Effects of Low-Temperature and Low-Salt Fermentation on the Physicochemical Properties and Volatile Flavor Substances of Chinese Kohlrabi Using Gas Chromatography–Ion Mobility Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of HSCK and LSCK
2.2. GC-IMS Analysis
2.3. E-Nose Analysis
2.4. Physicochemical Analysis
2.5. Microbiological Analysis
2.6. Texture Analysis
2.7. Data Analysis
3. Results
3.1. Physicochemical Properties and Microbial Indicators Analysis
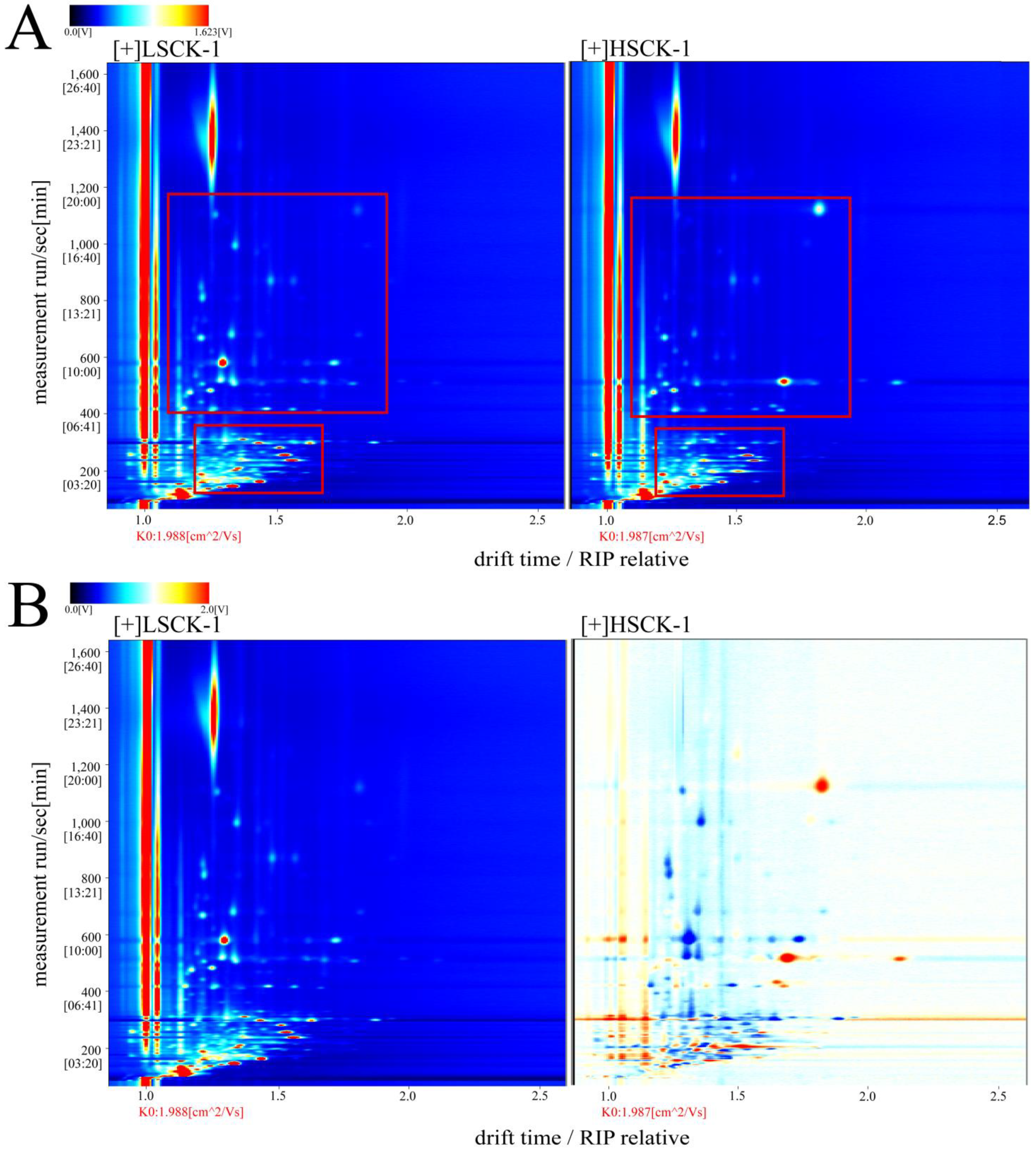
3.2. GC-IMS Analysis for Volatile Flavor Substances
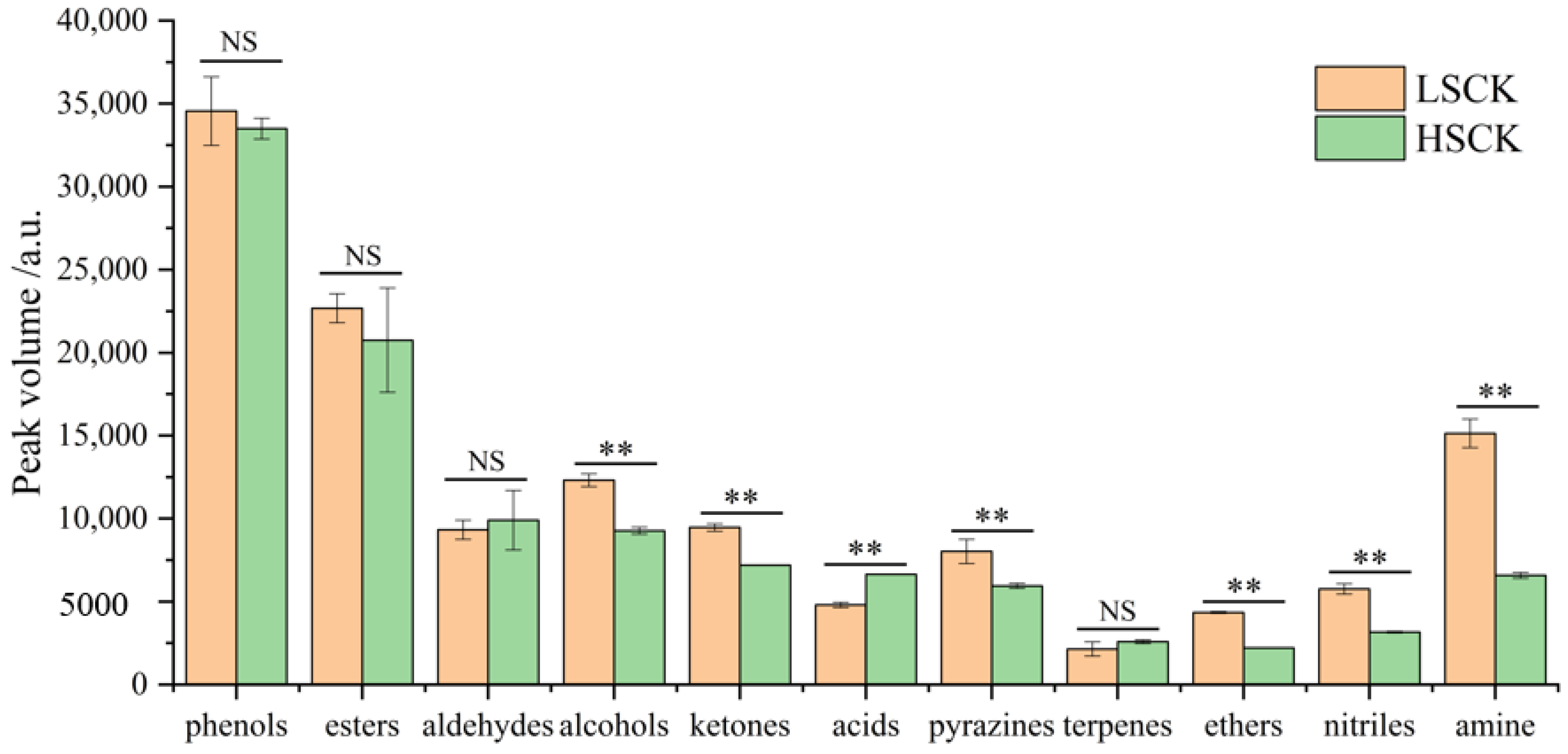
3.3. Qualitative and Quantitative Analysis of Volatile Flavor Substances
3.4. Fingerprints of Volatile Flavor Substances for LSCK and HSCK
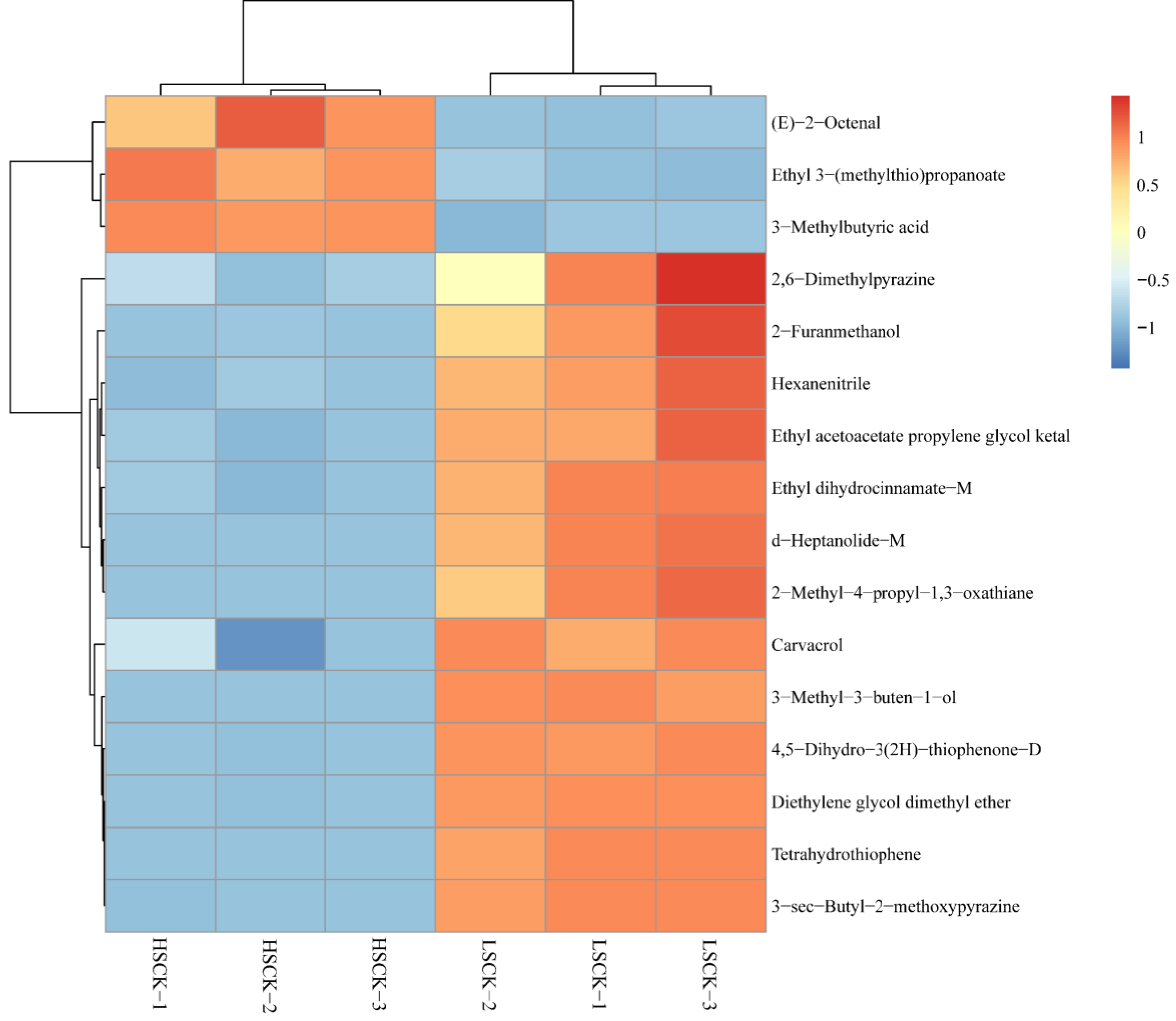
3.5. Differential Volatile Flavor Substances between LSCK and HSCK
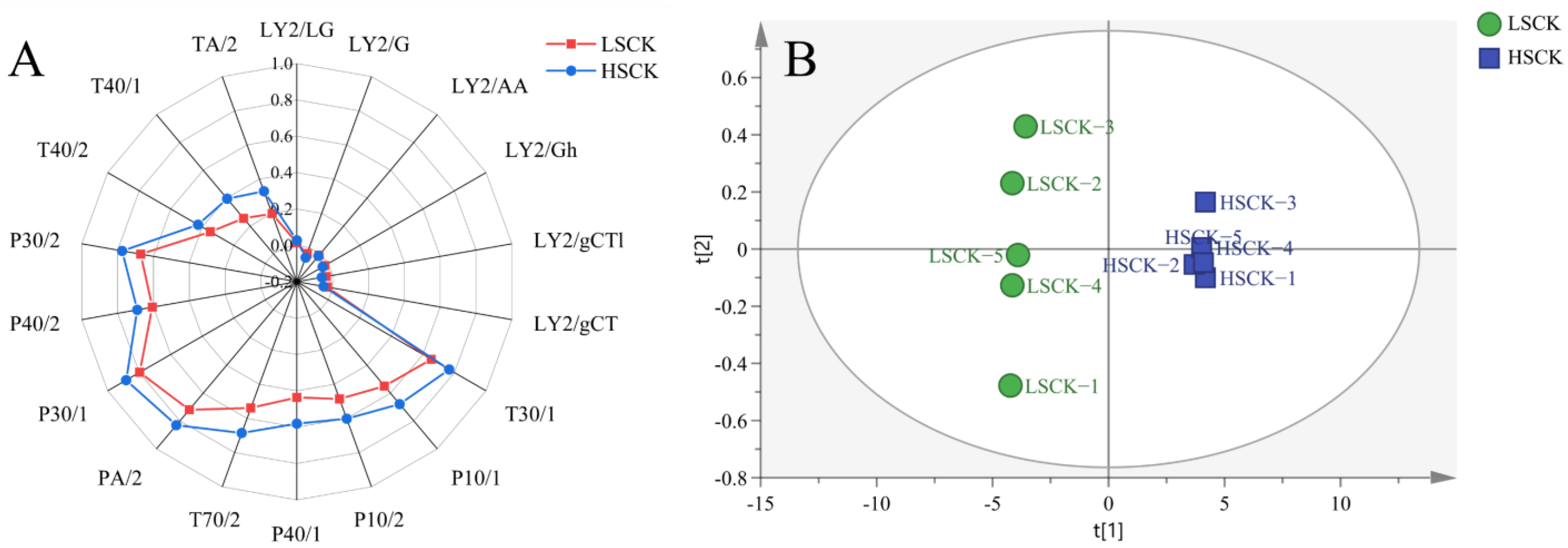
3.6. E-Nose Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paśko, P.; Galanty, A.; Żmudzki, P.; Gdula-Argasińska, J.; Zagrodzki, P. Influence of Different Light Conditions and Time of Sprouting on Harmful and Beneficial Aspects of Rutabaga Sprouts in Comparison to Their Roots and Seeds. J. Sci. Food Agric. 2018, 99, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Chen, H.; Xiang, L.; Zhang, Y.; Liu, D.; Zhao, Z. GC-TOF-MS-Based Non-Targeted Metabolomic Analysis of Differential Metabolites in Chinese Ultra-Long-Term Industrially Fermented Kohlrabi and Their Associated Metabolic Pathways. Metabolites 2022, 12, 991. [Google Scholar] [CrossRef] [PubMed]
- Koltun, S.J.; MacIntosh, A.J.; Goodrich-Schneider, R.M.; Klee, H.J.; Hutton, S.F.; Junoy, L.J.; Sarnoski, P.J. Effects of thermal processing on flavor and consumer perception using tomato juice produced from Florida grown fresh market cultivars. J. Food Process. Preserv. 2022, 46, e16164. [Google Scholar] [CrossRef]
- Israr, T.; Rakha, A.; Sohail, M.; Rashid, S.; Shehzad, A. Salt reduction in baked products: Strategies and constraints. Trends Food Sci. Technol. 2016, 51, 98–105. [Google Scholar] [CrossRef]
- Lee, M.-A.; Choi, Y.-J.; Lee, H.; Hwang, S.; Lee, H.J.; Park, S.J.; Chung, Y.B.; Yun, Y.-R.; Park, S.-H.; Min, S.; et al. Influence of Salinity on the Microbial Community Composition and Metabolite Profile in Kimchi. Fermentation 2021, 7, 308. [Google Scholar] [CrossRef]
- He, Z.; Chen, H.; Wang, X.; Lin, X.; Ji, C.; Li, S.; Liang, H. Effects of different temperatures on bacterial diversity and volatile flavor compounds during the fermentation of suancai, a traditional fermented vegetable food from northeastern China. LWT 2020, 118, 108773. [Google Scholar] [CrossRef]
- Liang, H.; Chen, H.; Zhang, W.; Yu, C.; Ji, C.; Lin, X. Investigation on microbial diversity of industrial Zhacai paocai during fermentation using high-throughput sequencing and their functional characterization. LWT 2018, 91, 460–466. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Drabińska, N.; Ogrodowczyk, A.M. Crossroad of Tradition and Innovation—The Application of Lactic Acid Fermentation to Increase the Nutritional and Health-Promoting Potential of Plant-Based Food Products—A Review. Pol. J. Food Nutr. Sci. 2021, 71, 107–134. [Google Scholar] [CrossRef]
- Wu, R.; Yu, M.; Liu, X.; Meng, L.; Wang, Q.; Xue, Y.; Wu, J.; Yue, X. Changes in flavour and microbial diversity during natural fermentation of suancai, a traditional food made in Northeast China. Int. J. Food Microbiol. 2015, 211, 23–31. [Google Scholar] [CrossRef]
- Shi, D.; Yin, C.; Fan, X.; Yao, F.; Qiao, Y.; Xue, S.; Lu, Q.; Feng, C.; Meng, J.; Gao, H. Effects of ultrasound and gamma irradiation on quality maintenance of fresh Lentinula edodes during cold storage. Food Chem. 2022, 373, 131478. [Google Scholar] [CrossRef]
- GB 4789.35-2016; Microbiological Examination of Food—Examination of lactic acid bacteria, National Medical Products Administration, National Health and Family Planning Commission of the People’s Republic of China. China Standards Press: Beijing, China, 2016.
- Hnin, K.K.; Zhang, M.; Devahastin, S.; Wang, B. Combined Infrared Freeze Drying and Infrared Drying of Rose-Flavored Yogurt Melts—Effect on Product Quality. Food Bioprocess Technol. 2020, 13, 1356–1367. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Fu, Y.; Shi, Y.-G.; Chen, F.-L.; Guan, H.-N.; Liu, L.-L.; Zhang, C.-Y.; Zhu, P.-Y.; Liu, Y.; et al. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2021, 346, 128880. [Google Scholar] [CrossRef]
- Liu, H.; Wen, J.; Xu, Y.; Wu, J.; Yu, Y.; Yang, J.; Liu, H.; Fu, M. Evaluation of dynamic changes and formation regularity in volatile flavor compounds in Citrus reticulata ‘chachi’ peel at different collection periods using gas chromatography-ion mobility spectrometry. LWT 2022, 171, 114126. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, N.; Liu, M.; Liu, Y.; Zan, X.; Tan, B.; Sun, Y.; Zhai, X. Effects of Low Temperature Plasma Treatment on Flavor Characteristics of Raw and Cooked Brown Rice. Food Sci. 2021, 42, 74–80. [Google Scholar] [CrossRef]
- Bel-Rhlid, R.; Berger, R.G.; Blank, I. Bio-mediated generation of food flavors—Towards sustainable flavor production inspired by nature. Trends Food Sci. Technol. 2018, 78, 134–143. [Google Scholar] [CrossRef]
- Aghili, N.S.; Rasekh, M.; Karami, H.; Azizi, V.; Gancarz, M. Detection of fraud in sesame oil with the help of artificial intelligence combined with chemometrics methods and chemical compounds characterization by gas chromatography–mass spectrometry. LWT 2022, 167, 113863. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Wu, Y.; Qu, F.; Liu, L.; Wang, B.; Wang, P.; Zhang, X. HS-SPME/GC-MS Reveals the Season Effects on Volatile Compounds of Green Tea in High-Latitude Region. Foods 2022, 11, 3016. [Google Scholar] [CrossRef]
- Bai, J.; Jiang, H.; Tao, G.; Zhang, X.; Li, Y.; Peng, Y. Analysis of the Aromatic Components of Potato Flour Incorporated Instant Congee Processed by Explosion Puffing by Combined Use of SPME-GC-MS and PCA. Food Sci. 2020, 41, 217–224. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Chang, M.; He, W.; Lu, X.; Fei, S.; Lu, G. Optimization of Electronic Nose Sensor Array for Tea Aroma Detecting Based on Correlation Coefficient and Cluster Analysis. Chemosensors 2021, 9, 266. [Google Scholar] [CrossRef]
- Banerjee, M.B.; Roy, R.B.; Tudu, B.; Bandyopadhyay, R.; Bhattacharyya, N. Black tea classification employing feature fusion of E-Nose and E-Tongue responses. J. Food Eng. 2019, 244, 55–63. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Duncan, A.; Bah, C.S.F.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Amin, H.F. Impact of fermentation conditions on the physicochemical properties, fatty acid and cholesterol contents in salted-fermented hoki roe. Food Chem. 2018, 264, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control. 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Mi, T.; Wang, D.; Yao, S.; Yang, H.; Che, Y.; Wu, C. Effects of salt concentration on the quality and microbial diversity of spontaneously fermented radish paocai. Food Res. Int. 2022, 160, 111622. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.; Ayele, A. Pectinase from Microorganisms and Its Industrial Applications. Sci. World J. 2022, 2022, 1881305. [Google Scholar] [CrossRef]
- Hu, J.; Bi, J.; Li, X.; Wu, X.; Jin, X.; Guo, C. Understanding the mechanism of moisture migration impact on the texture and color characters of dried apple cubes. J. Food Process. Preserv. 2021, 45, e16031. [Google Scholar] [CrossRef]
- Chun, B.H.; Kim, K.H.; Jeong, S.E.; Jeon, C.O. The effect of salt concentrations on the fermentation of doenjang, a traditional Korean fermented soybean paste. Food Microbiol. 2020, 86, 103329. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, C.; Shan, W.; Zheng, F.; Liu, C.; Wang, J.; Li, Q. Physicochemical, flavor and microbial dynamic changes during low-salt doubanjiang (broad bean paste) fermentation. Food Chem. 2021, 351, 128454. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Wang, A.; Wang, J.; Wu, X.; Wu, Y.; Fu, Y.; Sun, H. Insights into interactions between food polyphenols and proteins: An updated overview. J. Food Process. Preserv. 2022, 46, e16597. [Google Scholar] [CrossRef]
- Ferreyra, S.; Bottini, R.; Fontana, A. Temperature and light conditions affect stability of phenolic compounds of stored grape cane extracts. Food Chem. 2023, 405, 134718. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, C.; Lu, H.; Pei, Z.; Yan, H. The Succession of Bacterial Flora and the Variation of Volatile Flavor Components during the Production of Fermented Sausage. J. Chin. Inst. Food Sci. Technol. 2022, 22, 282–290. [Google Scholar] [CrossRef]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and Flavor Perceptions of Glucosinolates, Isothiocyanates, and Related Compounds. Mol. Nutr. Food Res. 2018, 62, e1700990. [Google Scholar] [CrossRef]
- Li, H.; Zhou, F.; Pan, S.; Xu, X. Effect of Vacuum Impregnation on Quality Changes of Turnip during the Pickling Process. Food Sci. 2018, 39, 36–41. [Google Scholar] [CrossRef]
- Yuanfeng, W.; Chengzhi, L.; Ligen, Z.; Juan, S.; Xinjie, S.; Yao, Z.; Jianwei, M. Approaches for enhancing the stability and formation of sulforaphane. Food Chem. 2021, 345, 128771. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Ji, Y.; Guan, Y.; Feng, K.; Sarengaowa. Microbial dynamics and volatilome profiles during the fermentation of Chinese northeast sauerkraut by Leuconostoc mesenteroides ORC 2 and Lactobacillus plantarum HBUAS 51041 under different salt concentrations. Food Res. Int. 2020, 130, 108926. [Google Scholar] [CrossRef]
- Ying, W.; Ya-Ting, J.; Jin-Xuan, C.; Yin-Ji, C.; Yang-Ying, S.; Xiao-Qun, Z.; Dao-Dong, P.; Chang-Rong, O.; Ning, G. Study on lipolysis-oxidation and volatile flavour compounds of dry-cured goose with different curing salt content during production. Food Chem. 2016, 190, 33–40. [Google Scholar] [CrossRef]
- Hong, S.P.; Lee, E.J.; Kim, Y.H.; Ahn, D.U. Effect of Fermentation Temperature on the Volatile Composition of Kimchi. J. Food Sci. 2016, 81, C2623–C2629. [Google Scholar] [CrossRef]
- Yu, J.; Lu, K.; Zi, J.; Yang, X.; Xie, W. Characterization of aroma profiles and aroma-active compounds in high-salt and low-salt shrimp paste by molecular sensory science. Food Biosci. 2022, 45, 101470. [Google Scholar] [CrossRef]
- Qiu, D.; Duan, R.; Wang, Y.; He, Y.; Li, C.; Shen, X.; Li, Y. Effects of different drying temperatures on the profile and sources of flavor in semi-dried golden pompano (Trachinotus ovatus). Food Chem. 2023, 401, 134112. [Google Scholar] [CrossRef]
- Jin, G.; He, L.; Zhang, J.; Yu, X.; Wang, J.; Huang, F. Effects of temperature and NaCl percentage on lipid oxidation in pork muscle and exploration of the controlling method using response surface methodology (RSM). Food Chem. 2012, 131, 817–825. [Google Scholar] [CrossRef]
- Zhu, W.; Luan, H.; Bu, Y.; Li, X.; Li, J.; Ji, G. Flavor characteristics of shrimp sauces with different fermentation and storage time. LWT 2019, 110, 142–151. [Google Scholar] [CrossRef]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of immobilized Lactobacillus casei on the evolution of flavor compounds in probiotic dry-fermented sausages during ripening. Meat Sci. 2015, 100, 41–51. [Google Scholar] [CrossRef] [PubMed]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on dihydromyrcenol. Food Chem. Toxicol. 2010, 48, S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Tronchoni, J.; Querol, A.; Belloch, C. Production of aroma compounds by cryotolerant Saccharomyces species and hybrids at low and moderate fermentation temperatures. J. Appl. Microbiol. 2013, 114, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Ye, D.; Zang, X.; Nan, H.; Liu, Y. Effect of low temperature on the shaping of yeast-derived metabolite compositions during wine fermentation. Food Res. Int. 2022, 162, 112016. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, H.; Xing, S.; Liu, W.; Li, H. Advances in Research on Microbial Succession and Flavor Changes in Pickles. Food Sci. 2021, 42, 294–305. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, W.; Guo, S.; Sun, Y.; Yao, K.; Liu, Z.; Sun, Z.; Kwok, L.-Y.; Peng, C. Different growth behaviors and metabolomic profiles in yogurts induced by multistrain probiotics of Lactobacillus casei Zhang and Bifidobacterium lactis V9 under different fermentation temperatures. J. Dairy Sci. 2021, 104, 10528–10539. [Google Scholar] [CrossRef]
- Thierry, A.; Maillard, M.-B.; Richoux, R.; Kerjean, J.-R.; Lortal, S. Propionibacterium freudenreichii strains quantitatively affect production of volatile compounds in Swiss cheese. Lait 2005, 85, 57–74. [Google Scholar] [CrossRef]
- Thierry, A.; Richoux, R.; Kerjean, J.-R. Isovaleric acid is mainly produced by Propionibacterium freudenreichii in Swiss cheese. Int. Dairy J. 2004, 14, 801–807. [Google Scholar] [CrossRef]
- Park, H.S.; Reinbold, G.W.; Hammond, E.G.; Clark, W.S. Growth of Propionibacteria at Low Temperatures. J. Dairy Sci. 1967, 50, 589–591. [Google Scholar] [CrossRef]
- Castada, H.Z.; Park, C.; Harper, W.J.; Barringer, S.A. Suppression of propanoic acid, acetic acid and 3-methylbutanoic acid production by other volatiles in a Swiss cheese curd slurry system. Int. Dairy J. 2016, 54, 29–32. [Google Scholar] [CrossRef]
- Wu, S.; Du, H.; Xu, Y. Daqu microbiota adaptability to altered temperature determines the formation of characteristic compounds. Int. J. Food Microbiol. 2023, 385, 109995. [Google Scholar] [CrossRef]
- Askari-Khorasgani, O.; Pessarakli, M. Relationship between signaling and metabolic pathways of grapevines under temperature and light stresses—A review. J. Plant Nutr. 2019, 42, 2164–2175. [Google Scholar] [CrossRef]
- Mayobre, C.; Pereira, L.; Eltahiri, A.; Bar, E.; Lewinsohn, E.; Garcia-Mas, J.; Pujol, M. Genetic dissection of aroma biosynthesis in melon and its relationship with climacteric ripening. Food Chem. 2021, 353, 129484. [Google Scholar] [CrossRef]
- Akkad, R.; Buchko, A.; Johnston, S.P.; Han, J.; House, J.D.; Curtis, J.M. Sprouting improves the flavour quality of faba bean flours. Food Chem. 2021, 364, 130355. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Wang, S.-L.; Luo, R.-M. Evaluation of key aroma compounds and protein secondary structure in the roasted Tan mutton during the traditional charcoal process. Front. Nutr. 2022, 9, 1003126. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, G.; Yin, F.; Liu, Z.; Wang, J.; Qin, L.; Zhou, D.; Shahidi, F.; Zhu, B. Effects of roasting temperature and time on aldehyde formation derived from lipid oxidation in scallop (Patinopecten yessoensis) and the deterrent effect by antioxidants of bamboo leaves. Food Chem. 2022, 369, 130936. [Google Scholar] [CrossRef]


| Indicator | LSCK | HSCK |
|---|---|---|
| Lightness | 57.89 ± 1.70 a | 56.22 ± 1.20 a |
| Redness | 3.63 ± 0.50 a | 3.57 ± 0.17 a |
| Yellowness | 22.65 ± 0.41 a | 20.78 ± 0.65 b |
| pH | 4.76 ± 0.01 a | 4.27 ± 0.01 b |
| Total acid (%) | 0.39 ± 0.01 a | 0.59 ± 0.02 b |
| Reducing sugar (g/100 g) | 3.55 ± 0.03 a | 3.10 ± 0.07 b |
| Protein content (g/100 g) | 2.54 ± 0.09 a | 2.13 ± 0.10 b |
| Brittleness (mm−1) | 0.14 ± 0.01 a | 0.11 ± 0.01 b |
| Colonies number (log10CFU/g) | 4.18 ± 0.08 a | 5.60 ± 0.04 b |
| Lactic acid bacteria count (log10CFU/g) | 4.40 ± 0.17 a | 5.23 ± 0.02 b |
| Data Origin | The Principal Components | R2X | R2X (cum) |
|---|---|---|---|
| GC-IMS | PC1 | 0.8350 | 0.835 |
| PC2 | 0.0704 | 0.905 |
| Data Origin | R2X (cum) | R2Y (cum) | Q2 (cum) |
|---|---|---|---|
| GC-IMS | 0.989 | 1 | 0.997 |
| Flavor Substance | CAS | VIP | Aroma Descriptions | |
|---|---|---|---|---|
| 1 | Tetrahydrothiophene | 110-01-0 | 7.28553 | Cabbage |
| 2 | Ethyl 3-(methylthio)propanoate | 13327-56-5 | 3.57834 | Meat, onion, and garlic |
| 3 | 3-Methylbutyric acid | 503-74-2 | 2.44778 | Putrid and sweaty smell |
| 4 | Hexanenitrile | 628-73-9 | 2.35979 | |
| 5 | 3-Methyl-3-buten-1-ol | 763-32-6 | 2.24654 | Sweet fruit |
| 6 | Diethylene glycol dimethyl ether | 111-96-6 | 2.14249 | |
| 7 | (E)-2-Octenal | 2548-87-0 | 2.10519 | Citrus |
| 8 | 4,5-Dihydro-3(2H)-thiophenone-D | 1003-04-9 | 2.09335 | Garlic and Onion |
| 9 | 3-sec-Butyl-2-methoxypyrazine | 24168-70-5 | 1.91523 | Fresh green pea |
| 10 | δ-Heptanolide-M | 713-95-1 | 1.77461 | Peach |
| 11 | 2,6-Dimethylpyrazine | 108-50-9 | 1.42207 | Coffee and fried peanut |
| 12 | Ethyl dihydrocinnamate-M | 103-36-6 | 1.38867 | Orange and grape |
| 13 | 2-Methyl-4-propyl-1,3-oxathiane | 59323-76-1 | 1.38086 | Spicy herb |
| 14 | Carvacrol | 499-75-2 | 1.23141 | |
| 15 | Ethyl acetoacetate propylene glycol ketal | 6290-17-1 | 1.15615 | Fruit |
| 16 | 2-Furanmethanol | 623-19-8 | 1.12531 | Green and burnt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Nie, X.; Peng, T.; Xiang, L.; Liu, D.; Luo, H.; Zhao, Z. Effects of Low-Temperature and Low-Salt Fermentation on the Physicochemical Properties and Volatile Flavor Substances of Chinese Kohlrabi Using Gas Chromatography–Ion Mobility Spectrometry. Fermentation 2023, 9, 146. https://doi.org/10.3390/fermentation9020146
Chen H, Nie X, Peng T, Xiang L, Liu D, Luo H, Zhao Z. Effects of Low-Temperature and Low-Salt Fermentation on the Physicochemical Properties and Volatile Flavor Substances of Chinese Kohlrabi Using Gas Chromatography–Ion Mobility Spectrometry. Fermentation. 2023; 9(2):146. https://doi.org/10.3390/fermentation9020146
Chicago/Turabian StyleChen, Hongfan, Xin Nie, Tao Peng, Lu Xiang, Dayu Liu, Huailiang Luo, and Zhiping Zhao. 2023. "Effects of Low-Temperature and Low-Salt Fermentation on the Physicochemical Properties and Volatile Flavor Substances of Chinese Kohlrabi Using Gas Chromatography–Ion Mobility Spectrometry" Fermentation 9, no. 2: 146. https://doi.org/10.3390/fermentation9020146
APA StyleChen, H., Nie, X., Peng, T., Xiang, L., Liu, D., Luo, H., & Zhao, Z. (2023). Effects of Low-Temperature and Low-Salt Fermentation on the Physicochemical Properties and Volatile Flavor Substances of Chinese Kohlrabi Using Gas Chromatography–Ion Mobility Spectrometry. Fermentation, 9(2), 146. https://doi.org/10.3390/fermentation9020146






