Abstract
Enzyme mediated degradation of lignocellulosic biomass is an important step in waste-biorefineries. Multienzyme preparations can effectively degrade complex materials and, hence, can be applied in biorefineries. Here, an agro-industrial waste, sugarcane bagasse, was used to produce a bacterial multienzyme. The bacterial strains including B. thuringiensis B45, B. velezensis BF3 and B. amyloliquefaciens B987 exhibited their growth at temperatures from 30–50 °C in the presence of 2% salt. The isolates B45, BF3 and B987 were able to produce endoglucanase, xylanase and pectinase, respectively. Therefore, it was aimed to obtain a multienzyme preparation by cultivating the bacterial consortium under a solid-state fermentation of untreated and chemically treated sugarcane bagasse. The results showed that the titres of cellulase and xylanase were generally higher when the strain B45 cultivated at the start of the fermentation. Interestingly, the degradation of cellulose and hemicellulose present in sugarcane bagasse by the strains B45 and BF3 rendered the mere pectin component available to the pectinolytic strain B987. The degradation of SB by the consortium was confirmed by gravimetric analysis and scanning electron microscopy. The study showed that the bacterial strains can be cultivated under solid-state fermentation to obtain industrially important enzymes.
1. Introduction
Soil microbes play a diversified role in improving the fertility of soil, which provides growth promoting substances to plants and can degrade a variety of complexes in their habitat [1]. Bacteria are the most abundantly found microbes in soil and play vital roles in organic matter recycling. Exploring their diversity with a particular emphasis on finding industrial applications has remained a subject of research in microbial biotechnology. Indeed, soil bacterial strains have been reported for their plant growth promoting abilities and can be used for the sustainable development of agriculture [2] (Bauza-kaszewska et al., 2022). Soil bacteria have also been described for their positive impacts on plants thriving under a saline environment [3]. The environmental benefits of soil bacteria are evident as soil bacteria can degrade and detoxify pesticides, including Chlorantraniliprole and Flubendiamide [4]. Soil bacteria have also been described as sources of industrially relevant metabolites, including biosurfactant [5], bacteriocin [6], cellulase [7] and amylase [8].
Bacterial strains have been reported to degrade lignocellulosic biomass by producing a number of carbohydrases, including cellulase, xylanase and pectinase [9,10]. Cellulases are the main cell wall degrading enzymes that can act on cellulose either randomly (endoglucanase) or terminally (exoglucanase and β-glucosidase), leading to the release of sugars from the most abundant polymer on Earth [11] (Ejaz et al., 2021). In a plant cell wall, cellulose is attached with hemicellulose, which is a heterogenous polymer but predominantly contains xylan [12]. Xylanases degrade xylan into oligosaccharides and xylose as the main products [13]. Pectin, although a constituent of the plant cell wall, is mainly found in the middle lamella and present in negligible quantities in hardwoods [14] (Thite et al., 2020). Pectinases are a diversified group of enzymes that can attack on the main chain of galacturonic acid linked polymer as with all the side chains with various substituents [15] (Shrestha et al., 2021).
With recent innovations in biorefinery approaches, the interest towards plant cell wall degrading enzymes and their productions has renewed [16,17]. Filamentous fungi have remained the main source of these enzymes since the discovery of cellulases in Trichoderma [11]; however, lately, bacterial strains were recognized as a promising source of plant cell wall degrading enzymes [10,18]. Among bacteria, the members of the genus Bacillus have been particularly described as the house of industrially important metabolites, including plant cell wall degrading enzymes that can withstand extreme temperatures and pH [19,20,21]. The attributes of stability towards varying environmental conditions provide a prospect of utilizing Bacillus species in the fermentation of plant biomass [22,23], including sugarcane bagasse [19]. Since a single organism does not produce all the enzymes required to degrade a complex biomass, such as sugarcane bagasse (SB), microbial consortia are developed to obtain enzymes with desirable catalytic attributes [24]. Indeed, the degradation of lignocellulosic biomass is reportedly improved when a bacterial consortium is used compared to the degradation using a single strain [25].
SB is a fibrous residue that is generated after the extraction of sugar from the stalk and is considered one of the largest agro-industrial waste products, with approximately 1.7 billion tons of global annual production estimated in the year 2015 [26]. Although millions of tons of this waste are produced in sugar producing countries, it remains under-utilized. With a considerable proportion of fermentable components, SB can be assimilated by microorganisms; hence, it can serve as a source of low-cost feedstock for the production of valuable compounds. Fungi, through hyphae, can penetrate deeply into the substrates and, therefore, have frequently been reported for their use in the production of plant cell wall degrading enzymes, particularly cellulase and xylanase, under the solid state fermentation (SSF) of SB. Few researchers have reported the supplementation of pectic materials to SB for the production of pectinase using crude substrates [27]. A thorough literature survey did not reveal any report regarding the use of a bacterial consortium for the production of a multienzyme with pectinase rich activity under the SSF of SB without the supplementation of pectin. Earlier, we have reported about the improved production of cellulase and xylanase by the co-culture of S. cerevisiae MK-157 and C. tropicalis MK-118 under the SSF of SB [28]. Here, the production of pectinase-rich multienzyme preparation is being reported using a consortium of cellulolytic, xylanolytic and pectinolytic bacilli.
2. Materials and Methods
2.1. Isolation of Bacterial Strains
The soil samples were collected from Keerthar National Park (Coordinates: 25.274784214863075, 67.11471095337144) in sterile plastic bags, brought to the laboratory and kept at 4 °C until analyzed. The samples were processed through an elective and enrichment technique to isolate bacterial strain. The sample (100 g) was mixed with sugarcane bagasse (10 g), moistened with water and kept in plastic bags at 40 °C for one week while maintaining the moisture level. After the enrichment of the sample, the samples were separately transferred to Erlenmeyer flasks (250 mL) containing a mineral salt medium (MSM) [29] containing 1% (w/v) carboxymethyl cellulose (CMC), xylan and pectin as the sole carbon source and incubated at 40 °C for 48 h. The contents of these flasks were transferred (at 10% ratio) to flasks containing the corresponding substrates and incubated at 40 °C for 48 h. The step was repeated, the contents of the last flasks were spread over MSM containing corresponding substrates and the plates were incubated at 40 °C for 48 h. The isolated colonies were purified and maintained on Nutrient agar.
2.2. Screening of Bacterial Isolates for Cellulase, Xylanase and Pectinase Activities
The bacterial isolates were screened for cellulase, xylanase and pectinase activities using MSM supplemented with the corresponding substrates of the enzymes. A single isolated colony from a nutrient agar was transferred to a nutrient broth until OD600 reached 0.8–1.0. An aliquot (100 μL) from this inoculum was separately spread over MSM agar containing CMC, xylan or pectin and incubated at 40 °C for 24 h. The plates containing CMC and xylan were stained with congo red while the pectin containing plates were incubated with Ruthenium red [30]. The halos around the colonies were estimated and taken as the potential of the isolate to produce the corresponding enzyme. The isolates that appeared positive in the plate screening assay were further investigated in shake flask experiments [30]. Briefly, an inoculum was prepared as discussed earlier and separately transferred to MSM containing CMC, xylan or pectin and incubated at 40 °C for 24 h at 150 rpm. The cells were separated from the broth by centrifugation at 3000× g for 20 min, and the cell-free culture supernatant (CFCS) was taken as the enzyme preparation.
2.3. Enzyme Assays
Endoglucanase, xylanase and pectinase assays were performed as given earlier [10] by estimating the amount of reducing sugars using the dinitrosalicylic acid method [31]. One unit of the enzyme activity was defined as the μmol of the reducing sugars released by the 1 mL of the enzyme under standard assay conditions per minute. Protein estimation was carried out using Bradford’s method [32], and the specific activity (IU per μg of protein) was estimated.
2.4. Genomic DNA Extraction and Identification of the Promising Isolates
Based on the quantification of the endoglucanase, xylanase and pectinase, three isolates, B45, BF3 and B987, were selected for further studies. Genomic DNA was extracted as described elsewhere [33]. Universal primers 27F and 1492R were used to amplify 16S rDNA, and amplicons were sequenced by Macrogen, Korea. The sequences were submitted to the NCBI and accession numbers were obtained. The BLAST tool from NCBI was used to align the sequence with the database, and a phylogenetic tree was constructed using the Omega 6 tool.
2.5. Growth Profile of the Isolates
The promising isolates, B45, BF3 and B987, were investigated for their growth in the presence of cellulose, xylan and pectin, with and without salt at varying temperatures to determine the induction of various enzymes and the halo- and thermotolerance of the isolates. Briefly, a single isolated colony of each strain was transferred to a nutrient broth and cultivated at 37 °C for 24 h until OD600 reached 0.8–1.0. The inoculum was transferred (by maintaining the final OD600 of 0.3 corresponded to 2.8–3.2 × 108 cells per mL) to MSM containing CMC, xylan or pectin containing varying concentration of salt (0–4%) and incubated at different temperatures from 25–50 °C for 24–48 h. The final OD600 of the suspension was noted, and CFCS was prepared to analyze the amount of cellulase, xylanase and pectinase. The cell density was estimated using a calibrated curve of the bacterial cell count with the OD600.
2.6. Solid State Fermentation of Sugarcane Bagasse
The strains, (B45, BF3 and B987) were selected to grow under the SSF of SB in a consortium and to analyse the enzyme preparation. B45 was an endoglucanase (EG) producing strain, BF3 was xylanase producing strain while B987 had a potential to produce pectinase. The compatibility of the strain was confirmed by cross streaking on nutrient agar plates [28]. The inoculum of the three strains was prepared as given in the earlier section. The inoculation pattern of the strains was investigated by varying the time of the inoculation of the bacterial strains.
SB was obtained from a local fruit juice seller and pretreated using acid and alkali (Qadir et al. 2018). The SSF process was carried out as given in Shariq et al. (2020) with certain modifications. Briefly, 10 g of SB was autoclaved in a 500 mL Erlenmeyer flask and moistened with MSM without any carbon source to achieve a moisture level of 65%. The bacterial inoculum (5 mL) was transferred to this SB, mixed and the flasks were incubated at 45 °C for 48 h. At harvest, 100 mL of a 50 mM sodium citrate buffer (pH 5.0) containing 0.05% w/v Tween 80 was added and placed in a shaker at 150 rpm. The filtrate was separated by passing the contents through four layers of muslin cloth and then centrifuged at 2500× g for 15 min. The cell-free culture supernatant was assayed for the enzyme assays, and the residue was analysed for cellulose and hemicellulose contents gravimetrically. To use the consortium of the strains, five different experiments were designed for the inoculation of B45, BF3 and B987. With every experiment, the strains were also inoculated separately as controls. A summary of the inoculation pattern is given in Table 1.

Table 1.
Development of consortium for multienzyme preparation under Solid State fermentation of Sugarcane bagasse. Strain B45 of Bacillus thuringiensis, B. velezensis BF3 and B. amyloliqufaciens B987 were inoculated together (experiment 1) or with a different inoculation pattern and enzyme production in cell free culture supernatant was noted. (Inoculum of the strains was maintained at 2.8–3.2 × 108 cells per mL).
2.7. Gravimetric Analysis and Scanning Electron Microscopy
The gravimetric analysis of SB was carried out by adopting a previously described method [34], and the contents of lignin, cellulose and hemicelluloses were estimated [28]. Pretreated and fermented SB was visualized with scanning electron microscopy (SEM), JSM-6380 A, JEOL USA, after coating with a 250 A° thick gold using an Ion Sputtering Device.
2.8. Statistical Analysis
All the experiments were conducted in triplicate, and the mean values have been reported. Standard deviation and other analysis were performed using SPSS (Ed. 26).
3. Results and Discussion
The microbial diversity in soil has been explored widely for the production of industrially important metabolites, including plant cell wall degrading enzymes [30]. The discharge of lignocellulosic materials along with other industrial wastes render soil a diversified environment where microbial interactions can exhibit various degradative potential. Therefore, soil, particularly of less explored areas, has remained a source of microbial strains with desirable attributes for their exploitation on the commercial scale. Kirthar National Park, Sindh, Pakistan has been mainly described for its geographic pattern and floral diversity [35], and the studies related to the isolation of microbial strains are scarcely available. The current study was designed to isolate bacterial strains from the soil with a particular emphasis on their ability to produce plant cell wall degrading enzymes.
3.1. Isolation and Screening of Bacterial Strains
In all, 37 bacterial strains were isolated after the enrichment of the soil samples. The low number of isolates from a soil sample can be linked with the enrichment and selective pressure technique that favoured the growth of plant cell wall degrading bacteria at 40 °C. The isolated bacterial strains were screened using the plate assay and shake flask method. The plate assay showed that 16, 11 and 5 strains were capable to produce cellulase, xylanase and pectinase, respectively. As SB (which is rich in cellulose and hemicellulose) was used to enrich the sample, cellulolytic and hemicellulolytic organisms were favoured over pectinolytic bacteria.
The shake flask experiments showed that the strains generally produced low levels of endoglucanase, xylanase and pectinase; however, three strains (B45, BF3 and B987) released higher quantities of these enzymes and, hence, were selected for further studies. A total of 10 strains co-produced endoglucanase and xylanase (Table 2) that affirmed a previous finding about the co-expression of cellulase and xylanase [36]. None of the strains exhibited a co-expression of the studied enzymes with pectinase.

Table 2.
Endoglucanase, xylanase and pectinase production by the selected isolates. The isolates were separately cultivated in Mineral Salt medium containing carboxymethylcellulose, xylan and pectin and the enzyme activities were detected in cell-free culture supernatant.
3.2. Identification of the Promising Isolates
Three isolates, B45, BF3 and B987, exhibited the capability to over-produce endoglucanase, xylanase and pectinase, respectively, and were identified using the sequencing of their 16r RNA genes. The isolate, B45, was identified as Bacillus thuringiensis and found 100% similar to the strain SY of the same species (Figure 1). B. thuringiensis is a heterogenous group of various serotypes and has been reported as a common flora of forest soil [37]. Previously, various serotypes of B. thuringiensis were screened for hydrolytic enzyme production, and most of the strains were found amylolytic and cellulolytic [38]. The release of an insecticidal crystal (CRY) protein and hydrolytic activities provide prospects of this strain for its utilization in sustainable agricultural practices.

Figure 1.
Phylogenetic analysis of the isolates Bacillus velezensis BF3, B. amyloliquefaciens B987 and B. thuringiensis B45.
The strain BF3 was identified as B. velezensis and was found to be a producer of higher titres of xylanase. Ben Gharsa et al. has reported about the isolation of B. velezensis from the rhizospheric soil of a halophytic plant, Zygophyllum album, and investigated its antifungal potential [39]. Another study conducted by Lu et al. [40]. reported the presence of B. velezensis in the roots of a halophyte Suaeda glauca and described its potential anti-fungal role and growth promotion in Arabidopsis thaliana [41]. This organism has also been reported recently for xylanase production [42].
The strain B987 was found similar to the strain NIOB 109 of B. amyloliquefaciens and produced higher amounts of pectinase. Earlier, pectinase production from B. amyliquefaciens was reported [43]; however, after optimization, the authors could only obtain 0.76 U mL−1 of pectinase, which was much lower than the titers reported here, whereas the co-production of xylanase and pectinase has also been reported from B. amyloliquefaciens AD12 [44].
3.3. Growth Profile of the Isolates
The strains were separately cultivated in the presence of carboxymethylcellulose, xylan and pectin containing mineral salt media with a varying concentration of NaCl (0–4%; at 0.5% interval). Indeed, halotolerant or halophilic organisms produce enzymes that can perform their catalytic activities in the presence of salt. Such microbial strains and their enzymes are of particular interest to develop biorefineries utilizing plant biomass from halophytes. The strain BF45 and BF987 exhibited the highest growth in the medium containing 2% NaCl (Table 3) while the growth of BF3 was slightly less compared to the growth in presence of 1.5% NaCl. Nonetheless, the medium containing 2% NaCl was used in further experiments. The tolerance to salinity by B. thuringiensis is of particular interest as the genetically modified crops with the tox gene of B. thuringiensis remained a subject of interest for their salt tolerance [45]. Wekesa et al. (2022) [46] reported the growth of B. velezensis in the presence of ~2.9% NaCl and investigated its potential to control a phytopathogen. The enzyme production from the strains in the presence of salt was found to be growth-linked; more titres of the enzymes were obtained in the medium where growth was higher. The growth linked cellulase production was reported earlier by Sohail et al. [47]. Moreover, the production of the enzymes in a salt containing medium is an important finding as the tolerance of cellulase to salt has been associated with the tolerance to ionic liquids, and hence, such enzymes can saccharify a wide variety of substrates pretreated with ionic liquids [48].

Table 3.
Growth and enzyme production in presence of sodium chloride. The isolates, B45, BF3 and B987 were cultivated in Mineral salt medium containing carboxymethylcellulose, xylan or pectin and amended with sodium chloride. The OD600 (corresponded to 2.8–3.2 × 108 cells per mL) was taken as reflection of the growth. Endoglucanase, xylanase and pectinase was assayed from the strain B45, BF3 and B987, respectively. The enzyme titres (IU mL−1) are given in the parenthesis.
To further optimize the NaCl concentration, the strains were grown in (2%) salt containing media at varying temperatures, and the growth profile was noted. All the strains exhibited considerable growth at temperatures 50–55 °C (Table 4). However, the optimum growth was obtained at 45 °C, indicating the thermotolerant nature of the strains. The ability of the Bacillus species to grow at various temperatures has been utilized as an identification characteristic and included in the development of artificial intelligence-based algorithms [49]. Thermotolerant bacteria with the potential to produce industrially important enzymes are of particular interest as these can be used to design a process at high temperature that can resist contamination by mesophilic organisms [19]. Generally, the strains did not produce appreciable quantities of the enzymes at temperatures 55 or 60 °C except for the pectinolytic strain B987. The denaturation of the enzyme at a higher temperature may explain this observation [47]. Nonetheless, thermotolerant bacteria provide many advantages and are preferred in the lignocellulose biorefineries [24].

Table 4.
Growth of Bacillus thuringiensis B45, B. velezensis BF3 and B. amyloliquefaciens B987 at various temperatures in presence of NaCl. Inoculum maintained at 0.3 OD600 (corresponded to 2.8–3.2 × 108 cells per mL) was transferred, and the final OD was noted after 24 h of cultivation. The OD600 was taken as reflection of the growth. Endoglucanase, xylanase and pectinase from the strain B45, BF3 and B987 were assayed, respectively. The enzyme titres (IU mL−1) are given in the parenthesis.
3.4. Production of Multienzyme
The isolated strains were investigated for their compatibility while growing on an agar plate, and no inhibition was observed. Consequently, it was decided to use the three strains to develop a consortium for a simultaneous production of cellulase, xylanase and pectinase. It was of particular interest as sugarcane bagasse is not rich in pectin and, hence, the production of pectinase using SB is an additional advantage. Here, our main aim was to investigate the effect of the inoculation pattern of the members of the bacterial consortium on multienzyme preparation under the SSF of SB, and the utilization of pretreated SB was compared with the untreated SB. The results obtained from Experimental Set 1 revealed that when all three strains were cultivated together at 0 h into the fermentation, the SSF of alkali pretreated SB did not yield any titres of pectinase, yet high titres of endoglucanase (109.68 IU μg−1) and xylanase (202.68 IU μg−1) were obtained under these conditions (Table 5) while 35.82 IU μg−1 of pectinase were obtained when the experiment was conducted using acid pretreated SB. Earlier, Qadir et al. also described the production of higher titres of cellulase and xylanase under the SSF of alkali pretreated SB than acid pretreated SB [28]. The xylanase titres obtained in this study were comparable with the levels obtained earlier [24].

Table 5.
Production of endoglucanase (EG), xylanase (Xyl) and pectinase (Pec) by the co-culture of the bacterial strains (as mentioned in the methods section) under SSF of untreated (UTSB), acid pre-treated (ACSB) and alkali pre-treated SB (AKSB).
In Experimental Set 2, the strain B45 was cultivated with the commencement of the experiment (0 h) and the other two strains were inoculated at 8 h into the fermentation. It was known that B45 takes 10 h to enter into its exponential phase of growth, although endoglucanase production initiated earlier; therefore, the effect of the 8 h cultivation of this strain was noted on the production of the multienzyme by the consortium. In this set, untreated SB appeared to be the most suitable substrate for the production of pectinase (45.56 IU μg−1). This titre was much higher than those obtained previously by a fungal co-culture of A. niger MS23 and A. terreus MS105 when untreated banana peels were supplemented with pectin [27]. Nonetheless, the titres obtained in this study using untreated SB without pectin supplementation were promising, as the costs of pretreatment and supplementation can be avoided.
In Experimental Set 3, the strains B45 and B987 were co-cultured at the start of the fermentation (0 h). The strain B987 was a more rapid grower than B45, as its OD reached to its peak within 8–10 h with the concomitant pectinase production. In this pattern of inoculation, the titres of pectinase were the highest when untreated SB was fermented compared to the other substrates. The titres were even several folds higher than obtained earlier by the immobilized cells of a pectinolytic yeast, Geotrichum candidum AA15 [50].
When strains BF3 and B987 were co-cultured (Experimental Set 4), the fermentation of untreated SB yielded higher amounts of all the three enzymes than that of the fermentation of alkali pretreated SB or acid pretreated SB. However, the enzyme titres were lower from the experiments where B45 was inoculated, which may be attributed to the major role played by this strain in the degradation of crude substrates.
In yet another experiment (Experimental Set 5), though, the inoculation of the pectinolytic strain B45 was delayed until 16 h; however, the pectinase yield was not drastically affected. Indeed, this cultivation pattern yielded the highest titres of pectinase when untreated SB was utilized as the substrate. This finding could be attributed to the degradation caused by the cellulolytic and xylanolytic strains in the earlier phase of cultivation, facilitating the production of pectinase even when the inoculation of the strain B987 was delayed.
3.5. Gravimetric Analysis
The gravimetric analysis of SB showed that cellulose and hemicellulose contents in unfermented and fermented SB increased with chemical and biological treatments (Table 6). An increase in cellulose content was previously reported [28] and linked with the removal of the lignin seal by the alkaline pretreatment, whereas the pretreatment with the dilute acid was found to be less effective in removing lignin [34], although it increased cellulase accessibility. The lowest content of lignin and hemicellulose, along with the highest content of cellulose, was present in the fermented alkali pretreated SB analysed after Experimental Set 1. It indicated the maximum utilization of the substrate along with the production of the highest titres of xylanase. Indeed, alkaline pretreatment, by removing hemicelluloses and lignin, makes SB less dense and less rigid [51] and presents it as a suitable raw material. The cellulose content decreased substantially when untreated SB was fermented in Experimental Sets 2 and 5 that accompanied the higher levels of pectinase. Perhaps the utilization of cellulose allows the organism to access the pectic portions of the material and to produce more titres of pectinase.

Table 6.
Gravimetric analysis of untreated (UTSB), acid pretreated (ACSB), alkali pretreated (AKSB) and fermented Sugarcane bagasse. The details of experiments are given in method section.
3.6. SEM Analysis
The changes in the structure of SB brought by the pretreatment and/or fermentation were visualized with SEM and compared with the structure of untreated and unfermented SB that was having highly compact, rough, thick-walled fibres associated with pith (Figure 2). The pretreatment with acid or alkali damaged the compactness and separated the pith from the fibres. The acidic pretreatment resulted in the formation of pores in the structure as well. When acid pretreated SB was fermented in Experimental Sets 3 and 4 (Figure 3), the destruction in the matrix was more obvious than in alkali pretreated SB (Figure 4). The fermentation of alkali pretreated SB by the bacterial consortium caused loosening in the structure of SB and disrupted it more eloquently.
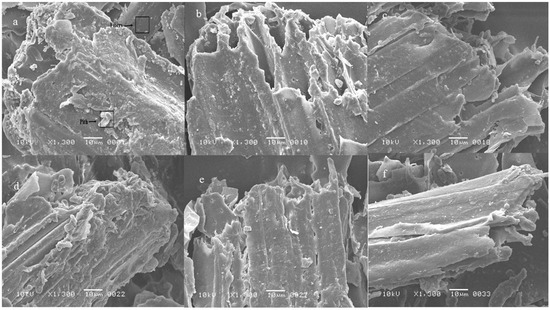
Figure 2.
Scanning electron microscopy of (a) untreated SB, (b) untreated SB obtained after experiment 1, (c) after experiment 2, (d) after experiment 3, (e) after experiment 4, (f) after experiment 5.
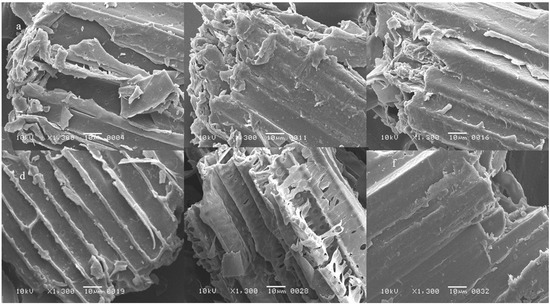
Figure 3.
Scanning electron microscopy of (a) acid pre-treated SB, (b) acid pre-treated SB obtained after experiment 1, (c) after experiment 2, (d) after experiment 3, (e) after experiment 4, (f) after experiment 5.

Figure 4.
Scanning electron microscopy of (a) alkali pre-treated SB, (b) alkali pre-treated SB obtained after experiment 1, (c) after experiment 2, (d) after experiment 3, (e) after experiment 4, (f) after experiment 5.
4. Conclusions
The Elective and Enrichment technique made the isolation of thermotolerant plant cell wall degrading bacteria possible. The genus Bacillus is a house for diversified industrially important enzymes. The species B. velezensis, B. amyloliquefaciens and B. thuringiensis exhibited considerable growth in the presence of 2% salt and at temperatures from 45–50 °C. The three strains were found compatible to grow together. The bacterial consortium developed in this study produced a pectinase-enriched multienzyme preparation containing cellulase and xylanase activities. Cellulase and xylanase activities varied with the variation in the inoculation of the strain. Therefore, the study provides a path to produce multienzymes with variable activities by varying the cultivation pattern of the bacterial consortium.
Author Contributions
O.M.A.: Methodology, Data curation, Funding acquisition; M.S., Conceptualization, Analysis, Writing initial and final draft; S.F.M., data curation; A.S.A., Methodology, Investigation; Y.E.-H., Investigation, analysis. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was obtained from Taif University through project number TURSP-2020/262.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data related to this article can be obtained from the corresponding author upon a reasonable request.
Acknowledgments
The assistance by Fasih Abro is acknowledged for the sample collection. The authors acknowledge the financial support of Taif University Researchers Supporting Project number TURSP-2020/262, Taif University, Taif, Saudi Arabia.
Conflicts of Interest
Authors do not declare any conflict of interest.
References
- Centuri, N.; Ulcuango, K.; Navas, M.; Mariscal-sancho, I.; Ib, M.A.; Moliner, A.; Hontoria, C. Soil Microbial Response to Cover Crop Termination Methods under Two Water Levels. Agronomy 2022, 12, 3002. [Google Scholar] [CrossRef]
- Bauza-kaszewska, J.; Breza-boruta, B.; Lamparski, R. Effects of Eco-Friendly Product Application and Sustainable Agricultural Management Practices on Soil Properties and Phytosanitary Condition of Winter Wheat Crops. Sustainability 2022, 14, 15754. [Google Scholar] [CrossRef]
- Javier, P.; Iturriaga, G.; Aguirre-mancilla, C.L.; Gabriel, J.; Dioselina, Á. Identification of Halophilic and Halotolerant Bacteria from the Root Soil of the Halophyte Sesuvium verrucosum Raf. Plants 2022, 11, 3355. [Google Scholar] [CrossRef]
- Fahmy, M.A.; Salem, S.H.; Qattan, S.Y.A.; Abourehab, M.A.S.; Ashkan, M.F.; Al-quwaie, D.A.; El-fattah, H.I.A.; Akl, B.A. Biodegradation of Chlorantraniliprole and Flubendiamide by Some Bacterial Strains Isolated from Different Polluted Sources. Processes 2022, 10, 2527. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, V.; Pandit, S.; Thapa, B.S.; Pant, K. Isolation of Biosurfactant-Producing Bacteria and Their Co-Culture Application in Microbial Fuel Cell for Simultaneous Hydrocarbon Degradation and Power Generation. Sustainability 2022, 14, 15638. [Google Scholar] [CrossRef]
- Naz, S.A.; Jabeen, N.; Sohail, M.; Rasool, S.A. Production and purification of pyocin from a soil associated pseudomonas aeruginosa strain sa 188. Pakistan J. Agric. Sci. 2015, 52, 873–879. [Google Scholar]
- Tariq, R.; Ansari, I.; Qadir, F.; Ahmed, A.; Shariq, M.; Zafar, U.; Ahmad, A.; Khan, S.A.; Sohail, M. Optimization of endoglucanase production from thermophilic strain of Bacillus licheniformis RT-17 and its application for saccharification of sugarcane bagasse. Pak. J. Bot. 2018, 50, 807–816. [Google Scholar]
- Sohail, M.; Ahmad, A.; Shahzad, S.; Khan, S.A. A survey of amylolytic bacteria and fungi from native environmental samples. Pak. J. Bot. 2005, 37, 155–161. [Google Scholar]
- Walia, A.; Guleria, S.; Mehta, P.; Chauhan, A.; Parkash, J. Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. 3 Biotech 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Rehman, I.; Ejaz, U.; Ansari, A.; Sohail, M. Production of multienzyme by Bacillus aestuarii UE25 using ionic liquid pretreated sugarcane bagasse. J. Basic Microbiol. 2021, 61, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From bioactivity to a variety of industrial applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Kaprelyants, L.; Zhurlova, O.; Shpyrko, T.; Pozhitkova, L. Xylooligosaccharides from agricultural by-products: Characterisation, production and physiological effects. Food Sci. Technol. 2017, 11, 25–34. [Google Scholar] [CrossRef]
- Viikari, L.; Alapuranen, M.; Puranen, T.; Vehmaanperä, J.; Siika-Aho, M. Thermostable enzymes in lignocellulose hydrolysis. Adv. Biochem. Eng. Biotechnol. 2007, 108, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Thite, V.S.; Nerurkar, A.S.; Baxi, N.N. Optimization of concurrent production of xylanolytic and pectinolytic enzymes by Bacillus safensis M35 and Bacillus altitudinis J208 using agro-industrial biomass through Response Surface Methodology. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Rahman, M.S.; Qin, W. New insights in pectinase production development and industrial applications. Appl. Microbiol. Biotechnol. 2021, 105, 9069–9087. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Zhang, F.; Bunterngsook, B.; Li, J.-X.; Zhao, X.-Q.; Champreda, V.; Liu, C.-G.; Bai, F.-W. Regulation and production of lignocellulolytic enzymes from Trichoderma reesei for biofuels production. Adv. Bioenergy 2019, 4, 79–119. [Google Scholar] [CrossRef]
- Behera, B.C.; Sethi, B.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Microbial cellulases—Diversity & biotechnology with reference to mangrove environment: A review. J. Genet. Eng. Biotechnol. 2017, 15, 197–210. [Google Scholar] [CrossRef]
- Hassan, M.; Sohail, M. Characterization of cellulases from thermophilic bacilli and their application for the saccharification of sugarcane bagasse. Pak. J. Bot. 2020, 52, 1785–1791. [Google Scholar] [CrossRef]
- Pandey, A.; Dhakar, K.; Sharma, A.; Priti, P.; Sati, P.; Kumar, B. Thermophilic bacteria that tolerate a wide temperature and pH range colonize the Soldhar (95 °C) and Ringigad (80 °C) hot springs of Uttarakhand, India. Ann. Microbiol. 2015, 65, 809–816. [Google Scholar] [CrossRef]
- Shrestha, S.; Khatiwada, J.R.; Zhang, X.; Chio, C.; Kognou, A.L.M.; Chen, F.; Han, S.; Chen, X.; Qin, W. Screening and molecular identification of novel pectinolytic bacteria from forest soil. Fermentation 2021, 7, 40. [Google Scholar] [CrossRef]
- Kang, S.-M.; Latif Khan, A.; Waqas, M.; Asaf, S.; Lee, K.-E.; Park, Y.-G.; Kim, A.-Y.; Khan, M.A.; You, Y.-H.; Lee, I.-J. Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 2019, 14, 416–423. [Google Scholar] [CrossRef]
- Rashid, R.; Sohail, M. Xylanolytic Bacillus species for xylooligosaccharides production: A critical review. Bioresour. Bioprocess. 2021, 8, 16. [Google Scholar] [CrossRef]
- Gavande, P.V.; Basak, A.; Sen, S.; Lepcha, K.; Murmu, N.; Rai, V.; Mazumdar, D.; Saha, S.P.; Das, V.; Ghosh, S. Functional characterization of thermotolerant microbial consortium for lignocellulolytic enzymes with central role of Firmicutes in rice straw depolymerization. Sci. Rep. 2021, 11, 3032. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Al-Tohamy, R.; Manni, A.; Luz, F.C.; Elsamahy, T.; Sun, J. Enhanced digestion of bio-pretreated sawdust using a novel bacterial consortium: Microbial community structure and methane-producing pathways. Fuel 2019, 254, 115604. [Google Scholar] [CrossRef]
- Arshad, M.; Ahmed, S. Cogeneration through bagasse: A renewable strategy to meet the future energy needs. Renew. Sustain. Energy Rev. 2016, 54, 732–737. [Google Scholar] [CrossRef]
- Rehman, S.; Aslam, H.; Ahmad, A.; Khan, S.A.; Sohail, M. Production of plant cell wall degrading enzymes by monoculture and co-culture of Aspergillus niger and Aspergillus terreus under SSF of banana peels. Braz. J. Microbiol. 2014, 1492, 1485–1492. [Google Scholar] [CrossRef]
- Qadir, F.; Shariq, M.; Ahmed, A.; Sohail, M. Evaluation of a yeast co-culture for cellulase and xylanase production under solid state fermentation of sugarcane bagasse using multivariate approach. Ind. Crops Prod. 2018, 123, 407–415. [Google Scholar] [CrossRef]
- Ejaz, U.; Muhammad, S.; Imran, F.; Ali, I.; Sohail, M. Cellulose extraction from methyltrioctylammonium chloride pretreated sugarcane bagasse and its application. Int. J. Biol. Macromol. 2020, 165, 11–17. [Google Scholar] [CrossRef]
- Sohail, M.; Naseeb, S.; Sherwani, S.K.; Sultana, S.; Aftab, S.; Shahzad, S.; Ahmad, A.; Khan, S.A. Distribution of hydrolytic enzymes among native fungi: Aspergillus the pre-dominant genus of hydrolase producer. Pak. J. Bot. 2009, 41, 2567–2582. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wahyuni, S.; Ode, W.; Purnamasari, G.; Pato, U.; Susilowati, P.E. Identification and Genetic Diversity of Amylase Producing Lactic Acid Bacteria from Brown Rice (Oryza nivara) Wakawondu Cultivar Based on 16S rRNA Gene. Fermentation 2022, 8, 691. [Google Scholar] [CrossRef]
- Sridevi, A.; Narasimha, G.; Ramanjaneyulu, G.; Dileepkumar, K.; Reddy, B.R.; Devi, P.S. Saccharification of pretreated sawdust by Aspergillus niger cellulase. 3 Biotech 2015, 5, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Finley, R.B. American Society of Mammalogists American Society of Mammalogists. J. Mammal. 1959, 40, 591–594. [Google Scholar] [CrossRef]
- Shariq, M.; Sohail, M. Production of cellulase and xylanase from Candida tropicalis (MK-118) on purified and crude substrates. Pak. J. Bot. 2020, 52, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Ammouneh, H.; Harba, M.; Makee, H.; Nuclear, C.; Commission, S. Isolation and characterization of native Bacillus thuringiensis isolates from Syrian soil and testing their insecticidal activities against some insect pests Isolation and characterization of native Bacillus thuringiensis isolates from Syrian soil and tes. Turk. J. Agric. For. 2011, 35, 421–431. [Google Scholar] [CrossRef]
- Al-momani, F.; Obeidat, M. Ecology, toxicity, and hydrolytic activities of Bacillus thuringiensis in forests. Turk. J. Agric. For. 2013, 37, 76–82. [Google Scholar] [CrossRef]
- Ben Gharsa, H.; Bouri, M.; Mougou Hamdane, A.; Schuster, C.; Leclerque, A.; Rhouma, A. Bacillus velezensis strain MBY2, a potential agent for the management of crown gall disease. PLoS ONE 2021, 16, e0252823. [Google Scholar] [CrossRef]
- Lu, P.; Jiang, K.; Hao, Y.-Q.; Chu, W.-Y.; Xu, Y.-D.; Yang, J.-Y.; Chen, J.-L.; Zeng, G.-H.; Gu, Z.-H.; Zhao, H.-X. Profiles of Bacillus spp. Isolated from the Rhizosphere of Suaeda glauca and Their Potential to Promote Plant Growth and Suppress Fungal Phytopathogens. J. Microbiol. Biotechnol. 2021, 31, 1231–1240. [Google Scholar]
- Lu, M.; Gao, Z.; Xing, S.; Long, J.; Li, C.; He, L.; Wang, X. Purification, characterization, and chemical modification of Bacillus velezensis SN-14 fibrinolytic enzyme. Int. J. Biol. Macromol. 2021, 177, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chandra, A.; Dhar, A.; Shukla, P.; Baishya, D. Multi-efficient thermostable endoxylanase from Bacillus velezensis AG20 and its production of xylooligosaccharides as efficient prebiotics with anticancer activity. Process Biochem. 2021, 109, 59–71. [Google Scholar] [CrossRef]
- Doan, C.T.; Chen, C.L.; Nguyen, V.B.; Tran, T.N.; Nguyen, A.D.; Wang, S.L. Conversion of pectin-containing by-products to pectinases by Bacillus amyloliquefaciens and its applications on hydrolyzing banana peels for prebiotics production. Polymers 2021, 13, 1483. [Google Scholar] [CrossRef]
- Nawawi, M.H.; Ismail, K.I.; Sa’ad, N.; Mohamad, R.; Tahir, P.M.; Asa’ari, A.Z.; Saad, W.Z. Optimisation of Xylanase–Pectinase Cocktail Production with Bacillus amyloliquefaciens ADI2 Using a Low-Cost Substrate via Statistical Strategy. Fermentation 2022, 8, 119. [Google Scholar] [CrossRef]
- Jiang, L.; Duan, L.; Tian, X.; Wang, B.; Zhang, H.; Zhang, M.; Li, Z. NaCl salinity stress decreased Bacillus thuringiensis (Bt) protein content of transgenic Bt cotton (Gossypium hirsutum L.) seedlings. Environ. Exp. Bot. 2006, 55, 315–320. [Google Scholar] [CrossRef]
- Wekesa, T.B.; Wekesa, V.W.; Onguso, J.M.; Wafula, E.N.; Kavesu, N. Isolation and Characterization of Bacillus velezensis from Lake Bogoria as a Potential Biocontrol of Fusarium solani in Phaseolus vulgaris L. Bacteria 2022, 1, 279–293. [Google Scholar] [CrossRef]
- Sohail, M.; Siddiqi, R.; Ahmad, A.; Khan, S.A. Cellulase production from Aspergillus niger MS82: Effect of temperature and pH. N. Biotechnol. 2009, 25, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Tantayotai, P.; Rattanaporn, K.; Tepaamorndech, S.; Cheenkachorn, K.; Sriariyanun, M. Analysis of an Ionic Liquid and Salt Tolerant Microbial Consortium Which Is Useful for Enhancement of Enzymatic Hydrolysis and Biogas Production. Waste Biomass Valorization 2019, 10, 1481–1491. [Google Scholar] [CrossRef]
- Bağcıoğlu, M.; Fricker, M.; Johler, S.; Ehling-Schulz, M. Detection and identification of Bacillus cereus, Bacillus cytotoxicus, Bacillus thuringiensis, Bacillus mycoides and Bacillus weihenstephanensis via machine learning based FTIR spectroscopy. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Ejaz, U.; Ahmed, A.; Sohail, M. Statistical optimization of immobilization of yeast cells on corncob for pectinase production. Biocatal. Agric. Biotechnol. 2018, 14, 450–456. [Google Scholar] [CrossRef]
- Chandel, A.K.; Antunes, F.A.; Silva, M.B.; Da Silva, S.S. Unraveling the structure of sugarcane bagasse after soaking in concentrated aqueous ammonia (SCAA) and ethanol production by Scheffersomyces (Pichia) stipitis. Biotechnol. Biofuels 2013, 6, 102. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).