Survey on Phthalates in Beer Packaged in Aluminum Cans, PET and Glass Bottles
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. GC–MS Analysis and Instrumentation
2.3. Calibration
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef] [PubMed]
- Holadová, K.; Prokůpková, G.; Hajšlová, J.; Poustka, J. Headspace solid-phase microextraction of phthalic acid esters from vegetable oil employing solvent-based matrix modification. Anal. Chim. Acta 2007, 582, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Cao, X.I. Phthalate esters in foods: Sources, occurrence and analytical methods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 21–43. [Google Scholar] [CrossRef]
- Horák, T.; Olšovská, J. Phthalates in Beverages—A Review. Kvas. Prum. 2020, 66, 264–269. [Google Scholar] [CrossRef]
- Rudel, R.A.; Perovich, L.J. Endocrine disrupting chemicals in indoor and outdoor air. Atmos. Environ. 2009, 43, 170–181. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Phthalates Action Plan, Revised 03/14/2012. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/phthalates_actionplan_revised_2012-03-14.pdf (accessed on 1 November 2022).
- Jurica, K.; Uršulin-Trstenjak, N.; Vukić Lušić, D.; Lušić, D.; Smit, Z. Exposure to phtalates and their presence in alcoholic beverages. Arh. Hig. Rada Toksikol. 2013, 64, 317–325. [Google Scholar] [CrossRef]
- Chatonnet, P.; Boutou, S.; Plana, A. Contamination of wines and spirits by phthalates: Types of contaminants present, contamination sources and means of prevention. Food Addit. Contam. Part A 2014, 31, 1605–1615. [Google Scholar] [CrossRef]
- Jurica, K.; Brcic, K.I.; Lasi’c, D.; Vukic, L.D.; Anic, J.S.; Lusic, D. Determination of phthalates in plum spirit and their occurrence during plum spirit production. Acta Aliment. 2016, 45, 141–148. [Google Scholar] [CrossRef]
- Pellegrino Vidal, R.; Ibañez, G.; Escandar, G. A green method for the quantification of plastics-derived endocrine disruptors in beverages by chemometrics-assisted liquid chromatography with simultaneous diode array and fluorescent detection. Talanta 2016, 159, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Gagula, G.; Mastanjević, K.; Mastanjević, K.; Krstanović, V.; Horvat, D.; Magdić, D. The influence of packaging material on volatile compounds of pale lager beer. Food Packag. Shelf Life 2020, 24, 100496. [Google Scholar] [CrossRef]
- Page, A.; Pira International Ltd. Plastic Packaging Market Trends and Forecasts. Available online: https://www.slideshare.net/adampage1976/plastic-packaging-market-trends-and-forecasts (accessed on 17 April 2022).
- Hunt, P.K. Los Microplásticos En El Agua Potable “No Parecen Representar Un Riesgo Para La Salud”, Dice La OMS. 2019. Available online: https://cnnespanol.cnn.com/2019/08/22/los-microplasticos-en-el-agua-potableno-parecen-representar-un-riesgo-para-la-salud-dice-la-oms/ (accessed on 6 April 2022).
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.; Carney Almroth, B.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Habschied, K.; Mastanjević, K.; Šibalić, M.; Krstanović, V.; Galić, V. A Survey on Detection of Plastic-Related Chemicals in Beer Packaged in PET Using FT-IR Technology. Beverages 2022, 8, 73. [Google Scholar] [CrossRef]
- Nurlatifah; Nakata, H. Monitoring of polymer type and plastic additives in coating film of beer cans from 16 countries. Sci. Rep. 2021, 11, 22115. [Google Scholar] [CrossRef]
- European Commission (EC). Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. 2011. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R0010 (accessed on 7 December 2022).
- Bičak, D. BiH Najveći Uvoznik Piva, Zbog Krize Svuda Raste Prodaja U Pet Ambalaži. Available online: https://www.poslovni.hr/svijet-i-regija/bih-najveci-uvoznik-piva-zbog-krize-svuda-raste-prodaja-u-pet-ambalazi-254488 (accessed on 1 November 2022).
- Yadav, S.; Rai, S.; Srivastava, A.K.; Panchal, S.; Patel, D.K.; Sharma, V.P.; Jain, S.; Srivastava, J.P. Determination of pesticide and phthalate residues in tea by QuEChERS method and their fate in processing. Environ. Sci. Pollut. Res. 2017, 24, 3074–3083. [Google Scholar] [CrossRef]
- Carnol, L.; Schummer, C.; Moris, G. Quantification of Six Phthalates and One Adipate in Luxembourgish Beer Using HS-SPME-GC/MS. Food Anal. Methods 2017, 10, 298–309. [Google Scholar] [CrossRef]
- Kartalović, B.; Vranešević, J.; Petrović, J.; Đurđević, B.; Ratajac, R. Detekcija ostataka mikroplastike—Razvoj metode za ftalate u medu. Arh. Vet. Med. 2021, 14, 19–33. [Google Scholar]
- Wittassek, M.; Koch, H.; Angerer, J.; Brüning, T. Assessing exposure to phthalates—The human biomonitoring approach. Mol. Nutr. Food Res. 2011, 55, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.-W.; Gao, J.; Yang, C.; Liu, X.-J.; Li, X.-J.; Pan, S.-Y. Development and application of an SPME/GC method for the determination of trace phthalates in beer using a calix [6] arene fiber. Anal. Chim. Acta 2009, 641, 64–74. [Google Scholar] [CrossRef]
- Olšovská, J.; Jandovská, J.; Běláková, S.; Kubizniaková, P.; Vrzal, T.; Štěrba, K. Monitoring of potential contaminants in beer from the Czech Republic. Kvas. Prum. 2019, 65, 84–96. [Google Scholar] [CrossRef]
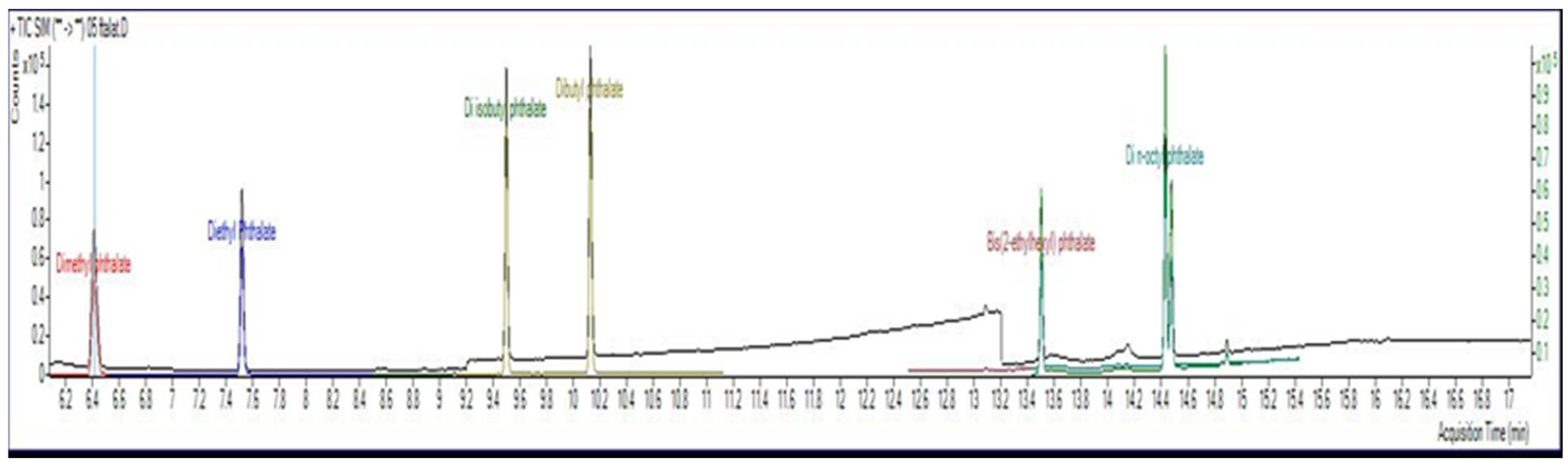
| Compound | LOQ µg/mL | LOD µg/mL |
|---|---|---|
| Dimethyl phthalate | 0.00469 | 0.00141 |
| Diethyl phthalate | 0.00418 | 0.00030 |
| Diisobutyl phthalate | 0.00101 | 0.00030 |
| Dibutyl phthalate | 0.00101 | 0.00030 |
| Bis (2-ethylhexyl) phthalate | 0.00141 | 0.00032 |
| Di-n-octyl phthalate | 0.001101 | 0.00031 |
| Sample | Dimethyl Phthalate | Diethyl Phthalate | Diisobutyl Phthalate | Dibutyl Phthalate | Bis (2-Ethylhexyl) Phthalate | Di-n-Octyl Phthalate | Sum |
|---|---|---|---|---|---|---|---|
| P1 | <LOQ | 0.46 p | 17.28 m | 29.73 o | 15.46 n | <LOQ | 62.93 p |
| P2 | <LOQ | 5.13 g | 25.93 g | 41.75 k | 27.298 i | <LOQ | 100.10 l |
| P3 | <LOQ | 9.16 d | 28.04 e | 51.44 g | 24.99 k | <LOQ | 113.62 h |
| P4 | <LOQ | 23.08 a | 25.97 g | 49.11 h | 29.11 g | <LOQ | 127.27 g |
| P5 | <LOQ | 9.97 c | 43.30 c | 73.29 c | 53.13 d | <LOQ | 179.69 c |
| P6 | <LOQ | 4.76 j | 22.73 j | 37.68 n | 9.12 p | <LOQ | 74.29 o |
| P7 | <LOQ | 4.28 l | 28.22 e | 61.10 e | 48.46 e | <LOQ | 142.06 f |
| P8 | <LOQ | 2.10 n | 25.46 h | 40.01 l | 23.07 l | <LOQ | 90.64 m |
| P9 | <LOQ | 5.61 f | 30.24 d | 62.75 d | 85.74 b | <LOQ | 184.33 b |
| P10 | <LOQ | 14.21 b | 43.72 b | 86.93 b | 74.96 c | <LOQ | 219.82 a |
| P11 | <LOQ | 4.63 k | 24.14 i | 54.65 f | 25.22 j | <LOQ | 108.64 i |
| P12 | <LOQ | 4.87 i | 20.65 l | 38.15 m | 11.15 o | <LOQ | 74.82 n |
| P13 | <LOQ | 4.95 h | 56.96 a | 92.17 a | 17.16 m | <LOQ | 171.24 e |
| P14 | <LOQ | 1.29 o | 26.88 f | 54.46 f | 95.06 a | <LOQ | 177.68 d |
| P15 | <LOQ | 6.53 e | 24.09 i | 47.61 i | 28.49 h | <LOQ | 106.72 k |
| P16 | <LOQ | 2.39 m | 22.30 k | 44.65 j | 37.95 f | <LOQ | 107.29 j |
| Sample | Dimethyl Phthalate | Diethyl Phthalate | Diisobutyl Phthalate | Dibutyl Phthalate | Bis (2-Ethylhexyl) Phthalate | Di-n-Octyl Phthalate | Sum |
|---|---|---|---|---|---|---|---|
| C1 | <LOQ | 2.46 bc | 2.63 a | 2.95 b | 326.81 a | <LOQ | 334.86 a |
| C2 | <LOQ | 2.36 c | 2.39 b | 2.79 c | 313.15 b | <LOQ | 318.64 c |
| C3 | <LOQ | 2.35 c | 2.35 bc | 2.49 d | 326.71 a | <LOQ | 333.90 a |
| C4 | <LOQ | 2.49 b | 2.75 a | 3.07 a | 313.15 b | <LOQ | 321.47 b |
| C5 | <LOQ | 1.72 f | 2.07 e | 2.22 e | 131.20 g | <LOQ | 137.22 h |
| C6 | <LOQ | 4.15 a | 2.75 a | 2.72 c | 241.26 d | <LOQ | 250.89 e |
| C7 | <LOQ | 2.07 d | 2.22 cd | 4.41 d | 121.19 h | <LOQ | 127.91 i |
| C8 | <LOQ | 1.95 e | 2.07 e | 2.22 e | 95.45 i | <LOQ | 101.60 j |
| C9 | <LOQ | 1.73 f | 2.11 de | 2.39 d | 141.05 f | <LOQ | 147.28 g |
| C10 | <LOQ | 1.84 ef | 1.92 f | 1.99 g | 229.62 e | <LOQ | 235.38 e |
| Sample | Dimethyl Phthalate | Diethyl Phthalate | Diisobutyl Phthalate | Dibutyl Phthalate | Bis (2-Ethylhexyl) Phthalate | Di-n-Octyl Phthalate | Sum |
|---|---|---|---|---|---|---|---|
| G1 | <LOQ | 0.76 m | 4.29 a | 3.99 bcd | 1.11 b | <LOQ | 10.14 cd |
| G2 | <LOQ | 0.74 m | 4.15 ab | 3.55 g | 3.65 a | <LOQ | 12.10 a |
| G3 | <LOQ | 1.52 i | 3.99 bc | 3.42 gh | 0.33 hi | <LOQ | 9.26 ef |
| G4 | <LOQ | 1.56 i | 3.90 cd | 3.60 fg | 0.39 gh | <LOQ | 9.45 e |
| G5 | <LOQ | 2.05 c | 2.80 hi | 3.05 ij | 0.35 ghi | <LOQ | 8.25 g |
| G6 | <LOQ | 2.55 a | 3.10 g | 2.90 j | 0.40 g | <LOQ | 8.95 f |
| G7 | <LOQ | 0.89 l | 3.55 ef | 4.10 bc | 1.01 c | <LOQ | 9.55 e |
| G8 | <LOQ | 0.99 k | 2.35 l | 3.85 de | 0.87 d | <LOQ | 8.06 gh |
| G9 | <LOQ | 2.45 b | 2.75 hij | 4.45 a | 0.41 g | <LOQ | 10.06 d |
| G10 | <LOQ | 1.61 hi | 3.95 bcd | 4.20 b | 0.71 e | <LOQ | 10.47 c |
| G11 | <LOQ | 1.89 d | 3.75 de | 3.95 cde | 0.58 f | <LOQ | 10.17 cd |
| G12 | <LOQ | 1.72 fg | 2.35 l | 3.25 hi | 0.52 f | <LOQ | 7.85 h |
| G13 | <LOQ | 1.83 de | 2.85 hij | 3.75 ef | 0.73 e | <LOQ | 9.06 f |
| G14 | <LOQ | 2.59 a | 2.94 gh | 4.65 a | 0.74 e | <LOQ | 10.92 b |
| G15 | <LOQ | 1.67 gh | 2.53 jkl | 3.11 ij | 0.83 d | <LOQ | 8.14 gh |
| G16 | <LOQ | 1.77 ef | 3.33 f | 3.93 cde | 0.97 c | <LOQ | 10.01 d |
| G17 | <LOQ | 1.27 j | 2.65 ijk | 2.88 j | 0.27 i | <LOQ | 7.07 i |
| G18 | <LOQ | 1.31 j | 2.45 kl | 3.07 ij | 0.40 g | <LOQ | 7.24 i |
| Sample | Dimethyl phthalate | Diethyl phthalate | Diisobutyl phthalate | Dibutyl phthalate | Bis (2-ethylhexyl) phthalate | Di-n-octyl phthalate | Sum |
|---|---|---|---|---|---|---|---|
| MIN_P | <LOQ | 0.46 c | 17.28 a | 27.73 a | 9.12 b | <LOQ | 62.93 b |
| MIN_G | <LOQ | 0.74 b | 2.35 b | 2.83 b | 0.27 c | <LOQ | 7.07 c |
| MIN_C | <LOQ | 1.72 a | 1.92 c | 1.99 c | 95.45 a | <LOQ | 101.60 a |
| MAX_P | <LOQ | 23.09 a | 56.96 a | 92.17 a | 95.06 b | <LOQ | 219.82 b |
| MAX_G | <LOQ | 2.59 c | 4.29 b | 4.65 b | 3.65 c | <LOQ | 12.10 c |
| MAX_C | <LOQ | 4.15 b | 2.75 c | 3.07 c | 326.90 a | <LOQ | 334.86 a |
| AVG_P | <LOQ | 6.46 a | 29.12 a | 54.09 a | 37.90 b | <LOQ | 127.57 b |
| AVG_G | <LOQ | 1.62 c | 3.20 b | 3.65 b | 0.79 c | <LOQ | 9.27 c |
| AVG_C | <LOQ | 2.31 b | 2.33 c | 2.52 c | 223.75 a | <LOQ | 230.92 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habschied, K.; Kartalović, B.; Lazić, D.; Krstanović, V.; Mastanjević, K. Survey on Phthalates in Beer Packaged in Aluminum Cans, PET and Glass Bottles. Fermentation 2023, 9, 125. https://doi.org/10.3390/fermentation9020125
Habschied K, Kartalović B, Lazić D, Krstanović V, Mastanjević K. Survey on Phthalates in Beer Packaged in Aluminum Cans, PET and Glass Bottles. Fermentation. 2023; 9(2):125. https://doi.org/10.3390/fermentation9020125
Chicago/Turabian StyleHabschied, Kristina, Brankica Kartalović, Dušan Lazić, Vinko Krstanović, and Krešimir Mastanjević. 2023. "Survey on Phthalates in Beer Packaged in Aluminum Cans, PET and Glass Bottles" Fermentation 9, no. 2: 125. https://doi.org/10.3390/fermentation9020125
APA StyleHabschied, K., Kartalović, B., Lazić, D., Krstanović, V., & Mastanjević, K. (2023). Survey on Phthalates in Beer Packaged in Aluminum Cans, PET and Glass Bottles. Fermentation, 9(2), 125. https://doi.org/10.3390/fermentation9020125









