Abstract
Bioconversion of the complex carbohydrates present in lignocellulosic biomass into simple sugars, in order to obtain biofuels and bio-based products, is still limited by the low performance of the enzymatic saccharification reaction and the high cost of cellulolytic enzymes. Low-cost additives such as soybean protein can reduce the unproductive adsorption of cellulases onto lignin, increasing conversion rates and reducing enzyme losses. Here, investigation was made of the effects of different soybean protein fractions, extracted in-house, on the enzymatic saccharification of hydrothermally pretreated sugarcane bagasse. The glucose released during biomass saccharification increased by up to 76% in the presence of the in-house extracted soybean protein, compared to the control (without additive). A remarkable finding was that the technique allowed the enzyme loading to be decreased four-fold. The results suggested that the alkali-extracted proteins presented high surface hydrophobicity, which enhanced their interaction with lignin and reduced the unproductive binding of cellulases. Among the main soybean protein fractions, glycinin had the best effect in improving saccharification, which could have been due to its higher hydrophobicity. Hence, in-house extracted soybean proteins seem to be interesting alternative additives capable of increasing the lignocellulosic biomass conversion efficiency in future biorefineries.
1. Introduction
The production of biofuels and other bio-based products from the bioconversion of lignocellulosic biomass is of paramount importance for reducing fossil fuel dependence and moving society towards a more sustainable bioeconomy [1,2]. Although promising, this process still has some technological challenges, such as the high cost of cellulolytic enzymes and low biomass conversion efficiency [3,4]. Some factors that can affect the biomass saccharification process are the biomass physical–chemical characteristics, the production of inhibitory compounds during the pretreatment and hydrolysis steps, the solids loading used in the process, enzyme–lignin unproductive binding, among others [5,6]. For instance, cellulose fibers can present amorphous and crystalline structures. The ordered regions of cellulose are more difficult to hydrolyze due to the presence of strong hydrogen bonds [5,6]. Additionally, some inhibitors generated during the pretreatment step (sugars, acids, furans, aldehydes, and phenolic compounds) can negatively affect both the enzyme’s performance and the fermentation process. These compounds release and their concentration will vary from the pretreatment characteristics and their severity [7].
Performing the enzymatic saccharification with a high solids loading is a very desirable process condition, since it will increase the glucose released, and decrease the water consumption and waste generation [5,8]. However, with the operation under a high solids loading, a few issues can arise in the process such as water constraints, enzyme inhibition (e.g., a high concentration of glucose can inhibit the activity of β-glucosidases, cellobiohydrolases, and endoglucanases), and mass transfer limitations due to the high viscosity slurry [5,8]. One possible way of overcoming these problems would be by using a fed-batch system [9].
Another issue that can impact the process efficiency is the enzyme–lignin unproductive binding. Cellulases can bind to lignin due to hydrophobic, hydrogen-bonding and electrostatic interactions [10]. From all the cellulases, β-glucosidases are the ones that can be most negatively affected by this process [11]. One interesting strategy for overcoming the unproductive binding is the use of lignin-blocking additives to prevent the unproductive adsorption of cellulases onto lignin, consequently decreasing enzyme losses and increasing the saccharification efficiency [12,13,14,15].
Additives such as bovine serum albumin (BSA), Tween 20 and 80, polyethylene glycol (PEG), and soybean protein have been previously investigated for this application [13,14,16,17]. A recent study showed that soybean protein and BSA, used as additives, had equivalent effects in enzymatic biomass hydrolysis, maintaining enzyme activity at levels higher than 90% [18]. However, the cost of BSA can reach around $560/kg, so soybean protein could be a better alternative, since it is much less expensive ($1.25/kg) [19]. Previous studies have highlighted the use of soybean protein as a lignin-blocking additive, providing positive results at low cost [20,21]. For example, the addition of soybean protein during the enzymatic hydrolysis of sugarcane bagasse allowed a 50% reduction of the enzymatic dosage in the process, while maintaining the same efficiency as the control (without any additive). An enzyme loading reduction is desirable since the high cost of cellulolytic enzymes has a negative impact on the economics of the biomass saccharification process [20,22]. However, despite this impressive result, the additional cost of the soybean protein still has an impact on the biorefinery’s economic viability [20].
Soybean protein is a mixture of different proteins, with glycinin and β-conglycinin constituting approximately 70% of them [23,24]. Each type of protein presents distinct characteristics, considering aspects such as solubility and surface hydrophobicity [24,25,26]. For instance, native glycinin has a lower hydrophobicity than β-conglycinin; however, when both proteins are incubated for 5 h in a buffer with pH 3.5 or 10, glycinin’s hydrophobicity can become higher compared to β-conglycinin [27]. The pH of the medium can also change the characteristics of the proteins. When exposed to alkaline pH, the soybean protein structure unfolds, its solubility increases, and the hydrophobic groups of the protein become more exposed [27,28]. Therefore, it would be of great interest to better understand the roles that these different soybean protein fractions could play when used as lignin-blocking additives during the enzymatic saccharification of lignocellulosic biomass, increasing the enzyme’s efficiency and possibly moving towards the feasibility of this process.
The present work investigates the effect of an in-house extracted soybean protein (by using an alkaline medium) as an additive to reduce the unproductive adsorption of enzymes during the enzymatic hydrolysis of hydrothermally pretreated sugarcane bagasse. Additionally, the soybean proteins were fractionated in order to determine which component (glycinin, β-conglycinin, and whey proteins) has the best effect on increasing the biomass saccharification. In this sense, our findings show that the extraction method can affect how the proteins act on enhancing the enzymatic hydrolysis of lignocellulosic biomass. Additionally, we also report for the first time that not all the soybean protein components have the same behavior as a lignin-blocking additive which are critical for understanding the enzymatic breakdown of lignocellulosic materials.
2. Materials and Methods
2.1. Materials
Sugarcane bagasse (with a moisture of ~9% and kindly donated by the Ipiranga Agroindustrial sugarcane mill, São Paulo state, Brazil) was ground in a knife mill (particle size < 2 mm) and then hydrothermally pretreated in a 5 L reactor (model 4580, Parr Instruments) at 195 °C for 10 min, using a solids loading of 15% (w/v). After the pretreatment, the bagasse was dried and characterized, presenting a composition of 52.8% glucan, 2.8% xylan, 25.2% lignin, and 0.8% ashes [29]. The commercial enzymatic cocktail used in the biomass saccharification reactions was Cellic CTec3 (Novozymes, Brazil). Its cellulolytic activity was determined according to the methodology reported by Ghose [30], in which the Filter Paper Activity (FPU/mL) of the commercial enzymatic cocktail was 152.3 FPU/mL of enzyme. This value is utilized for the precise and careful determination of all enzyme dosages during saccharification experiments. All the enzymatic hydrolysis reactions were carried out in sodium citrate buffer (50 mM, pH 4.8). Soybean protein was extracted in-house from soybean flour (without fat removal) or was obtained commercially (protein content ≥ 90%, Bremil, Brazil).
2.2. Soybean Protein Extraction Protocol
The extraction of protein from raw soybean flour was based on the methodology reported previously [31,32]. Briefly, raw soybean flour (10% w/v) was kept on a shaker under agitation (200 rpm) in a 3.5% (w/v) NaOH solution for 24 h, at 50 °C. The suspension was then centrifuged for 10 min at 5 °C and 8000 rpm. The supernatant containing the protein was separated and HCl solution (3.5% v/v) was added to reduce the pH to around 4, so that the protein was precipitated. The samples were centrifuged, and the precipitated protein was separated and resuspended in the enzymatic hydrolysis buffer (50 mM sodium citrate buffer, pH 4.8) for posterior use on the enzymatic saccharification. A schematic representation of the in-house soybean protein extraction and its effect during the enzymatic saccharification is shown in Figure 1a.
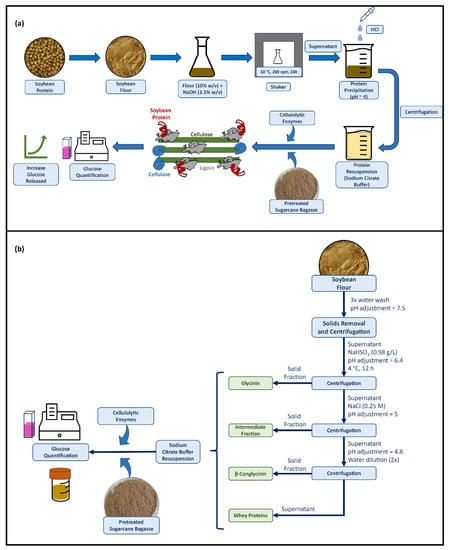
Figure 1.
(a) Schematic representation of the in-house soybean protein extraction and its effect during the enzymatic hydrolysis of the hydrothermally pretreated sugarcane bagasse. (b) Flowchart of the soybean protein compounds extraction.
For fractionation of the soybean proteins (glycinin, β-conglycinin, and whey proteins), the extraction protocol was based on the methodology reported by Wu et al. [24], without the oil extraction step. Firstly, the soybean flour was washed three times with distilled water, followed by adjusting the solution pH to 7.5 and keeping it under stirring for 1 h. The solution was passed through a steel sieve (120 mesh) to remove some solids and the resulting solution was centrifuged for 30 min at 8000 rpm and 5 °C. The supernatant was separated, NaHSO3 (0.98 g/L) was added to the solution, the pH was adjusted to 6.4, and the solution was stored at 4 °C for 12 h. After this period, the solution was centrifuged at 8000 rpm for 20 min, obtaining glycinin in the precipitate, which was resuspended in the sodium citrate enzymatic hydrolysis buffer. NaCl (0.25 M) was added to the supernatant, the pH was adjusted to 5, and stirring was continued for 1 h. The solution was centrifuged again for 30 min, obtaining an intermediate protein mixture in the precipitate. The supernatant was adjusted to pH 4.8 and diluted 2-fold with water, followed by a final centrifugation step (20 min, 5 °C, 8000 rpm). The resulting precipitate contained β-conglycinin and the supernatant contained whey protein. All three separated fractions were resuspended in the enzymatic hydrolysis buffer, with adjustment of the mass fraction to 12% (w/w), prior to their separate usage as additives in the saccharification reactions. The schematic representation of the soybean protein compounds extraction can be seen in Figure 1b.
2.3. Enzymatic Hydrolysis
Saccharification of the hydrothermally pretreated sugarcane bagasse with the commercial enzymatic cocktail Cellic CTec3 (Novozymes, Brazil) was carried out in the presence and absence of soybean protein (in-house extracted fractions and the commercial product) and whole soybean flour. The experiments were performed in 5 mL flasks, with incubation for 24 h in a rotational incubator at 50 °C and 30 rpm, using a solids loading of 15% (w/v), enzyme dosage of 10 FPU/g, and additive loading of 12% (w/w), according to the methodology of Brondi et al. [20]. Additional experiments were conducted with variation of the enzyme dosage (2.5, 5, and 10 FPU/g of bagasse), keeping the other hydrolysis conditions the same as described above.
After fractionation of the soybean protein, the components obtained (glycinin, β-conglycinin, an intermediate fraction, and whey proteins) were also evaluated as additives in sugarcane bagasse saccharification. The mass fractions of these components were previously set to 12% (w/w) in the hydrolysis buffer. The saccharification conditions were as described above: 15 (w/v) solids loading, 10 FPU/g enzymes loading, 24 h reaction time, and 50 °C.
After the enzymatic saccharification experiments, the supernatants were centrifuged (11,000 rpm, 5 °C, 15 min) to remove any solids present. All the experiments were performed in triplicate and the results were presented as mean ± standard deviation. The mean values were compared by the Tukey’s test (p < 0.05). Hydrolysis of the proteins alone (without the bagasse) was also performed, in order to account for any glucose released by the additive.
2.4. Glucose and Protein Quantification
The glucose released from the enzymatic hydrolysis reactions was determined using a glucose oxidase enzymatic assay kit (Labtest, Brazil), where the concentration was obtained from the absorbance of the sample at 505 nm, measured with a UV/Vis spectrophotometer. The protein quantification was carried out by a Pierce BCA (bicinchoninic acid) Protein Assay Kit (Thermo Scientific, Waltham, MA, USA), where the concentration was determined by measuring the sample absorbance using UV/Vis spectrophotometer (562 nm) [12].
3. Results and Discussion
3.1. Effects of In-House Extracted and Commercial Soybean Proteins
The positive effect of soybean protein in improving glucose release during the enzymatic hydrolysis of lignocellulosic biomass has been reported previously [12,17,20,21]. Here, instead of using a commercial source of soybean protein, the approach adopted was to use an in-house extracted soybean protein. Firstly, the protein was extracted from the whole soybean flour, without the oil extraction step (Figure 1a). Typically, a soybean seed contains approximately 36–40% protein in its composition [23,33]. The protein extraction method applied, using 3.5% (w/v) NaOH [23,31,32], resulted in extraction of approximately 48% (w/v) of the total protein content. Therefore, after the extraction process, the residual solid material containing fibers, oil, carbohydrates, and proteins (since half of the protein content was not extracted) could be used in other applications, such as human or animal feed, in the biorefinery context.
After the extraction process, the in-house soybean protein was applied as an additive during the enzymatic saccharification of hydrothermally pretreated sugarcane bagasse. Its effect was compared to the use of a commercial soybean protein and whole soybean flour (obtained after the grinding of the soybean seed, without the fat extraction step). The effects of the additives on the concentration of glucose released during the hydrolysis reaction of pretreated sugarcane bagasse are shown in Figure 2.
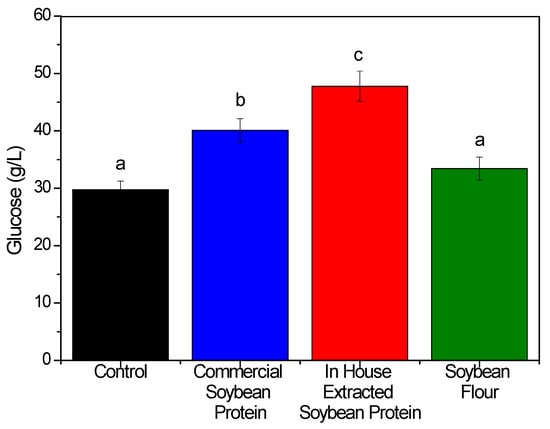
Figure 2.
Glucose released (g/L) after the enzymatic hydrolysis of hydrothermally pretreated sugarcane bagasse without additive (control) and in the presence of commercial soybean protein (blue bar), in-house extracted soybean protein (red bar), and soybean flour (green bar). The hydrolysis conditions were 15% (w/v) bagasse, 12% (w/w) soybean protein, 10 FPU/g enzyme loading, and 24 h of reaction at 50 °C. The different letters above each bar represent a significant difference between the values (Tukey’s test, p < 0.05).
The saccharification results obtained using the commercial soybean protein showed that its addition was able to increase glucose release by 34%, compared to the control (without any additive). On the other hand, the use of whole soybean flour did not result in a statistically significant increase compared to the control. This material consisted of a mixture of fibers, carbohydrates, proteins, soy lecithin, and soy saponin (components that can exhibit surfactant behavior). Although both the proteins and saponin could help to enhance the glucose production, no positive effect was observed here [16].
Surprisingly, the in-house extracted soybean protein was able to improve the glucose release by 61%, compared to the control (47.8 and 29.7 g/L, respectively). Hence, its addition was more effective in reducing the unproductive adsorption of cellulases and increasing the glucose release, compared to the commercial soybean protein reported previously (in which improvements of ~28% were obtained for the same conditions here evaluated) [20]. It is important to highlight that this protein extracted in-house would probably have a lower cost than the commercial product, since fat extraction using organic solvents was not employed here and a final drying step was not required, since the precipitated soybean protein was directly resuspended in the biomass enzymatic hydrolysis buffer. According to the Brazilian foreign trade reports for the year of 2022 (General Exports and Imports information—http://comexstat.mdic.gov.br/en/geral (accessed on 19 January 2023)), soybean protein presented an average export price of 3.6 US$/kg. It means that the in-house extracted protein will end up with a price lower than this one (because the fat extraction with hexane and the final protein spray-dryer will not be carried out). However, a more precise cost can be obtained only after a more detailed technical–economic evaluation.
The treatment of soybean protein at high (alkaline) pH can significantly change its structure [27,28], with the structure unfolding, its solubility increasing, and the hydrophobic groups of the protein becoming more exposed [27,28]. This increase in surface hydrophobicity could be the reason that the in-house extracted soybean protein was able to enhance the saccharification efficiency, in comparison to the control and use of the commercial soybean protein. Higher hydrophobicity could increase the interaction between the protein and lignin, reducing the unproductive binding of enzymes [34,35].
Since the effect of the in-house extracted soybean protein in enhancing the enzymatic hydrolysis of sugarcane bagasse was superior to that of the commercial product, evaluation was also made of the effect of the NaOH concentration (1, 2, and 3.5% w/v) used in the protein extraction procedure. After the extraction, the resulting proteins were used as additives during the biomass saccharification (Figure 3). The concentration of glucose released was improved using soybean protein from the different extraction protocols, but variation of the NaOH concentration in the extraction did not influence the soy protein performance. Therefore, the subsequent experiments employed soybean protein extracted using 1% (w/v) NaOH solution.
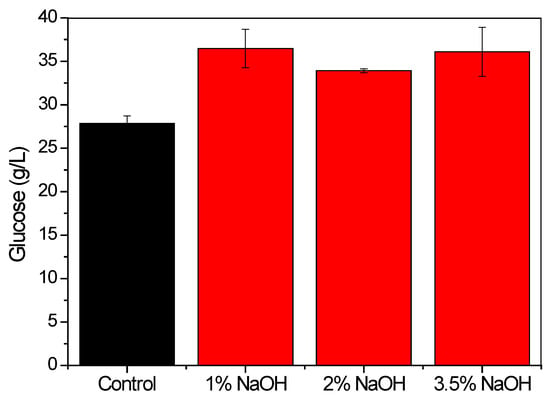
Figure 3.
Effect of the NaOH content (% w/v) used in the extraction of protein from the soybean flour. After extraction, the protein was used in the hydrolysis of sugarcane bagasse performed with bagasse loading of 15% (w/v), 12% (w/w) additive, and 10 FPU/g enzyme, for 24 h at 50 °C. Error bars represent the standard deviation.
3.2. Effect of Enzyme Loading
Experiments with variation of the enzymatic loading were carried out to determine whether the additives could enable higher glucose release when using low enzyme dosages. For this, enzyme loadings of 2.5, 5, and 10 FPU/g were tested, in the presence and absence of in-house soybean protein (12% w/v). As shown in Figure 4, all the hydrolysis conditions with the additive presented higher glucose release, compared to the corresponding controls with the same enzyme loading. Increases of 72, 76, and 55% were obtained for 2.5, 5, and 10 FPU/g, respectively, in comparison to the controls without additive.
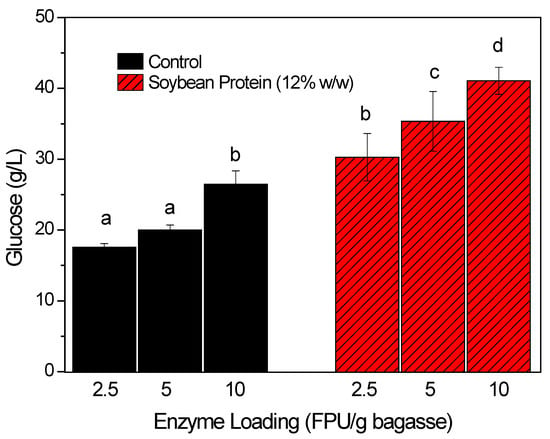
Figure 4.
Enzymatic saccharification using different enzyme loadings (2.5, 5, and 10 FPU/g) in the absence (black bars) and presence (red/stripped bars) of the in-house extracted soybean protein (12 w/w). The hydrolysis was carried out for 24 h at 50 °C, with 15% (w/v) bagasse. The different letters above each bar represent a significant difference between the values (Tukey’s test, p < 0.05).
The addition of in-house extracted soybean protein was able to maintain high glucose release, even at low enzyme dosages (Figure 4). For example, saccharification in the presence of the additive, with enzyme loading of 2.5 FPU/g, released a higher amount of glucose (30.3 g/L) than the control reaction using 10 FPU/g (26.5 g/L). This was an excellent result, since it showed that the addition of soybean protein enabled a 75% reduction of the enzyme loading (a four-fold decrease), while maintaining the same hydrolysis efficiency. A previous study with commercial soybean protein that employed the same hydrolysis conditions allowed a reduction of 50% (10 to 5 FPU/g) of the enzyme dosage maintaining the same glucose yield [20]. It means that the in-house extracted soybean protein allowed for the achievement of more significant improvements.
The enzyme dosage is the variable that most affects the feasibility of the second generation (2G) ethanol production process, due to the high cost of the cellulolytic enzymes [4,20]. Therefore, the use of in-house extracted protein could be a very attractive way to shift the enzymatic saccharification of lignocellulosic biomass towards economic feasibility. Furthermore, it is important to highlight that the extraction process proposed here should result in a low-cost additive, since it avoids the usual extraction of fat with organic solvents [23].
3.3. Effects of the Soybean Protein Fractions on Enzymatic Hydrolysis
Glycinin and β-conglycinin are the main compounds in soybean protein (around 70%). Each type of protein presents distinct characteristics, considering parameters such as surface hydrophobicity and solubility [24,25,26]. Therefore, in order to evaluate the effects of the two main proteins in the enzymatic hydrolysis of hydrothermally pretreated sugarcane bagasse, these components were extracted using the methodology reported by Wu et al. [24] and are also presented by Figure 1b. Figure 5 shows the glucose released after hydrolysis in the presence of the whole soybean protein, glycinin, an intermediate fraction, β-conglycinin, and the whey proteins. For each protein, the mass fraction was adjusted to 12% (w/w), prior to the biomass enzymatic hydrolysis reactions.
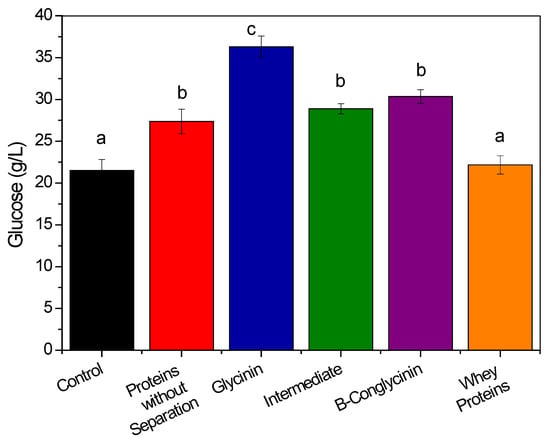
Figure 5.
Effects of different protein fractions in the hydrolysis of sugarcane bagasse. The protein extraction was performed under mild conditions. The different letters above each bar represent a significant difference between the values (Tukey’s test, p < 0.05).
Firstly, it is important to highlight that the effect of the whole protein present in Figure 5 (red bar) released approximately 27.4 g/L of glucose, the whole soybean protein from Figure 2 released ~47.8 g/L. This difference can be explained by the different extraction methods applied, in which the soybean flour was incubated in a mild pH buffer (7.5) and in a very alkaline pH (>12), respectively. This result corroborates the information presented previously (Section 3.1), in which the medium pH can change the proteins characteristics. In this sense, a more alkaline pH will enhance the protein effect during the enzymatic saccharification.
As shown in Figure 5, the addition of glycinin to the reaction medium led to the best performance in enhancing the glucose release, in comparison to the control (36.2 and 21.5 g/L, respectively). This result was even better than the effect of the whole soybean protein (glucose release of 27.4 g/L). The other protein compounds had either an effect similar to that of the protein before the separation (intermediate fraction and β-conglycinin, with 34 and 41% increases of glucose release, respectively, compared to the control) or an inferior effect (whey proteins, with a 3% increase). The better performance of glycinin (68% increase) could have been due to its higher surface hydrophobicity at the hydrolysis pH [27], which would increase its interaction with lignin and decrease the unproductive adsorption of enzymes, consequently enhancing the hydrolysis efficiency. Some characteristics of glycinin and β-conglycinin are summarized in Table 1 and as can be seen, when exposed for 4 h in a solution pH of 3.5 (near the 4.8 hydrolysis pH), glycinin presented a surface hydrophobicity of 290, while the one for β-Conglycinin was 260 [27].

Table 1.
Some characteristics of glycinin and β-conglycinin.
Therefore, the findings of the present work showed that although soybean protein has an excellent effect in increasing the release of glucose from the saccharification of lignocellulosic biomass, this positive effect is not related to all the proteins present in its structure. Surface hydrophobicity plays an important role in unproductive enzyme adsorption [25], so the differences in hydrophobicity among the soybean proteins are expected to be reflected in the effects of the proteins during the hydrolysis. As discussed above, soybean protein (all fractions) can significantly increase glucose release, maintaining up to 90% enzyme activity [18]. These results provide evidence that certain protein fractions can substantially enhance the release of glucose, while other fractions, due to their characteristics, are much less effective.
4. Conclusions
The results presented here show that in-house extracted soybean protein is an effective additive that can increase the release of glucose from hydrothermally pretreated sugarcane bagasse. Its addition led to increases of up to 76%, while enabling a four-fold decrease in the enzyme loading, maintaining the same glucose release obtained using a loading of 10 FPU/g. The extraction protocol is extremely important for the performance of soybean protein during the saccharification process, since the use of an alkaline medium can enhance the hydrophobicity of the protein and increase its effect in reducing the unproductive adsorption of enzymes. These findings can assist in overcoming the technological barriers related to lignocellulosic biomass conversion in future biorefineries.
Author Contributions
I.R.S.: Conceptualization, investigation, data analysis, writing; M.G.B.: Conceptualization, methodology, investigation, data analysis, writing. C.S.F.: Conceptualization, methodology, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for financial support provided by the following Brazilian research funding agencies: National Council for Scientific and Technological Development (CNPq, grants 141303/2019-0, 126802/2021-1, and 407314/2018-0), Coordination for the Improvement of Higher Education Personnel (CAPES, Finance Code 001) and State of São Paulo Research Foundation (FAPESP, grants 2016/10636-8 and 2019/25261-8).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Freitas, J.V.; Bilatto, S.; Squinca, P.; Pinto, A.S.S.; Brondi, M.G.; Bondancia, T.J.; Batista, G.; Klaic, R.; Farinas, C.S. Sugarcane biorefineries: Potential opportunities towards shifting from wastes to products. Ind. Crops Prod. 2021, 172, 114057. [Google Scholar] [CrossRef]
- Melendez, J.R.; Mátyás, B.; Hena, S.; Lowy, D.A.; El Salous, A. Perspectives in the production of bioethanol: A review of sustainable methods, technologies, and bioprocesses. Renew. Sustain. Energy Rev. 2022, 160, 112260. [Google Scholar] [CrossRef]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; Dong, C.-D.; Patel, A.K.; Puri, M. Global status of lignocellulosic biorefinery: Challenges and perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef] [PubMed]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Verma, A.; Singhania, R.R.; Varjani, S.; Di Dong, C.; Kumar Patel, A. Current understanding of the inhibition factors and their mechanism of action for the lignocellulosic biomass hydrolysis. Bioresour. Technol. 2021, 332, 125042. [Google Scholar] [CrossRef] [PubMed]
- Kumar Saini, J.; Himanshu; Hemansi; Kaur, A.; Mathur, A. Strategies to enhance enzymatic hydrolysis of lignocellulosic biomass for biorefinery applications: A review. Bioresour. Technol. 2022, 360, 127517. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Mukasekuru, M.R.; Hu, J.; Zhao, X.; Sun, F.F.; Pascal, K.; Ren, H.; Zhang, J. Enhanced High-Solids Fed-Batch Enzymatic Hydrolysis of Sugar Cane Bagasse with Accessory Enzymes and Additives at Low Cellulase Loading. ACS Sustain. Chem. Eng. 2018, 6, 12787–12796. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017, 35, 466–489. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Adsorption of enzyme onto lignins of liquid hot water pretreated hardwoods. Biotechnol. Bioeng. 2015, 112, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Brondi, M.G.; Pinto, A.S.; Farinas, C.S. Combining additives improves sugars release from hydrothermally pretreated sugarcane bagasse in integrated 1G-2G biorefineries. Bioresour. Technol. Rep. 2021, 15, 100819. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Lu, Y.; Zhang, H.; Hua, Q.; Bi, R.; Rojas, O.; Renneckar, S.; Fan, S.; Xiao, Z.; et al. The pre-addition of “blocking” proteins decreases subsequent cellulase adsorption to lignin and enhances cellulose hydrolysis. Bioresour. Technol. 2023, 367, 128276. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef]
- Bhagia, S.; Dhir, R.; Kumar, R.; Wyman, C.E. Deactivation of Cellulase at the Air-Liquid Interface Is the Main Cause of Incomplete Cellulose Conversion at Low Enzyme Loadings. Sci. Rep. 2018, 8, 1350. [Google Scholar] [CrossRef] [PubMed]
- Brondi, M.G.; Vasconcellos, V.M.; Giordano, R.C.; Farinas, C.S. Alternative Low-Cost Additives to Improve the Saccharification of Lignocellulosic Biomass. Appl. Biochem. Biotechnol. 2019, 187, 461–473. [Google Scholar] [CrossRef]
- Almeida, R.M.R.G.; Pimentel, W.R.O.; Santos-Rocha, M.S.R.; Buffo, M.M.; Farinas, C.S.; Ximenes, E.A.; Ladisch, M.R. Protective effects of non-catalytic proteins on endoglucanase activity at air and lignin interfaces. Biotechnol. Prog. 2021, 37, e3134. [Google Scholar] [CrossRef] [PubMed]
- Madadi, M.; Song, G.; Sun, F.; Sun, C.; Xia, C.; Zhang, E.; Karimi, K.; Tu, M. Positive role of non-catalytic proteins on mitigating inhibitory effects of lignin and enhancing cellulase activity in enzymatic hydrolysis: Application, mechanism, and prospective. Environ. Res. 2022, 215, 114291. [Google Scholar] [CrossRef]
- Brondi, M.G.; Elias, A.M.; Furlan, F.F.; Giordano, R.C.; Farinas, C.S. Performance targets defined by retro-techno-economic analysis for the use of soybean protein as saccharification additive in an integrated biorefinery. Sci. Rep. 2020, 10, 7367. [Google Scholar] [CrossRef]
- Pinto, A.S.S.; Brondi, M.G.; de Freitas, J.V.; Furlan, F.F.; Ribeiro, M.P.A.; Giordano, R.C.; Farinas, C.S. Mitigating the negative impact of soluble and insoluble lignin in biorefineries. Renew. Energy 2021, 173, 1017–1026. [Google Scholar] [CrossRef]
- Longati, A.A.; Lino, A.R.A.; Giordano, R.C.; Furlan, F.F.; Cruz, A.J.G. Defining research & development process targets through retro-techno-economic analysis: The sugarcane biorefinery case. Bioresour. Technol. 2018, 263, 1–9. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Zuidam, N.J. Whole soybean protein extraction processes: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 163–172. [Google Scholar] [CrossRef]
- Wu, S.; Murphy, P.A.; Johnson, L.A.; Fratzke, A.R.; Reuber, M.A. Pilot-plant fractionation of soybean glycinin and β-conglycinin. J. Am. Oil Chem. Soc. 1999, 76, 285–293. [Google Scholar] [CrossRef]
- Maruyama, N.; Prak, K.; Motoyama, S.; Choi, S.; Yagasaki, K.; Ishimoto, M.; Utsumi, S. Structure−Physicochemical Function Relationships of Soybean Glycinin at Subunit Levels Assessed by Using Mutant Lines. J. Agric. Food Chem. 2004, 52, 8197–8201. [Google Scholar] [CrossRef]
- Tezuka, M.; Yagasaki, K.; Ono, T. Changes in Characters of Soybean Glycinin Groups I, IIa, and IIb Caused by Heating. J. Agric. Food Chem. 2004, 52, 1693–1699. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L.; Chen, J. Role of β-Conglycinin and Glycinin Subunits in the pH-Shifting-Induced Structural and Physicochemical Changes of Soy Protein Isolate. J. Food Sci. 2011, 76, C293–C302. [Google Scholar] [CrossRef]
- Yan, S.; Xu, J.; Zhang, X.; Xie, F.; Zhang, S.; Jiang, L.; Qi, B.; Li, Y. Effect of pH-shifting treatment on the structural and functional properties of soybean protein isolate and its interactions with (–)-epigallocatechin-3-gallate. Process Biochem. 2021, 101, 190–198. [Google Scholar] [CrossRef]
- Pinto, A.S.S.; Elias, A.M.; Furlan, F.F.; Ribeiro, M.P.A.; Giordano, R.C.; Farinas, C.S. Techno-Economic Feasibility of Biomass Washing in 1G2G Sugarcane Biorefineries. Bioenergy Res. 2021, 14, 1253–1264. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Deak, N.A.; Johnson, L.A.; Lusas, E.W.; Rhee, K.C. Soy Protein Products, Processing, and Utilization. In Soybeans; Elsevier: Amsterdam, The Netherlands, 2008; pp. 661–724. [Google Scholar]
- Wagner, J.R.; Sorgentini, D.A.; Añón, M.C. Relation between Solubility and Surface Hydrophobicity as an Indicator of Modifications during Preparation Processes of Commercial and Laboratory-Prepared Soy Protein Isolates. J. Agric. Food Chem. 2000, 48, 3159–3165. [Google Scholar] [CrossRef]
- Liu, K. Chemistry and Nutritional Value of Soybean Components. In Soybeans; Springer: Boston, MA, USA, 1997; pp. 25–113. [Google Scholar]
- Börjesson, J.; Peterson, R.; Tjerneld, F. Enhanced enzymatic conversion of softwood lignocellulose by poly(ethylene glycol) addition. Enzyme Microb. Technol. 2007, 40, 754–762. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, Z.; Zhang, R.; Wang, D.; Jenkins, B. Non-ionic Surfactants and Non-Catalytic Protein Treatment on Enzymatic Hydrolysis of Pretreated Creeping Wild Ryegrass. Appl. Biochem. Biotechnol. 2008, 146, 231–248. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, J.; Mei, L.; Wang, Q. Ionic Strength and pH Responsive Permeability of Soy Glycinin Microcapsules. Langmuir 2018, 34, 9711–9718. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, J.R.; Li, K.K.; Yin, S.W.; Wang, J.M.; Zhu, J.H.; Yang, X.Q. Characterization of soy β-conglycinin-dextran conjugate prepared by Maillard reaction in crowded liquid system. Food Res. Int. 2012, 49, 648–654. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).