Abstract
The role of soil-borne microalgae in the edaphic ecosystem is barely known, especially concerning their plant-growth-promoting traits, although they are used as biofertilizers. In this study, a microalgal strain isolated from soil cultivated with maize was evaluated as an exogenous producer of indole-3-acetic acid (IAA) in potato dextrose broth—PD—and sugarcane molasses, with or without tryptophan, and phosphate solubilizer (in ‘National Botanical Research Institute’s phosphate’—NBRIP—medium with tricalcium phosphate)with plant-growth-promoting traits, under heterotrophic conditions. The species of microalga was identified, by sequencing the ITS region in the rDNA and the morphological characteristics, as Chlorella vulgaris. Its growth was significantly higher in the PD medium, with slower growth in molasses. The addition of tryptophan did not influence the growth of C. vulgaris in either medium, but it increased the production of IAA, mainly in the PD medium, to 265 µg mL−1. The microalga grew in the medium with insoluble phosphate, releasing phosphorus into the medium (30 mg L−1 after 72 h). This is the first study on the application of C. vulgaris as a phosphate solubilizer. More studies should be performed on C. vulgaris as a prospective plant-growth-promoting microorganism, besides its ability to produce exogenous IAA, and further investigations should be conducted on developing inexpensive culture media.
1. Introduction
Soil microorganisms promote plant development by increasing the availability of nutrients in the soil and producing phytohormone-like compounds, which stimulate root development. Thus, studying soil-borne microorganisms and their interactions with plants is important for developing sustainable agronomic practices to reduce or replace toxic and polluting chemicals by stimulating ecological processes.
Microalgae occur in arable and non-arable soils, and due to their high growth rates and ability to survive under extreme conditions, they are commonly studied for their application as agricultural bioproducts to stimulate plant growth and disease control [1,2]. Microalgae are members of the plant microbiome and influence plants by activating signaling pathways [1].
Species of microalgae have diverse energetic metabolisms, ranging from autotrophy to heterotrophy, which supports their ability to survive under different environmental conditions [3]. Microalgae might also be used for treating liquid residues, such as domestic sewage, industrial effluents, and agricultural wastewaters, which provide minerals and organic carbon sources for the growth of microalgae [4,5]. Considering the conditions and nutrients in the residues, and also the mixotrophic and heterotrophic metabolisms of the microalgae, there is a potential to explore the sources with decreased costs to produce biomass and improving the production of targeted compounds.
Mixotrophy combines certain characteristics of autotrophy and heterotrophy, where photoautotrophy is supplemented with some form of organic carbon [6]. Microalgae produce energy more efficiently via heterotrophic (0.00924 g cells per kJ input) and mixotrophic (0.00749 g cells per kJ input) metabolism than via autotrophic metabolism (0.00177 g cells per kJ input) [7]. This ability of heterotrophic and mixotrophic metabolisms in microalgae might be used in biotechnological applications in various ways.
Microalgae provide plant biostimulant compounds in agricultural settings. Algae stimulate the bioactivity of plants by producing auxin-like compounds or substances that promote the synthesis of indole-3-acetic acid (IAA) endogenously in plants [8]. IAA is synthesized by various organisms, such as bacteria, microalgae, fungi, and plants, and is among the most common hormones that regulates growth and development in plants. In microorganisms, IAA regulates physiological responses and gene expression and might influence microbial interactions [9]. Auxin and auxin-like compounds are better documented in cyanobacteria than in green algae, and studies have focused more on the endogenous production instead of the exogenous production aspect [2]. A study by Mazur et al. [10] found that the green algae Scenedesmus armatus and Chlorella pyrenoidosa can produce extracellular IAA, which is the most common plant hormone in the auxin family [11]. The general pathway to produce IAA requires tryptophan, although some tryptophan-independent pathways also occur in plants and bacteria [2].
The production of phytohormones, such as auxins, is one of the mechanisms of plant-growth-promoting traits, besides biological nitrogen fixation and phosphate solubilization [12]. Fungi, bacteria, and cyanobacteria are the most common phosphorus solubilizers [13], but green microalgae are not known to act as phosphorus solubilizers.
In this study, the production of exogenous IAA by a strain of Chlorella vulgaris, a green algal species, which was isolated from soil cultivated with maize, was evaluated under heterotrophic conditions. The capacity of this strain to solubilize phosphate in vitro was also determined. Algae are a new source of biofertilizers, bactericides, and fungicides for plants [1], and hence in this context, the search for plant-growth-promoting traits in algae is interesting for crop yield improvement.
2. Materials and Methods
2.1. Isolation and Identification of the Microalga
The strain of the microalgal species C. vulgaris (MGA10) was from the culture collection of the Laboratory of Agricultural and Molecular Microbiology (LAMAM), Universidade Federal de São Carlos, Araras, São Paulo State, Brazil. This strain was isolated from the soil in which maize was cultivated in an experiment conducted in the field area of the university, using yeast extract–peptone–dextrose (YPD) medium (10 g L−1 yeast extract, 20 g L−1 peptone, 20 g L−1 glucose, and 20 g L−1 agar). The pure culture of C. vulgaris was stored in slants of YPD medium at 8 °C.
The strain was identified based on its morphological characteristics, such as cell and colony morphology. The microalga was punctually inoculated in the center of Petri dishes containing potato dextrose (PD) agar medium (Kasvi®, São José do Pinhais, Brazil) and incubated in dark (inside an incubator without light) and light conditions (at room temperature under natural sunlight) for 3 days. The cells were transferred to the PD broth medium (Kasvi®), incubated at 30 °C under constant shaking for 12–16 h, and analyzed under an optical microscope (at 400×) and a scanning electron microscope (SEM). Molecular identification was conducted by sequencing the internal transcribed spacer (ITS) region of the rDNA.
To obtain the SEM images, 1 mL of the microalga suspension cultured in PD broth medium containing approximately 107 cells mL−1 was centrifuged at 5000 rpm for 5 min. Then, the supernatant was discarded, and the pellet was resuspended in 1 mL of 2.5% glutaraldehyde. After 3 h of fixation, the material was homogenized and placed on stubs covered with carbon tape. The stubs were kept in an oven at 30 °C for 3 h, followed by incubation of the material for 12 h at room temperature. For imaging, the stubs were inserted without metallization into a Thermo Fisher® Prisma E Scanning Electron Microscope (Waltham, MA, USA), and the material was analyzed under high vacuum conditions.
DNA from the cells was extracted using the DNeasy®PowerSoil® Pro Kit (Qiagen®, Venlo, The Netherlands), and then, the ITS region (including the ITS1 and ITS2 regions separated by the 5.8S gene) and part of the 18S and 28S genes were amplified via PCR using the primers ITS5–1737F (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS4–2409R (5′-TCCTCCGCTTATTGATATGC-3′). The fragment (750–950 bp) amplified via PCR was used for sequencing in MinION (Oxford Nanopore). The reads were filtered for quality and size, and then, used for making consensus fragments, which were analyzed using the BlastN tool in the NCBI database to determine the microbial species.
2.2. Characterization of the Growth of Microalga with Different Carbon Sources
The growth of microalga was characterized in potato broth (PB) medium, which was prepared by cooking 1 kg of peeled potatoes in 1 L of distilled water. About 30 min after cooking, when the potatoes were very soft, the mass was discarded, and 200 mL of the broth was filtered through a funnel with cotton and diluted to 1 L with distilled water to prepare the PB medium. Three different carbon sources at 20 g L−1 (glucose, fructose, and sucrose) were added to the PB medium, separately. The PB medium without any added carbon source was also analyzed. Clarified sugarcane molasses with 20 g L−1 of total reducing sugar (TRS) was also prepared. For clarification, molasses (obtained from an ethanol-producing unit nearby) was diluted and homogenized in distilled water, heated at 60–65 °C in a water bath for 15 min, filtered through a funnel with cotton, and supplemented with NaH2PO4 (2.5 g L−1). The filtrate was left in a fluent steam for 15 min, and then incubated for 48 h [14]. The concentration of reducing sugars was determined by performing the 3,5-dinitrosalicylic acid assay [15], with acid hydrolysis (TRS) or without acid hydrolysis (reducing sugars). The molasses medium was adjusted to 20 g L−1 of TRS with distilled water. The concentration of reducing sugars (with and without acid hydrolysis) was also determined in the PB-based medium. All media were autoclaved for 15 min at 120 °C.
The inoculum was prepared by growing the microalga in the YPD medium to a final volume of 10 mL at 30 °C for 12–16 h. Next, the absorbance of the cell suspension was adjusted to 1.0 (at 600 nm). The suspension was centrifuged at 5000 rpm for 5 min and washed with distilled water. The supernatant was discarded, and the cell pellet was suspended in the culture media as described above. Next, 20 µL of the inoculum was added to a 96-well microplate (Costar flat bottom with lid culture plate) with 180 µL of each medium in triplicate. The microplate was incubated at 30 °C under constant agitation. Absorbance readings (at 600 nm) were taken every 15 min for 24 h using the Tecan Infinite® M200 microplate reader.
The growth curves were plotted against time for the microalga grown in different culture media using absorbance values. The exponential growth phase was identified, and the maximum specific growth rate (µmax) in the linear region of the semi-log curves was determined.
2.3. Quantification of Exogenous IAA Production by C. vulgaris Using Molasses and Potato Dextrose Broth Media
First, the production of IAA by the strain of C. vulgaris was analyzed using a qualitative colorimetric assay [16]. The strain was cultured in PD broth supplemented with tryptophan (0.5 g L−1) and incubated in a shaker at 30 °C and 160 rpm for 48 h. Next, 1 mL of Salkowski reagent (a solution consisting of 35% perchloric acid and 0.5 M ferrous chloride) was added to 1 mL of the culture supernatant (obtained after centrifugation of the microalgal cultures at 3000 rpm for 3 min) and incubated for 30 min at room temperature in the dark. The presence of IAA in the medium was confirmed when the solution turned pink.
To quantify the IAA produced, initially, the microalgal inoculum was prepared in YPD medium as described above. The suspension was centrifuged, and the cells were suspended in a saline solution (0.85% NaCl). Cell counting was performed in a Neubauer chamber to standardize the cell concentration to ~1 × 106 cells mL−1. The production of exogenous IAA was evaluated in PD broth medium (containing 20 g L−1 glucose) or clarified molasses medium (containing 20 g L−1 TRS), with or without the addition of tryptophan (0.5 g L−1). The flasks with 50 mL of medium (in triplicate) were inoculated with cells at an initial concentration of 1 × 105 cells mL−1, incubated at 30 °C for 48 h with constant shaking at 160 rpm. Sampling was performed every 12 h.
Cell growth was evaluated by analyzing the optical density (absorbance) at 600 nm. Then, the cell suspension was centrifuged, and 0.5 mL of the supernatant was added to 0.5 mL of Salkowski reagent. The intensity of the color generated during the reaction was determined by spectrophotometry at 530 nm, and the concentration of IAA was estimated from a standard curve.
2.4. Phosphate Solubilization by C. vulgaris Using NBRIP Medium
Initially, a qualitative assay was performed to determine the ability of the C. vulgaris strain to solubilize phosphate. The strain was punctually inoculated onto the PDYA medium (Potato dextrose agar medium, Kasvi®, supplemented with 5 g L−1 yeast extract, 50 mL L−1 10% K2HPO4, and 10 mL L−1 10% CaCl2). The plate was incubated for 48 h at 30 °C. A translucent halo around the colony indicated a positive result for phosphate solubilization.
To quantify the solubilization of tricalcium phosphate by the microalga, an assay was performed in the NBRIP medium (10 g L−1 glucose, 5 g L−1 MgCl2·6H2O, 0.25 g L−1 MgSO4·7H2O, 0.2 g L−1 KCl, and 0.1 g L−1 (NH4)2SO4), with or without 5 g L−1 Ca3(PO4)2 (insoluble phosphate), following the method described in another study [17]. The pH of the medium was adjusted to 7.0 before autoclaving.
Erlenmeyer flasks containing NBRIP medium (final volume: 50 mL) were inoculated with the microalgal inoculum prepared as described above at an initial concentration of 1 × 105 cells mL−1. The flasks (in triplicate) were maintained at 30 °C for 72 h, with constant shaking at 160 rpm. Sampling was performed every 24 h using 2 mL of the sample to determine the cell and phosphorus concentration. Three treatments were established, including NBRIP medium + microalgae + insoluble phosphate, NBRIP medium + microalgae, and NBRIP medium + insoluble phosphate.
Cell growth was evaluated by analyzing the optical density (absorbance) at 600 nm. Then, the supernatant obtained after centrifugation of the cell suspension was used to determine the concentration of soluble phosphorus, via the molybdenum blue method at 650 nm using a spectrophotometer [18]. The concentration of soluble phosphorus was estimated from a standard curve, and the pH was measured in the supernatant using a pH meter.
The results of phosphorus solubilization were analyzed by comparing the time periods after subtracting the concentration of phosphorus obtained in the non-inoculated NBRIP medium with insoluble phosphate from the concentration obtained in the inoculated NBRIP medium with insoluble phosphate, at each time period. Following this, the concentration of phosphorus obtained at the initial time (0 h) was discounted from the concentration of phosphorus obtained at 24, 48, and 72 h.
2.5. Statistical Analysis
The assays performed to determine IAA production and phosphate solubilization had a completely randomized experimental design. All statistical analysis was performed using the software STATISTICA 7.0. For IAA production and optical density (cell growth), an analysis of variance (n = 3) was performed to determine significant differences between the PD broth and molasses media, between the presence and absence of tryptophan, and between multiple time periods. Phosphate solubilization (n = 3) was compared across multiple time periods.
3. Results and Discussion
The microalgal strain was identified by sequencing the ITS1-ITS2 region of genomic rDNA. The obtained sequences were aligned with those from the NCBI GenBank, and the results indicated that the most likely species for this microalgal strain was Chlorella vulgaris (details of alignments in Figure 1).
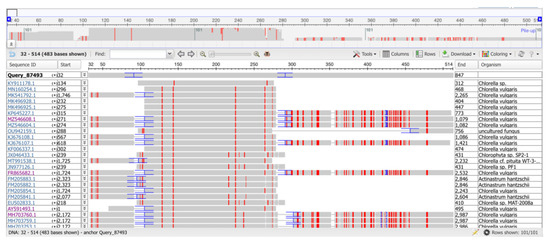
Figure 1.
Multiple sequence alignment (MSA) of the BlastN result using the rRNA amplicon sequence. The consensus of the Oxford Nanopore sequencing was used for the BlastN analysis. The MSA shows the first 25 hits, among which most are Chlorella vulgaris sequences.
Regarding cell morphology, the images obtained via optical microscopy and SEM indicated that the cells were spherical, approximately 4 to 8 µm in diameter, and autospores were present inside the cell (autosporangium), which is associated with the most common type of asexual reproduction in algae (Figure 2). C. vulgaris is found in aquatic and terrestrial environments. These microalgae have a high photosynthetic ability and autotrophic, mixotrophic, or heterotrophic metabolisms, which makes them suitable for large-scale cultivation for commercial purposes [19,20].
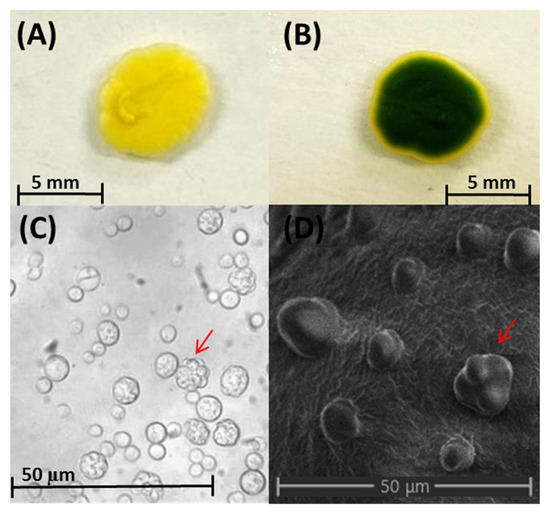
Figure 2.
Colony and cell morphology of the C. vulgaris strain isolated from soil. (A) A colony grown in the dark. (B) A colony grown under natural sunlight. (C) Cells imaged under an optical microscope at 400×. (D) Cells imaged under a scanning electron microscope. The red arrows in (C,D) indicate the autospores inside the autosporangium. The cells were cultivated in potato dextrose broth (for microscopy) or potato dextrose agar (for colony viewing).
This strain was isolated in YPD medium along with several species of yeasts from soil cultivated with maize. However, it was identified as microalga mainly based on the coloration of the colony (greenish-yellow to green). The green color was more evident when the strain was grown under natural sunlight rather than in the dark (inside an incubator), as shown in Figure 2. As the strain of C. vulgaris grew well in the YPD medium under heterotrophic conditions, the inoculum was developed and the microalgal strain was conserved in the YPD medium rather than in the mineral medium.
Several studies have reported the beneficial effects of C. vulgaris on plants, especially as a biological control agent, increasing tolerance to abiotic stress, promoting plant growth, and as an anti-aging agent delaying senescence [1]. For example, adding C. vulgaris to the culture medium or soil substantially increases the fresh and dry weight of lettuce seedlings [21]. The combined seed and soil inoculation of C. vulgaris accelerates the germination and maturity of Hibiscus esculentus and also increases pod yield and plant height [22].
These beneficial effects on plant growth and crop yield might be attributed to the plant-growth-promoting traits, such as the production of phytohormones and siderophores, inhibition of the growth of phytopathogens, mineral solubilization, etc. [12]. Species of Chlorella produce endogenous IAA; however, few studies have investigated the exogenous production of this auxin that promotes plant growth and development, although it is the most popular and physiologically active auxin [10,23,24]. Another important plant-growth-promoting trait is the capacity to solubilize phosphorus. Besides bacteria and fungi, cyanobacteria also exhibit this trait [13]. However, no published study has reported the role of Chlorella as a phosphorus solubilizer. Preliminary and qualitative assays indicated that the strain of C. vulgaris produced exogenous IAA and solubilized phosphate under heterotrophic conditions in the PD broth medium and PDYA medium, respectively (Figure 3).
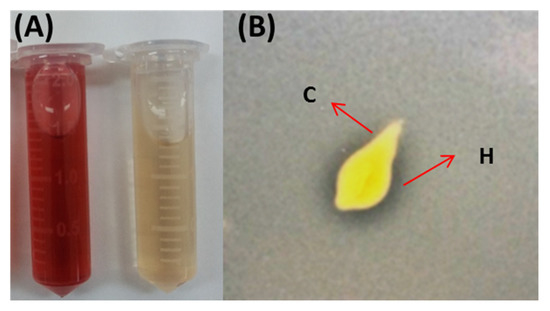
Figure 3.
Qualitative tests to evaluate the production of exogenous IAA (A) and phosphorus solubilization (B) by a strain of C. vulgaris isolated from soil. In (A), the tube on the left shows the reaction with the microalga compared to the tube on the right (negative control). In (B), ‘C’ indicates the colony, and ‘H’ indicates the solubilization halo in the medium with insoluble phosphate.
Several studies have indicated that algal extracts might be effective in promoting plant growth because they contain macronutrients, trace elements, metabolites, amino acids, and phytohormones [25]. Auxins are either present in algal extracts or are secreted into the medium; however, studies on the secretion of auxins by green algae are limited [10,26,27]. Evaluating the production of exogenous IAA by algae grown in different culture media might provide interesting results. Thus, the production of IAA was assayed using two culture media, potato broth and molasses. These media were selected because they are cheap and easily available; additionally, molasses is a byproduct of the sugarcane industry. It is a liquid rich in nutrients, growth factors, minerals, and sugars (40–60%), and thus, it acts as a valuable substrate for microbial processes [28]. As molasses contains sugars, such as glucose, fructose, and sucrose, the growth of C. vulgaris in potato broth with glucose, fructose, and sucrose and in molasses was analyzed to evaluate whether these sugars can be used as carbon sources under heterotrophic conditions. The results are presented in Table 1.

Table 1.
Maximum specific growth rate (µmax) of C. vulgaris grown in potato broth with different carbon sources and in molasses, at 30 °C, under constant shaking, and the concentration of reducing sugars in each medium.
The growth rate of C. vulgaris was higher in potato broth with glucose than in potato broth with fructose or sucrose. Similar growth rates were observed in potato broth without additional carbon sources and with fructose or sucrose, indicating that these sugars were not assimilated satisfactorily, and algal growth was supported by the low concentration of reducing sugars present in the potato broth. The growth rate in molasses was the lowest, and it was most probably based on the glucose present as a reducing sugar in the culture medium (Table 1).
A previous work [29] with C. vulgaris showed that the highest µmax (0.030 h−1) was obtained when glucose (10 g L−1) was added to the Basal Bold medium rather than fructose or sucrose at the same concentration (0.015 or 0.020 h−1, respectively). Microalgae lack the enzyme invertase used for hydrolyzing sucrose to glucose and fructose [30]. Additionally, the initial sugar concentration might affect sugar uptake and growth, as the maximum growth rate of the biomass of a strain of C. vulgaris was observed with glucose at a range of 1–5 g L−1, but the same effect was achieved with a lower concentration of fructose (0.1–1.0 g L−1) [31].
The growth of C. vulgaris in potato broth without an additional carbon source might also be influenced by the starch present in the medium. However, this strain showed low amylolytic activity in a qualitative assay (data not shown). Amylase activity was detected in C. vulgaris grown in a mineral medium under illumination, and seven putative genes encoding α-amylases, β-amylases, and isoamylase were also found in an in silico study [32].
The production of exogenous IAA by C. vulgaris in potato broth with 20 g L−1 glucose (PD) was compared to the results of IAA production in molasses containing 20 g L−1 of TRS, with or without the addition of tryptophan, a precursor of IAA in most microorganisms. The results of cell growth and IAA production are shown in Figure 4 and Table 2 and Table 3.
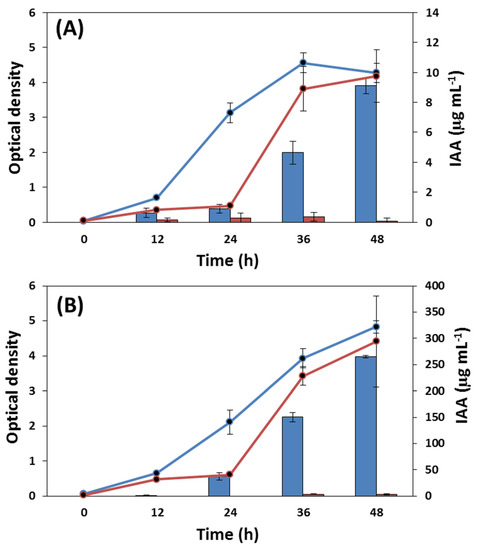
Figure 4.
Growth (line) and exogenous IAA production (bar) by C. vulgaris was determined using potato dextrose broth medium (in blue) and molasses (in red), without (A) and with (B) tryptophan.

Table 2.
Statistical results of growth (optical density) of C. vulgaris in potato dextrose broth (PD) and molasses, with or without tryptophan, by Tukey’s test (p < 0.05) 1.

Table 3.
Statistical results of IAA production (µg mL−1) by C. vulgaris in potato dextrose broth (PD) and molasses, with or without tryptophan, by Tukey´s test (p < 0.05) 1.
The growth of C. vulgaris was significantly higher in the PD medium than in the molasses medium. The growth was slower in molasses, confirming the preliminary test in microplates (Table 1), although it did not differ from that in the PD medium after 36 h. The addition of tryptophan did not influence the growth of C. vulgaris in either medium (Figure 4 and Table 2).
The production of IAA differed significantly between the PD medium and molasses, and with or without the addition of tryptophan, but the effect of tryptophan was only significant in the PD medium (Figure 4 and Table 3).
Chlorella minutissima supplemented with 5 g L−1 of glucose under dark conditions produced six times more auxins than cultures under light or dark conditions without supplementation. These findings suggested that organic energy reserves are more critical for IAA production than light [33,34]. Although the concentration of total reducing sugars was similar, the difference between the PD medium and molasses in IAA production, following the addition of tryptophan to the medium, was that molasses had a smaller fraction of reducing sugars (glucose mainly) than PD broth with glucose, which should have been directed to cell growth. The surplus of reducing sugars (glucose) in the PD broth promoted the production of IAA, while only a small amount of sucrose in molasses was used by C. vulgaris. The high exogenous production of IAA by C. vulgaris in a PD medium with tryptophan (265 µg mL−1) is quite promising for processes to synthesize bioproducts, and further optimization of the culture conditions might cost-effectively enhance the production.
The solubilization of phosphate by C. vulgaris is another plant-growth-promoting trait. As shown in Figure 5, the microalga grew substantially in the medium with insoluble phosphate and released significant quantities of phosphorus into the medium. Thus, the phosphate was solubilized to release phosphorus, which supported the growth and accumulated in the medium to a concentration of 30 mg L−1 after 72 h. The concentration of phosphorus in the medium doubled from 48 to 72 h, whereas the growth of C. vulgaris entered the stationary phase (Figure 5).
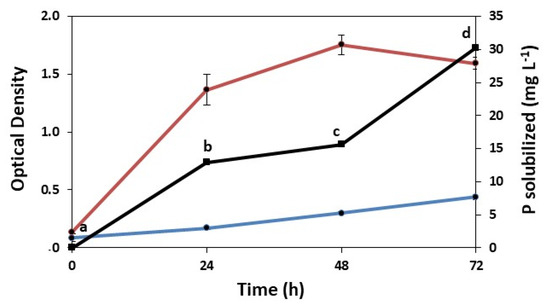
Figure 5.
Growth of C. vulgaris in NBRIP medium without insoluble phosphate (blue line) and with insoluble phosphate (red line), along with phosphorus solubilization (black line). Different letters on the black line indicate significant difference determined by Tukey’s test at each time period (p < 0.05).
The mechanisms by which phosphorus is solubilized from insoluble sources, such as tricalcium phosphate, include the release of compounds such as organic acids, siderophores, protons, and carbon dioxide, or the release of extracellular enzymes such as phosphatases and phytases [13]. The activities of the enzymes involved in phosphorus solubilization were not quantified here, but acids were probably produced by C. vulgaris during growth in the NBRIP medium. Without the addition of insoluble phosphate, the pH of the medium decreased from 7.0 to 3.2 after C. vulgaris was grown for 72 h (the optical density varied from 0.1 to 0.4). With insoluble phosphate, the optical density varied from 0.1 to 1.7, and the pH decreased only slightly (from 7.0 to 6.2). Although acids were produced and phosphorus was solubilized, the pH did not decrease substantially, probably because of the increase in chelation of the cations bound to phosphorus or due to the formation of soluble complexes with metal ions (Ca, Al, and Fe) associated with insoluble phosphate [13].
Several genera of cyanobacteria, such as Anabaena, Calothrix, Nostoc, and Scytonema, can solubilize phosphate [13], but no study has reported C. vulgaris as a phosphate solubilizer. Further studies are required to enhance the solubilization index using other sources of insoluble phosphate. Coupled with the ability to produce exogenous IAA, this strain of C. vulgaris should receive more attention as a prospective plant-growth-promoting microorganism. Simultaneously, inexpensive culture media also need to be developed.
Author Contributions
Conceptualization, R.L.B.B., M.M.R.-M. and S.R.C.-A.; methodology, R.L.B.B., M.M.R.-M. and S.R.C.-A.; investigation, R.L.B.B., M.M.R.-M. and S.R.C.-A.; writing—original draft preparation, M.M.R.-M., M.A.T. and S.R.C.-A.; writing—review and editing, M.M.R.-M., M.A.T. and S.R.C.-A.; funding acquisition, M.M.R.-M. and S.R.C.-A. All authors have read and agreed to the published version of the manuscript.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (Finance code 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request to the corresponding author.
Acknowledgments
We thank ByMyCell Inova Simples Ltd.a for the molecular identification of the microbial strain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, S.M.; Ryu, C.M. Algae as new kids in the beneficial plant microbiome. Front. Plant Sci. 2021, 12, 599742. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Dodd, C.D.; Chen, J.E.; Phang, S.M.; Chin, C.F.; Yow, Y.Y.; Ratnayeke, S. Regulation of algal and cyanobacterial auxin production, physiology, and application in agriculture: An overview. J. Appl. Phycol. 2021, 33, 2995–3023. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Maryjoseph, S.; Ketheesan, B. Microalgae based wastewater treatment for the removal of emerging contaminants: A review of challenges and opportunities. Case Stud. Chem. Environ. Eng. 2020, 2, 100046. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.H.H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2022, 13, 100205. [Google Scholar] [CrossRef]
- Verma, R.; Kumari, K.V.L.K.; Srivastava, A.; Kumar, A. Photoautotrophic, mixotrophic, and heterotrophic culture media optimization for enhanced microalgae production. J. Environ. Chem. Eng. 2020, 8, 104149. [Google Scholar] [CrossRef]
- Yang, C.; Hua, Q.; Shimizu, K. Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem. Eng. J. 2000, 6, 87–102. [Google Scholar] [CrossRef]
- Mógor, A.F.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Fu, S.F.; Wei, J.Y.; Chen, H.W.; Liu, Y.Y.; Lu, H.Y.; Chou, J.Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef]
- Mazur, H.; Konop, A.; Synak, R. Indole-3-acetic acid in the culture medium of two axenic green microalgae. J. Appl. Phycol. 2001, 13, 35–42. [Google Scholar] [CrossRef]
- Simon, S.; Petrásek, J. Why plants need more than one type of auxin. Plant Sci. 2011, 180, 454–460. [Google Scholar] [CrossRef]
- Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bactéria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.B.; Sunhiga Filho, R.F.; Mattos, E.C.; Pereira, E.B.; Baptista, A.S. Produção de etanol a partir de melaço de cana. Rev. Est. Amb. 2019, 21, 38–45. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening of phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Colzani, H.; Rodrigues, Q.E.G.; Fogaça, C.; Gelinski, J.M.L.N.; Pereira-Filho, E.; Borges, E.M. Determinação de fosfato em refrigerantes utilizando um scanner de mesa e análise automatizada de dados: Um exemplo didático para ensino de química. Quim. Nova 2017, 40, 833–839. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Faheed, F.A.; El-Fattah, Z.A. Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J. Agric. Soc. Sci. 2008, 4, 165–169. [Google Scholar]
- Agwa, O.K.; Ogugbue, C.J.; Williams, E.E. Field evidences of Chlorella vulgaris potentials as a biofertilizer for Hibiscus esculentus. Int. J. Agric. Res. 2017, 12, 181–189. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, L.; Lu, M.; Chen, G.; Zhang, L. Extraction and analysis of auxins in plants using dispersive liquid−liquid microextraction followed by high-performance liquid chromatography with fluorescence detection. J. Agric. Food Chem. 2010, 58, 2763–2770. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Ördög, V.; Novák, O.; Rolčík, J.; Strnad, M.; Bálint, P.; van Staden, J. Auxin and cytokinin relationships in 24 microalgal strains. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Labeeuw, L.; Khey, J.; Bramucci, A.R.; Atwal, H.; de la Mata, A.P.; Harynuk, J.; Case, R.J. Indole-3-acetic acid is produced by Emilianiahuxleyi coccolith-bearing cells and triggers a physiological response in bald cells. Front. Microbiol. 2016, 7, 828. [Google Scholar] [CrossRef]
- Khasin, M.; Cahoon, R.R.; Nickerson, K.W.; Riekhof, W.R. Molecular machinery of auxin synthesis, secretion, and perception in the unicellular chlorophyte alga Chlorella sorokiniana UTEX 1230. PLoS ONE 2018, 13, e0205227. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, H. Microbial production of value-added bioproducts and enzymes from molasses, a byproduct of sugar industry. Food Chem. 2021, 346, 128860. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.A.; Fonseca, G.G. Screening of an adapted culture medium composed by different carbon sources for heterotrophic cultivation of Chlorella vulgaris using a microplate assay. Acta Sci. Biol. Sci. 2018, 40, e39401. [Google Scholar]
- Perez-Garcia, O.; Escalante, F.M.E.; Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Dolganyuk, V.; Michaud, P.; Ivanova, S. Influence of carbohydrate additives on the growth rate of microalgae biomass with an increased carbohydrate content. Mar. Drugs 2021, 19, 381. [Google Scholar] [CrossRef] [PubMed]
- Hlima, H.B.; Karray, A.; Dammak, M.; Elleuch, F.; Michaud, P.; Fendri, I.; Abdelkafi, S. Production and structure prediction of amylases from Chlorella vulgaris. Environ. Sci. Pollut. Res. 2021, 28, 51046–51059. [Google Scholar] [CrossRef] [PubMed]
- Sairanen, I.; Novák, O.; Pěnčík, A.; Ikeda, Y.; Jones, B.; Sandberg, G.; Ljung, K. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 2012, 24, 4907–4916. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Maróti, G.; Ljung, K.; Turečková, V.; Strnad, M.; Ordög, V.; van Staden, J. Effect of light on growth and endogenous hormones in Chlorella minutissima (Trebouxiophyceae). Plant Physiol. Biochem. 2014, 79, 66–76. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).