. [Google Scholar] [CrossRef] [PubMed]


 ,
,  ,
,  = viable cells;
= viable cells;  ,
,  ,
,  = total sugar and
= total sugar and  ,
,  ,
,  = ethanol concentration).
= ethanol concentration).
 ,
,  ,
,  = viable cells;
= viable cells;  ,
,  ,
,  = total sugar and
= total sugar and  ,
,  ,
,  = ethanol concentration).
= ethanol concentration).
 ), viable cells ((A):
), viable cells ((A):  ), total sugar ((B):
), total sugar ((B):  ), and ethanol ((B):
), and ethanol ((B):  ) concentrations during repeated-batch ethanol fermentation by immobilized cells in a CR. The circled numbers indicate the cycle number of the fermentation.
) concentrations during repeated-batch ethanol fermentation by immobilized cells in a CR. The circled numbers indicate the cycle number of the fermentation.
 ), viable cells ((A):
), viable cells ((A):  ), total sugar ((B):
), total sugar ((B):  ), and ethanol ((B):
), and ethanol ((B):  ) concentrations during repeated-batch ethanol fermentation by immobilized cells in a CR. The circled numbers indicate the cycle number of the fermentation.
) concentrations during repeated-batch ethanol fermentation by immobilized cells in a CR. The circled numbers indicate the cycle number of the fermentation.
 ,
, ), non-viable cells ((A):
), non-viable cells ((A):  ,
, ), sugar ((B):
), sugar ((B):  ,
, ), and ethanol ((B):
), and ethanol ((B):  ,
, ) concentrations during repeated-batch fermentation by immobilized cells in System I: STR (black symbols) coupled with CR-I (white symbols). The circled numbers indicate the cycle number of the repeated-batch fermentation.
) concentrations during repeated-batch fermentation by immobilized cells in System I: STR (black symbols) coupled with CR-I (white symbols). The circled numbers indicate the cycle number of the repeated-batch fermentation.
 ,
, ), non-viable cells ((A):
), non-viable cells ((A):  ,
, ), sugar ((B):
), sugar ((B):  ,
, ), and ethanol ((B):
), and ethanol ((B):  ,
, ) concentrations during repeated-batch fermentation by immobilized cells in System I: STR (black symbols) coupled with CR-I (white symbols). The circled numbers indicate the cycle number of the repeated-batch fermentation.
) concentrations during repeated-batch fermentation by immobilized cells in System I: STR (black symbols) coupled with CR-I (white symbols). The circled numbers indicate the cycle number of the repeated-batch fermentation.
 ,
, ,
, ), non-viable cells ((A):
), non-viable cells ((A):  ,
, ,
, ), sugar ((B):
), sugar ((B):  ,
, ,
, ), and ethanol ((B):
), and ethanol ((B):  ,
, ,
, ) concentrations during repeated-batch fermentation by immobilized cells in System II: STR (black symbols) coupled with CR-F (gray symbols) and CR-I (white symbols), respectively. The circled numbers indicate the cycle number of the repeated-batch fermentation.
) concentrations during repeated-batch fermentation by immobilized cells in System II: STR (black symbols) coupled with CR-F (gray symbols) and CR-I (white symbols), respectively. The circled numbers indicate the cycle number of the repeated-batch fermentation.
 ,
, ,
,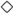 ), non-viable cells ((A):
), non-viable cells ((A):  ,
, ,
, ), sugar ((B):
), sugar ((B):  ,
, ,
, ), and ethanol ((B):
), and ethanol ((B):  ,
, ,
, ) concentrations during repeated-batch fermentation by immobilized cells in System II: STR (black symbols) coupled with CR-F (gray symbols) and CR-I (white symbols), respectively. The circled numbers indicate the cycle number of the repeated-batch fermentation.
) concentrations during repeated-batch fermentation by immobilized cells in System II: STR (black symbols) coupled with CR-F (gray symbols) and CR-I (white symbols), respectively. The circled numbers indicate the cycle number of the repeated-batch fermentation.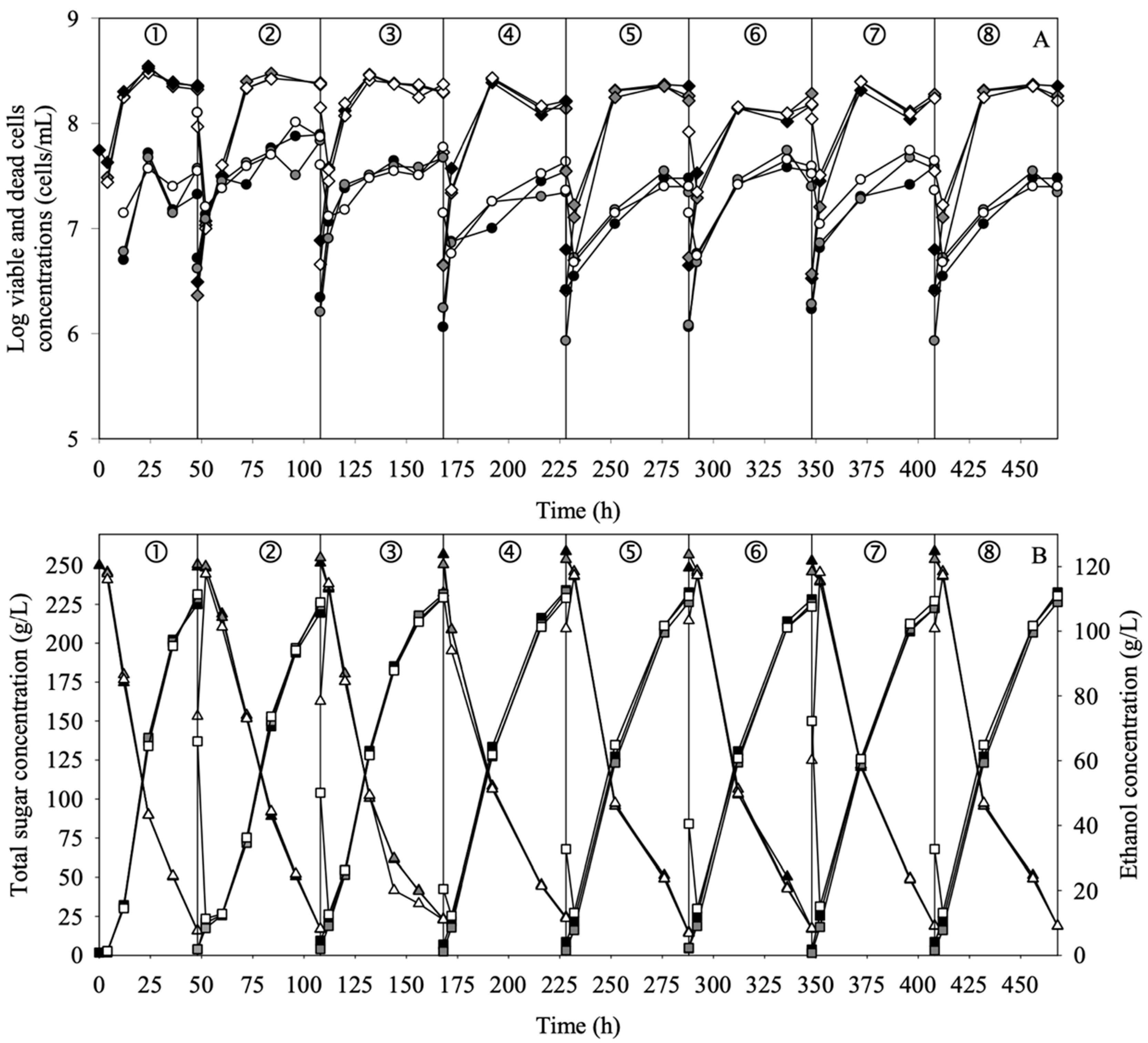

| Percentage of Rattan (%) | PE (g/L) | QP (g/L⋅h) | SC (%) | YP/S (g/g) | t (h) |
|---|---|---|---|---|---|
| 30 | 108.70 ± 1.91 a | 2.01 ± 0.04 a | 93.42 ± 0.06 a | 0.46 ± 0.01 b | 54 |
| 50 | 106.15 ± 2.42 a | 1.97 ± 0.05 a | 90.35 ± 0.02 c | 0.48 ± 0.01 a | 54 |
| 70 | 106.25 ± 0.95 a | 1.93 ± 0.02 a | 90.38 ± 0.07 b | 0.47 ± 0.01 ab | 54 |
| System | PE (g/L) | QP (g/L⋅h) | Total SC (%) | Total YP/S(g/g) | PE * (g) | t * (h) | QP * (g/h) |
|---|---|---|---|---|---|---|---|
| CR | 109.85 ± 3.17 b | 1.88 ± 0.14 a | 89.59 ± 1.64 b | 0.49 ± 0.01 a | - | - | - |
| System I (STR & CR-I) | 108.51 ± 1.98 b | 1.87 ± 0.17 a | 93.83 ± 1.37 a | 0.46 ± 0.01 b | 1563 | 468 | 3.34 |
| System II (STR & CR-F & CR-I) | 109.44 ± 1.50 b | 1.88 ± 0.16 a | 92.61 ± 1.34 ab | 0.47 ± 0.01 ab | 1576 | 468 | 3.37 |
| Free cells in STR & CR * [32] | 116.52 ± 2.21 a | 2.00 ± 0.19 a | 92.98 ± 0.78 a | 0.49 ± 0.02 a | 1532 | 471.5 | 3.25 |
| Substrate | Initial Sugar (g/L) | S. cerevisiae | Carrier | Cycles | PE (g/L) | QP (g/L⋅h) | SC (%) | YP/S (g/g) |
|---|---|---|---|---|---|---|---|---|
| Sugarcane molasses [42] | 231 | SC90 | Delignified sugarcane molasses | 5 | - | - | - | 0.34–0.42 |
| Blackstrap molasses [43] | 240 | M30 | Thin-shell silk cocoons | 5 | 77.6–88.3 | 1.62–1.84 | 86.11–92.89 | 0.44–0.48 |
| Glucose [44] | 200 | 1308 | Fibrous matrix | 22 | 91.57 | 11.75 | 100 | 0.46 |
| Glucose and sucrose [45] | 280 | 3013 | Sweet sorghum pith | 10 | 130.12 | 2.00 | 98.21 | 0.48 |
| Sweet sorghum stem juice (from this study, System II) | 250 | SSJ01 | Rattan | 8 | 109.44 | 1.88 | 92.61 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriputorn, B.; Laopaiboon, L.; Laopaiboon, P. Repeated-Batch Ethanol Fermentation from Sweet Sorghum Stem Juice under a Very High Gravity Condition Using a Stirred Tank Bioreactor Coupled with a Column Bioreactor by Immobilized Saccharomyces cerevisiae. Fermentation 2023, 9, 159. https://doi.org/10.3390/fermentation9020159
Sriputorn B, Laopaiboon L, Laopaiboon P. Repeated-Batch Ethanol Fermentation from Sweet Sorghum Stem Juice under a Very High Gravity Condition Using a Stirred Tank Bioreactor Coupled with a Column Bioreactor by Immobilized Saccharomyces cerevisiae. Fermentation. 2023; 9(2):159. https://doi.org/10.3390/fermentation9020159
Chicago/Turabian StyleSriputorn, Benjaporn, Lakkana Laopaiboon, and Pattana Laopaiboon. 2023. "Repeated-Batch Ethanol Fermentation from Sweet Sorghum Stem Juice under a Very High Gravity Condition Using a Stirred Tank Bioreactor Coupled with a Column Bioreactor by Immobilized Saccharomyces cerevisiae" Fermentation 9, no. 2: 159. https://doi.org/10.3390/fermentation9020159
APA StyleSriputorn, B., Laopaiboon, L., & Laopaiboon, P. (2023). Repeated-Batch Ethanol Fermentation from Sweet Sorghum Stem Juice under a Very High Gravity Condition Using a Stirred Tank Bioreactor Coupled with a Column Bioreactor by Immobilized Saccharomyces cerevisiae. Fermentation, 9(2), 159. https://doi.org/10.3390/fermentation9020159





