Abstract
This study demonstrated the bioconversion of lignocellulosic by-product corn stover (CS) to the value-added fermentative product L-lactic acid using the furfural tolerant Enterococcus mundtii WX1 and Lactobacillus rhamnosus SCJ9. The efficacy of dilute acid pretreatment by sulfuric and formic acids varying from 1% to 4% (v/v) concentration was compared. CS pretreated with 1% (v/v) sulfuric acid was selected for L-LA fermentation regarding the highest efficacy of fermentable sugar release when combined with the enzymatic hydrolysis process. Optimal conditions achieved a highest sugar release of 24.5 g/L glucose and 11.2 g/L of xylose from 100 g/L pretreated CS with 1% (v/v) sulfuric acid at 121 °C for 30 min, followed by enzymatic hydrolysis with Cellic CTec2 30 FPU/g pretreated CS at 50 °C for 48 h. The maximum L-LA titer, yield, and average productivity reached 31.4 g/L, 0.90 g/g, and 1.73 g/L/h, respectively. Moreover, addition of a hemicellulose-degrading enzyme complex combined with Cellic CTec2 led to an increase in xylose release, which resulted in a higher L-LA titer of 36.7 g/L at 48 h fermentation. Moreover, the purification of LA from culture broth by a process of electrodialysis with 331 g/L of LA and purity of 99.7% (w/w), was successful, with an optically pure L-LA of 99.9%. This study not only presents a feasible process for L-LA production from lignocellulose hydrolysate derived from abundant corn stover; this study also showed an alternative approach for solving the problem of haze air pollution caused by inappropriate management of corn production residuals.
1. Introduction
Corn stover (CS) refers to husks, cobs, leaves, and stalks that remain on the field after corn harvest [1]. As a typical lignocellulose, CS is composed of cellulose, hemicellulose, and lignin forming a complex and recalcitrant structure. CS is the most promising and abundant agricultural by-product that remains in the field after corn harvest, which can be used to produce value-added products, via chemical and biological processes, such as organic acid and ethanol. In northern Thailand, corn is widely planted for industrial purposes, and large amounts of post-harvest by-product of CS are produced, which can be used as an energy source. Regarding the recent situation of air pollution in northern Thailand and neighboring countries, by-products from corn production such as CS were included in the causes of the haze problem because of inappropriate corn by-product management. This agricultural by-product can be hydrolyzed to achieve fermentable sugars and used as the substrate for lactic acid fermentation. Various pretreatment methods for lignocellulose substrate have been applied, including alkali pretreatment, dilute acid pretreatment, and steam explosion. The hydrolysis of lignocelluloses under acid conditions normally generates hydrolysates rich in pentose sugar. However, harsh conditions during the pretreatment process activate the formation of inhibitory compounds, which negatively influence the fermentation performance by decreasing production yield and affecting microbial growth [2]. Therefore, microorganisms used in the fermentation of lignocellulose hydrolysate to produce lactic acid should be tolerant towards inhibitors produced by the pretreatment process.
Lactic acid (LA) is a natural organic acid with known applications in the food and non-food industries such as beverages, cosmetics, textiles, and pharmaceuticals [3,4,5]. Approximately 90% of recent industrial LA production worldwide is carried out using various microorganisms, especially lactic acid bacteria (LAB) [6]. Recent developments in industrial bioconversion technology and the advantage of the microbes’ ability to selectively produce only D- or L-LA provide efficient strategies for the industrial production of LA through microbial fermentation [7,8]. Nutritional factors, particularly carbon and nitrogen sources, are the most important factors that directly affect lactic acid productivity and economic production cost [9,10]. A variety of simple sugars, i.e., glucose and sucrose derived from low-cost food-related sources such as cassava starch and sugar cane molasses, have been used for industrial lactic acid production (around 90% of lactic acid in the market) [11,12]. Nonetheless, using starch as a substrate for industrial production of a biochemical has recently been recognized as an inappropriate substrate because of food security concerns; therefore, lignocellulosic substrates are the targeted alternative [5,13]. From published techno-economic analyses, LA production from lignocellulosic material has attracted significant interest because the minimum selling price of lignocellulosic lactic acid was estimated to be 1.38–1.91 $/kg, which is lower than the market price (around 1.7–2.1 $/kg) and could be further lowered to 1.09 $/kg [12]. Lignocellulose is composed of three main fractions: cellulose (30–60% of dry matter, DM), hemicellulose (14–40% of DM), and lignin (7–25% of DM) [14,15]. The difficulties in the degradation of lignocellulose complex structure associated with lignin have been found to be the classical obstacle for industrial utilization of lignocelluloses. Special pretreatments, using physical, chemical, and/or biological processes, are required for achieving fermentable sugars including hexose (mainly glucose) and pentose (xylose and other pentoses) which are derived from cellulose and hemicellulose, respectively [16,17]. However, the main problem in the lactic acid production of most LAB from fermentable sugars derived from lignocelluloses is their inability to consume xylose for their metabolism. This leads to a decrease in fermentation efficiency and a low lactic acid yield. Alternatively, microbial co-fermentation by pentose- and hexose-utilizing strains provides a more feasible and effective way to increase lactic acid yield from lignocellulose hydrolysate. Most of homofermentative LAB, including L. delbrueckii, L. paracasei and L. lactis, cannot convert xylose to LA. In contrast, some heterofermentative LAB such as L. brevis CHCC 2097 and L. pentosus CHCC 2355 have been reported for their ability to produce lactic acid from xylose released from wheat straw [18]. Therefore, the efficiency of microbes in LA production is an important key to the utilization of mixed sugars. Moreover, the pretreatment of lignocellulose feedstocks by a harsh chemical pretreatment process may generate toxic compounds, wherein xylose is further degraded into furfural (FF) and forms of 5-hydroxymethyl furfural (HMF) by hexose degradation [19]. Thus, the fermentation process of lignocellulosic hydrolysate needs a detoxification process to remove growth inhibition compounds such as FF and HMF. Even though the detoxification process can be successfully operated by a variety of techniques, most are complicated processes and increase production costs [5,20,21]. Therefore, inhibitor-tolerant microbes—both FF and HMF-tolerant strains—used in fermentation processes are beneficial because they avoid or reduce detoxification costs. Regarding our previous study, co-culture fermentation of E. mundtii WX1 (xylose utilizing LAB) and L. rhamnosus SCJ9 (glucose utilizing LAB) for L-lactic acid production, in the optimized medium using mixed sugars of glucose and xylose as carbon sources, was successful. The co-culture showed the ability to produce L-LA in the presence of 2 g/L furfural [22]. Therefore, the detoxification process was not necessary for L-LA production using this co-cultured system. However, the previous experiment was performed based on standard sugar, which is different from fermentation using cellulose hydrolysate as the substrate. Therefore, co-culture fermentation using lignocellulose hydrolysate directly derived from CS has to be investigated.
This paper describes L-LA production from CS hydrolysate in a 2.5-L fermenter using a co-culture of HMF and FF E. mundtii WX1 and L. rhamnosus SCJ9. The proper pretreatment and enzyme dosage were also elucidated to achieve a high yield of optically pure L-LA via the fermentation of mixed sugars derived from CS. This finding also provides the potential of a CS valorization process, which is an alternative approach to eliminate the agricultural by-product involved in haze formation from CS burning during the corn-harvesting season in northern Thailand and neighboring countries.
2. Materials and Methods
2.1. Raw Materials, Chemicals, and Enzymes
Fresh corn stover (CS) collected from Chiang Mai province in Thailand was washed in order to remove dirt and chopped into small pieces prior to drying at 60 °C until a constant dry weight. The air-dried CS was milled using a hammer mill and passed through a 10-mesh sieve. The milled CS was packed into vacuum sealer plastic storage bags and stored at room temperature (30 °C) for further use. Standard sugars used for HPLC calibration, including glucose, xylose, and arabinose, were purchased from Sigma-Aldrich (Steinem, Germany). Furfural (FF) and hydroxymethyl furfural (HMF) were of analytical grade, commercially available from Sigma-Aldrich. Furthermore, Cellic CTec2 (cellulose degrading enzyme complex, approximately 150 FPU/g) was purchased from Sigma-Aldrich. Accellerase XY (hemicellulose degrading enzyme complex, approximately 20,000 ABXU/g) was obtained from Genecor International (Rochester, NY, USA).
2.2. Microorganisms and Inoculum Preparation
The optically pure L-lactic acid-producing bacteria, Lactobacillus rhamnosus SCJ9 and Enterococcus mundtii WX1, were isolated from sugarcane juice and silkworm, respectively, from our previous studies [22,23] and kept as a stock culture collection at the Laboratory of Microbial Resources Development and Enzyme Technology, Faculty of Agro-Industry, Chiang Mai University. These microorganisms were chosen because they performed well in the co-culture fermentation of L-lactic acid production from mixed hexose-pentose sugars in the earlier study [22]. L. rhamnosus SCJ9 and E. mundtii WX1 were maintained in de Man Rogosa and Sharpe (MRS) broth (10 g/L glucose, 10 g/L beef extract, 10 g/L peptone, 5 g/L yeast extract, 1 g/L Tween 80, 2 g/L tri-ammonium hydrogen citrate, 5 g/L sodium acetate, 2 g/L K2HPO4, 0.1 g/L MgSO4, 0.1 g/L MnSO4) containing 15% (v/v) glycerol and stored at −80 °C. To prepare seed inoculum, a single colony of L. rhamnosus SCJ9 and E. mundtii WX1 were inoculated into 10 mL MRS broth and further incubated at 37 °C under static conditions for 12–18 h to achieve an OD600 of 0.8–0.9.
2.3. Dilute Acid Pretreatment of Corn Stover
Dilute acid pretreatment of CS was conducted with sulfuric and formic acid in varied concentrations of 1–4% (v/v). Briefly, 1 g of milled CS was placed into a 250-mL Erlenmeyer flask containing 10 mL of different concentrations of pretreatment liquid (the final concentration was equivalent to 100 g/L milled CS). Each CS suspension was incubated at 30 °C on a 150 rpm rotary shaker for 1 h, and then heated by autoclave at 80 °C for 30 min. The sample was neutralized with 20% (w/v) NaOH and filtered through filter paper to separate the solid and liquid fractions. The liquid fractions were analyzed for HMF and FF as well as the released glucose, xylose, and arabinose by HPLC. The liquid fractions were analyzed for HMF and FF as well as the released glucose, xylose, and arabinose by HPLC The control experiment was performed by using water as the pretreated solution. The solid fractions were washed twice with distilled water prior to further determining hemicellulose and cellulose using an ANKOM A2000 fiber analyzer (Ankom Technology, Macedon, NY, USA). Total carbohydrate recovery was calculated based on a combination of hemicellulose and cellulose.
2.4. Enzymatic Hydrolysis of Pretreated Corn Stover
Enzymatic hydrolysis of pretreated CS was incubated with a Cellic CTec2. Briefly, 10 g of the pretreated CS was suspended in 100 mL sterile distilled water (equivalent to 100 g/L). Then, various Cellic CTec2 dosages were applied to the suspension. Based on weight of the pretreated CS, the enzyme was varied into four concentrations including 10, 20, 30, and 40 FPU/g pretreated CS (equivalent to 100, 200, 300, and 400 FPU/100 mL suspension). The reaction was performed at 50 °C for 48 h and then stopped by placing it in an ice-bath. After that, it was centrifuged at 12,000 rpm, 4 °C for 10 min. The supernatant was quantitatively analyzed for glucose, xylose, and arabinose by HPLC.
2.5. Optimization of Process Parameters on Corn Stover Pretreatment
The selected chemicals, with their proper concentrations, were fixed to further find the proper temperatures and times for CS pretreatment. Here, temperatures were varied between 80 and 121 °C while times were varied between 30 and 60 min. After the pretreatment step was completed, each pretreated CS was then neutralized, and enzymatic hydrolysis using the proper enzyme dosages was added. After 48 h, the samples were centrifuged and analyzed by HPLC for glucose, xylose and arabinose released as well as the formation of HMF and FF
2.6. L-Lactic Acid Fermentation from Pretreated Corn Stover in 2.5-L Bioreactor
Milled CS was suspended in the selected chemical for pretreatment (1% sulfuric acid) to achieve a final concentration of 100 g/L. Then, the pretreatment step was conducted according to the proper conditions selected from the previous experiment (121 °C, 30 min). The pretreated CS was neutralized by adding 20% (w/v) NaOH prior to enzymatic hydrolysis, and 30 FPU of Cellic CTec2/g pretreated CS was transferred into the pretreated CS, followed by incubation at 50 °C for 48 h. In the meantime, pretreated CS hydrolyzed with 30 FPU of Cellic CTec2, combined with 4000 ABXU of Accellerase XY/g pretreated CS, was also studied; the amount of Accellerase XY was set for the sufficient hydrolysis of the substrate according to the previous study [24]. The CS hydrolysate was prepared for 1000 mL, which was then placed into a 2.5-L BIOSTAT stirred tank bioreactor (Sartorius AG, Germany) and used as the sole carbon source. Approximately 500 mL of other optimized medium components (20.6 g/L yeast extract, 2.5 g/L peptone, 2.5 g/L beef extract, 1.4 g/L Tween 80, 2.0 g/L Tri-ammonium citrate, 1.0 g/L sodium acetate, 1.2 g/L MnSO4∙H2O, 1.5 g/L K2HPO4 and 0.1 g/L MgSO4∙7H2O) for co-culture L-lactic acid production by E. mundtii WX1 and L. rhamnosus SCJ9, according to the previous study [22], were separately prepared and autoclaved before transferring to fermentation. Then, 4% (v/v) of E. mundtii WX1 and 4% (v/v) of L. rhamnosus SCJ9 inocula were transferred to the bioreactor. The conditions were set to 37 °C with an agitation speed of 150 rpm. The pH was maintained at pH 6.5 by adding 20% (w/v) NaOH. Samples were taken at 3 h intervals to determine the lactic acid content, glucose, xylose, and viable cells.
2.7. Purification of L-Lactic Acid
The purification of L-lactic acid from fermentation broth was performed using a combination of ultra- and nanofiltration, electrodialysis, and ion-exchange chromatography techniques, which are more environmentally friendly than the conventional process (lactic acid precipitation using calcium hydroxide), following the method of Olszewska-Widdrat et al. [25] with some modification. Briefly, to remove the solid compound and biomass, the culture broth was initially carried out using filter bags (Schwegmann Filtrations-Technik GmbH, Grafschaft, Germany), with 150 μm pore size, and the permeate was then subjected to 1.5 bar ultra-filtration (ABB ceramic membranes, 2 μm pore size) using a UFI-TEC crossflow filtration system (Oranienburg, Germany). To remove the cations, the pH value of the permeate stream was adjusted to 10 by using 20% (w/v) NaOH, and was introduced into the expanded bed column containing PUROLITE S950 acid chelating resin (Purolite, Ratingen, Germany), from below, at a flow rate of 6 bed volumes per h. The conductivity of the purified water was monitored, and the separation was complete when conductivity was below 1 mS/cm. After that, the free lactic acid solution was concentrated via monopolar and bipolar electrodialysis, which were carried out under constant polarity at a temperature of 35 °C [25]. The concentrated L-lactic acid solution was passed through a strong cation exchanger (RELITE EXC 08, Resindion S.R.L., Binasco, Italy) followed by a weak anion exchanger (RELITE EXA 133, Resindion S.R.L.) to remove the remaining cations and anions. The removal of color impurities was accomplished using strong acid resin PUROLITE MN-502 (Purolite). In the final step, the filtrate was vacuum evaporated using a vacuum distillation plant (Büchi Labortechnik, Essen, Germany) at 55 °C, 200 rpm and 0.05 bar.
2.8. Analytical Methods
The determination of organic acids and sugars from each fermentation sample were analyzed using HPLC (Dionex, Sunnyvale, CA, USA) equipped with Eurokat H column (300 mm × 8 mm × 10 μm, Knauer, Berlin, Germany). An aqueous solution of 5 mM sulfuric acid was used as the mobile phase, at a flow rate of 0.80 mL/min. The injection volume was 10 μL and the detection was carried out by a RI-71 refractive index detector (Shodex, Tokyo, Japan). The concentration of FF and HMF were analyzed using a C18 EurophereII column (Knauer, Berlin, Germany). The mobile phase was acetonitrile in water (1:8) with 1% (v/v) of acetic acid. The flow rate was 1.0 mL/min at 23 °C and detected using UV/VIS detector at 280 nm. The composition of untreated CS and the residues, including cellulose, hemicellulose, and lignin, were determined using an ANKOM A2000 fiber analyzer (Ankom Technology, Macedon, NY, USA). The structure and surface characteristics of untreated and pretreated CS samples were observed by a scanning electron microscope JEOL model JSM-5910LV (JEOL Corporation, Tokyo, Japan). The analysis of lactic acid optical purity was carried out using HPLC (Knauer, Berlin, Germany) equipped with a Chiralpak®MA(+) column (50 mm × 4.6 mm × 3 μm, Daicel, Tokyo, Japan). An aqueous solution of 2 mM CuSO4 was used as the mobile phase at a flow rate of 0.80 mL/min, and an ultraviolet detector was used for detection.
2.9. Statistical Analysis
Statistical analysis of the experimental data was performed using analysis of variance (ANOVA), and Tukey’s multiple range test was used to compare the differences among treatments at a 95% confidence level using the statistical program SPSS, version 17.0 (SPSS Inc. Chicago, IL, USA).
3. Results and Discussion
3.1. Composition of Corn Stover
To investigate the potential resource in CS that could expect to be used as a substrate for LA production, the compositions of CS were measured. The average composition of CS contained 33.80% hemicellulose and 42.43% cellulose, which make up a total carbohydrate content of 76.23% on a dry basis (Table 1). From the previously reported study, the contents of hemicellulose and cellulose in CS can vary based on its type and geographical location However, CS generally consists of 70% carbohydrate content and 15–20% lignin [1]. The higher content of total carbohydrate in CS from this study (76.23%) indicates an advantage, as cellulose and hemicellulose are the two main components that could be further converted to fermentable monomeric sugars which are important substrates for bacterial growth and fermentation.

Table 1.
Composition of corn stover on dry solid basis.
3.2. Effect of Acid Concentration on Carbohydrate Recovery and Inhibitor Generation
To investigate the effect of different pretreatment conditions, the residual contents, after pretreatment with acid dosages (1–4%) of sulfuric acid and formic acid at 80 °C for 30 min, were determined; the results are presented in Figure 1. The residual contents of total carbohydrate recovery in CS after 1% sulfuric acid pretreatment (72%) was higher than those from 2%, 3% and 4% sulfuric acid pretreatments (58.8%, 59.1% and 57.2%); however, the total carbohydrate recovery amounts in CS after 1–4% formic acid pretreatments (approximately 70%) were not significantly different (p < 0.05). However, the total carbohydrate recovery from the control was found to be at the highest level, at 78%. Moreover, sugar released from the pretreatment process in the liquor part of the 1–4% sulfuric acid pretreatment was in a range of 13.74–15.88 g/L, which was also higher than the sugar released from formic acid pretreated substrates (7.54–12.64 g/L) (Table 2). Additionally, a high content of inhibitors (HMF and FF), the by-product of hemicellulose and cellulose degradation, was also found from sulfuric acid pretreatment at a higher level when compared to formic acid pretreated CS. This finding indicates that strong acid (sulfuric acid) causes the higher degradation of hemicellulose and cellulose in CS during pretreatment, compared to weak acid (formic acid) pretreatments. Previous reports have also shown that using formic acid pretreatment results in the formation of less inhibitor than sulfuric acid pretreatment, and also results in an increase in cellulose and hemicellulose recovery [26]. Therefore, it is critical to set a balance between carbohydrate recovery and by-product and inhibitor generation.
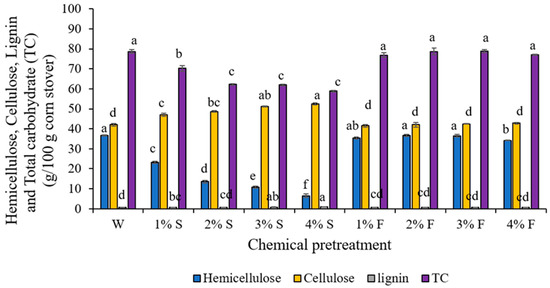
Figure 1.
Effect of different chemicals and concentrations on the extraction efficiency of hemicellulose, cellulose, lignin, and the total carbohydrates (TC) contents. The conditions for all chemical pretreatments were 80 °C for 30 min. Water (W) was used as the control. (F = formic acid, S = sulfuric acid). Superscripted alphabets refer to significant different mean values (p < 0.05) of hemicellulose, cellulose, and lignin among pretreatment conditions.

Table 2.
Effect of dilute acid pretreatment on released sugar, furfural and HMF concentration from corn stover at 80 °C for 30 min.
3.3. Effect of Enzyme Dosage on Sugar Released from Pretreated Corn Stover
Due to the high recovery of total carbohydrate from residual solids after pretreatment, pretreated CS from 1% of sulfuric and formic acid was selected for enzymatic saccharification by different dosages of Cellic CTec2 (10–40 FPU/g pretreated CS). Table 3 shows the released sugar and inhibitor after hydrolyzing with different enzyme dosages at 50 °C for 48 h. Overall, the released sugar concentration was slightly increased when the enzyme dosage was increased from 10–40 FPU/g pretreated CS, and the maximum sugar released was obtained from 40 FPU/g pretreated CS after it was pretreated with 1% sulfuric acid. However, the fermentable sugar obtained from this condition was not significantly different (p < 0.05) when compared with 30 FPU/g pretreated CS. Furthermore, a high released sugar concentration was also observed in the 1% formic pretreated CS hydrolyzed with a Cellic CTec2, in the range of 30–40 FPU/g pretreated CS. Due to the high cost of the hydrolyzing enzyme, 30 FPU/g pretreated CS was selected for further experimentation. In addition, a lower concentration of released sugar was detected in water-pretreated CS. These results could suggest that damage to the CS structure caused by sulfuric acid might play an important role in increasing and/or enhancing enzymatic hydrolysis performance, and may lead to a higher released sugar when compared to formic acid pretreatment. The Cellic CTec2 enzymes, including cellulase, β-glucosidase, and hemicellulose, are general enzymes necessary to convert cellulose and hemicellulose into soluble sugars. The direct factor that affects sugar release is probably the accessible surface area of cellulose, because the prerequisite step for enzymatic hydrolysis to occur is intimate contact between the cellulase and the reactive cellulose surface [27]. Moreover, with Cellic CTec2, an earlier stabilization in the hydrolysis efficiency was observed which may be related to changes in the composition of the lignocellulosic material [28].

Table 3.
Effect of Cellic CTec2 enzyme dosages on released sugar content in pretreated corn stover.
3.4. Effect of Pretreatment Temperature and Reaction Time on Sugar Release and Inhibitor Generation
To increase the fermentable sugar released from CS, the increasing temperature and reaction time of pretreatment were investigated, and the results are presented in Figure 2. An increase in the sugar released and inhibitor concentration when the temperature and reaction time increased was observed in sulfuric acid pretreatment. As shown in Figure 2A, a dramatic increase was observed in glucose concentration (22.1 and 27.6 g/L) when pretreated at 80 °C for 30 min, compared to 121 °C with the same reaction time, respectively. When compared to formic acid, pretreatment at 80 and 121 °C for 30 min slightly increased glucose concentrations to 17.0 and 18.7 g/L, respectively. Thus, CS pretreated with 1% sulfuric acid at 121 °C for 30 min achieved a higher glucose yield than formic acid pretreatment. In the case of water pretreatment, although some advantages of water pretreatment were observed, such as inexpensive process, and no pollution and corrosion, the total sugar released at 121 °C for 30 min was markedly low when compared to the use of acid pretreatments (Figure 2B). Moreover, CS pretreated with 1% sulfuric acid at 121 °C for 30 min generated 2.6 and 0.4 g/L of HMF and FF, respectively. Interestingly, these inhibitor concentrations have no effect on the lactic acid fermentation of both L. rhamnosus SCJ9 and E. mundtii WX1 strains, which tolerated to FF up to 2 g/L, as mentioned in the previous study [22]. The degradation of lignin and hemicellulose before enzymatic hydrolysis of lignocellulosic biomass is highly necessary because it increases the digestibility of cellulose [29,30]. The success of hot water pretreatment in the removal of lignin from lignocellulose increased cellulose accessibility, and minimal production of potentially inhibitory products has been also reported; however, the process required high pressures and extreme temperatures ranging from 160 to 240 °C [31,32]. Dilute sulfuric acid pretreatment is usually performed over a temperature range of 140–210 °C, with sulfuric acid concentration less than 4% (w/v) and a residence time ranging from a few minutes to hours in different types of pretreatment reactors [33].
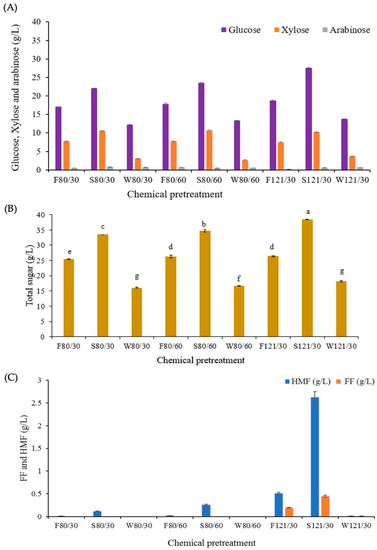
Figure 2.
Effect of 1% extraction solvents, temperatures, and times on the content of sugar composition (A), total sugar (B), and content of HMF and FF (C) in the pretreated corn stover. The X-axis presents the type of extraction solvent at 1% (v/v)/temperature (°C)/time (min) used for each pretreatment of corn stover prior to enzymatic hydrolysis (F = formic acid, S = sulfuric acid, W = water/80 and 121 °C/30, 60 min). Superscripted alphabets (Figure 2B) refer to significant different mean values (p < 0.05) of total sugar among pretreatment conditions.
The morphology of raw and pretreated CS at 121 °C for 30 min was conducted by SEM analysis (Figure 3). Raw CS showed a compact surface structure with fibers arranged in bundles, which obstruct the enzymatic hydrolysis. Whereas the surfaces of acid-pretreated CS were clearly observed for the damage and physical appearance of destructive structure, especially in the case of sulfuric acid pretreatment, the CS structure became more physically loose when compared to the formic acid pretreatment. These results demonstrated that pretreatment could destroy the structure of CS, which is composed of a cellulose, hemicellulose and lignin network. The revelation of the internal structure of CS samples increases accessibility for the enzymes and lead to the increase in fermentable sugar released [34]. However, water pretreatment only caused minimal changes on the surface of the CS sample.
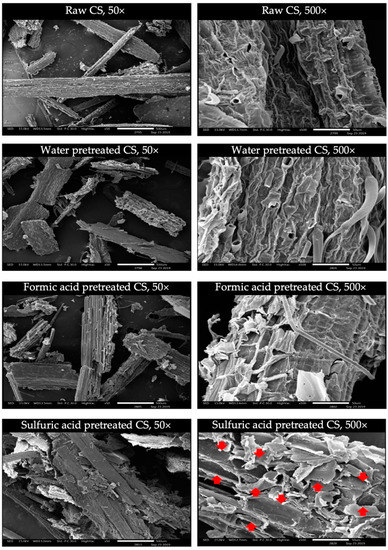
Figure 3.
The SEM images of the pretreated corn stover (CS) by water, formic acid, and sulfuric acid at 121 °C for 30 min compared with the raw corn stover at 50× and 500× magnification. The damage structures were indicated by red arrows.
3.5. Co-Culture of L-Lactic Acid Fermentation Using Corn Stover Hydrolysate
The ability of co-culture of E. mundtii WX1 and L. rhamnosus SCJ9 to produce L-LA from CS hydrolyzed by either Cellic CTec2 (CC) or Cellic CTec2 combined with Accellerase XY (CA) was further examined (Figure 4). The glucose and xylose contents in the CC medium were 24.5 and 11.2 g/L, respectively, whereas 27.2 and 18.5 g/L were detected in the CA medium. It could be suggested that the higher level of glucose and xylose in CA was effectively assisted by the additional commercial enzyme Accellerase XY, which is mainly composed of xylosidase that can release more xylose from xylan or xylan-related substrates in CS. Due to the hemicellulose remaining in the solid fraction, accessory enzymes such as xylanases, esterases, and some oxidases are necessary for the enzyme mixture [35]. A maximum L-LA titer of 31.4 and 36.7 g/L at 48 h was present in CC and CA media, respectively, since the total sugar of CA was higher than that from CC media. However, a maximum yield of 0.88 and 0.80 g/g of glucose and xylose consumed was achieved in CC and CA media, respectively, which corresponds to 88 and 80% of maximum theoretical yield (1.0 g/g sugar consumed), respectively. The average LA productivity in CC medium (1.73 g/L/h) was slightly lower than that in CA medium (2.20 g/L/h) between 6 and 30 h. The average consumption rates of xylose in CC and CA medium between 6 and 30 h were 0.32 and 0.56 g/L/h, respectively; meanwhile, the average glucose consumption rates between 3 and 9 h were about 2.89 and 3.31 g/L/h. These results could confirm that E. mundtii WX1 (xylose-glucose utilizing bacterium) and L. rhamnosus SCJ9 (glucose utilizing bacterium) have the ability to ferment CS hydrolysate into L-LA. Lactic acid production by co-fermentation was also reported by Cui et al. [36], using L. rhamnosus and L. brevis to produce LA from CS, which showed a yield of 0.7 g/g. Liu et al. [37] studied the pretreatment process of CS using 5% sulfuric acid at 175 °C for 5 min, and then detoxified using A. resinae ZN1. This resulted in 4.81 mg of FF, 3.34 mg of HMF and a 74.3% yield of LA from 30% (w/w) solids’ loading of CS. Moreover, improvement of the fermentation process, or modification of the metabolic pathways of microbial stains for higher LA yield may be explored in further investigations.
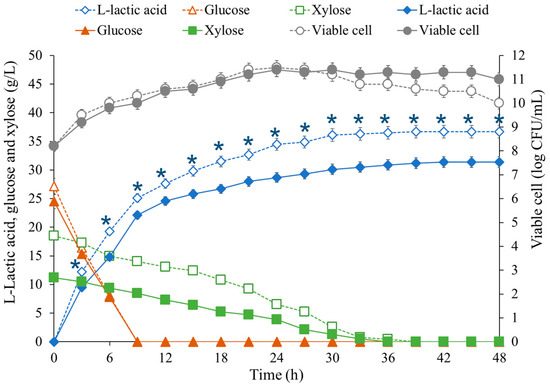
Figure 4.
Profile of lactic acid production from diluted sulfuric acid pretreated (1%, (v/v) acid 121 °C for 30 min) and enzymatically saccharified (50 °C for 48 h) with Cellic CTec2 (solid line) and Cellic CTec2 and Accellerase XY (dashed line) corn stover (10%, w/v) by co-culture of E. mundtii WX1 and L. rhamnosus SCJ9 in 2.5-L stirred tank fermenter at 37 °C for 48 h. The data are presented with symbols: ( ,
,  ) lactic acid, (
) lactic acid, ( ,
,  ) glucose, (
) glucose, ( ,
,  ) xylose and (
) xylose and ( ,
,  ) viable cell. Asterisks indicate statistically significant differences (p < 0.05) for lactic acid titers of both treatments.
) viable cell. Asterisks indicate statistically significant differences (p < 0.05) for lactic acid titers of both treatments.
 ,
,  ) lactic acid, (
) lactic acid, ( ,
,  ) glucose, (
) glucose, ( ,
,  ) xylose and (
) xylose and ( ,
,  ) viable cell. Asterisks indicate statistically significant differences (p < 0.05) for lactic acid titers of both treatments.
) viable cell. Asterisks indicate statistically significant differences (p < 0.05) for lactic acid titers of both treatments.
3.6. Purification of L-Lactic Acid
The results of the purification steps of L-lactic acid produced by co-culture of E. mundtii WX1 and L. rhamnosus SCJ9 using corn stover hydrolysate are shown in Table 4. After fermentation, a volume of 6.68 L of fermentation broth containing 34.7 g/L of lactic acid was subjected to a coarse filtration step followed by an ultra-filtration step. It was observed that more than 87% of LA remained in the permeate stream of the ultra-filtration step; additionally, the total nitrogen and total phosphorus reduced by approximately 44 and 35%, respectively. After ultra-filtration, the removal of divalent ions, mainly Mg2+ and Ca2+, was carried out in a softening step. As seen in the results in Table 4, the concentrations of Mg2+ and Ca2+ after the softening step were 0.14 and 3.91 mg/L, respectively, which equaled 99.9 and 97.6% removals, respectively. Before applying chromatography, a bipolar electrodialysis and a decolorization step were employed for the removal of residual compounds. Cation and anion exchange chromatography were then employed to remove the residual ions. It was found that a recovery percentage of 77.8% of lactic acid was observed; in addition, more than 98% of the monovalent ions were successfully removed. In the final step, a volume of 0.25 L solution containing 331 g/L of lactic acid was obtained, whereas the concentration of impurity compounds was less than 0.8 g/L, which equaled a lactic acid purity of around 99.7% (w/w), with an optical purity of 99.9%. However, the overall recovery yield of lactic acid was 50.2%, which was lost during the different processes. Among the separation steps used in this study, a low recovery yield of lactic acid was detected at the cation and anion exchange chromatography steps, as presented in Table 4. This is caused by the weakened resin-exchange capacity with extended time, as mentioned by Aljundi et al. [38]. Moreover, the existence of nutrients and other ions may decrease the capacity of resin [39]. The ion-removal steps such as the softening step and electrodialysis step, could be modified to remove other ions before passing through the ion-exchange step.

Table 4.
Composition analysis of L-lactic acid purification processes of the fermentation broth.
4. Conclusions
The effect of varied concentrations of sulfuric and formic acid pretreatment on CS was analyzed to find a suitable dilute pretreatment process of CS for further use as the substrate in L-LA production. CS pretreatment with 1% (v/v) sulfuric acid at 121 °C for 30 min followed by the enzymatic hydrolysis with Cellic CTec2 30 FPU/g pretreated CS at 50 °C for 48 h was chosen. Moreover, the fermentation inhibitors found in CS hydrolysate did not show any negative effect on bacterial performances. In addition, the released fermentable sugar can be enhanced when Cellic CTec2 is combined with hemicellulose-degrading enzyme. This study confirms that the L-LA production by a co-culture of E. mundtii WX1 and L. rhamnosus SCJ9 in a 2.5-L fermenter using CS hydrolysate as substrate was successful. Moreover, the purification of lactic acid from culture broth by electrodialysis processes was also successful, with 50.2% recovery. This study not only presents a feasible process for L-LA production from lignocellulose hydrolysate derived from abundant corn stover, but also showed an alternative approach for solving the problem of air pollution by the haze caused from CS burning during the corn-harvesting season; this is an inappropriate management of corn-production residuals.
Author Contributions
Conceptualization, A.K. (Augchararat Klongklaew) and C.K.; methodology and formal analysis, A.K. (Augchararat Klongklaew), K.U., D.K., A.K. (Apinun Kanpiengjai), H.A., L.S., R.S., J.V. and C.K.; investigation, A.K. (Augchararat Klongklaew), K.U., D.K., A.K. (Apinun Kanpiengjai), H.A., L.S., R.S., J.V. and C.K.; writing—original draft preparation, A.K. (Augchararat Klongklaew) and C.K.; writing—review and editing, A.K. (Augchararat Klongklaew), K.U., A.K. (Apinun Kanpiengjai), J.V. and C.K.; supervision, C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Chiang Mai University, the National Research Council of Thailand (NRCT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by Chiang Mai University’s 50th Anniversary Scholarship. We acknowledge Chiang Mai University and Leibniz Institute for Agricultural Engineering and Bioeconomy, Department of Microbiome Biotechnology, Germany for the research facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aghaei, S.; Karimi Alavijeh, M.; Shafiei, M.; Karimi, K. A comprehensive review on bioethanol production from corn stover: Worldwide potential, environmental importance, and perspectives. Biomass Bioenergy 2022, 161, 106447. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Schneider, R.; Mehlmann, K.; Venus, J. Recent advances in D-lactic acid production from renewable resources: Case studies on agro-industrial waste streams. Food Technol. Biotechnol. 2019, 57, 293–304. [Google Scholar] [CrossRef]
- Eş, I.; Khaneghah, A.M.; Barba, F.J.; Saraiva, J.A.; Sant’Ana, A.S.; Hashemi, S.M.B. Recent advancements in lactic acid production-a review. Food Res. Int. 2018, 107, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Yankov, D. Fermentative lactic acid production from lignocellulosic feedstocks: From source to purified product. Front. Chem. 2022, 10, 823005. [Google Scholar] [CrossRef] [PubMed]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological advances in lactic acid production by lactic acid bacteria: Lignocellulose as novel substrate. Biofuel Bioprod. Biorefin. 2018, 12, 290–303. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Direct lactic acid fermentation: Focus on simultaneous saccharification and lactic acid production. Biotechnol. Adv. 2009, 27, 145–152. [Google Scholar] [CrossRef]
- Mora-Villalobos, J.A.; Montero-Zamora, J.; Barboza, N.; Rojas-Garbanzo, C.; Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; Lopez-Gomez, J.P. Multi-product lactic acid bacteria fermentations: A review. Fermentation 2020, 6, 23. [Google Scholar] [CrossRef]
- Enamala, M.K.; Pasumarthy, D.S.; Gandrapu, P.K.; Chavali, M.; Mudumbai, H.; Kuppam, C. Production of a variety of industrially significant products by biological sources through fermentation. In Microbial Technology for the Welfare of Society; Springer: Berlin/Heidelberg, Germany, 2019; pp. 201–221. [Google Scholar]
- Hofvendahl, K.; Hahn–Hägerdal, B. Factors affecting the fermentative lactic acid production from renewable resources. Enzym. Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Clemens, R.A.; Jones, J.M.; Kern, M.; Lee, S.Y.; Mayhew, E.J.; Slavin, J.L.; Zivanovic, S. Functionality of sugars in foods and health. Compr. Rev. Food Sci. Food Saf. 2016, 15, 433–470. [Google Scholar]
- Li, Y.; Bhagwat, S.S.; Cortés-Peña, Y.R.; Ki, D.; Rao, C.V.; Jin, Y.-S.; Guest, J.S. Sustainable lactic acid production from lignocellulosic biomass. ACS Sustain. Chem. Eng. 2021, 9, 1341–1351. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Block, D.E.; Mills, D.A. Simultaneous consumption of pentose and hexose sugars: An optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2010, 88, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [PubMed]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, X.; Wu, L.; Li, Y.; Li, F.; Xiu, Z.; Tong, Y. The advanced performance of microbial consortium for simultaneous utilization of glucose and xylose to produce lactic acid directly from dilute sulfuric acid pretreated corn stover. Biotechnol. Biofuels 2021, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.B.; Romaní, A.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Industrial robust yeast isolates with great potential for fermentation of lignocellulosic biomass. Bioresour. Technol. 2014, 161, 192–199. [Google Scholar] [PubMed]
- Larsson, S.; Reimann, A.; Nilvebrant, N.-O.; Jönsson, L.J. Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl. Biochem. Biotechnol. 1999, 77, 91–103. [Google Scholar] [CrossRef]
- Lenihan, P.; Orozco, A.; O’Neill, E.; Ahmad, M.; Rooney, D.; Walker, G. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010, 156, 395–403. [Google Scholar] [CrossRef]
- Klongklaew, A.; Unban, K.; Kanpiengjai, A.; Wongputtisin, P.; Pamueangmun, P.; Shetty, K.; Khanongnuch, C. Improvement of enantiomeric L-lactic acid production from mixed hexose-pentose sugars by coculture of Enterococcus mundtii WX1 and Lactobacillus rhamnosus SCJ9. Fermentation 2021, 7, 95. [Google Scholar] [CrossRef]
- Unban, K.; Puangkhankham, N.; Kanpiengjai, A.; Govindarajan, R.K.; Kalaimurugan, D.; Khanongnuch, C. Improvement of polymer grade L-lactic acid production using Lactobacillus rhamnosus SCJ9 from low-grade cassava chips by simultaneous saccharification and fermentation. Processes 2020, 8, 1143. [Google Scholar]
- Morrison, J.M.; Elshahed, M.S.; Youssef, N.H. Defined enzyme cocktail from the anaerobic fungus Orpinomyces sp. strain C1A effectively releases sugars from pretreated corn stover and switchgrass. Sci. Rep. 2016, 6, 29217. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Widdrat, A.; Alexandri, M.; López-Gómez, J.P.; Schneider, R.; Mandl, M.; Venus, J. Production and purification of L-lactic acid in lab and pilot scales using sweet sorghum juice. Fermentation 2019, 5, 36. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Z.; Fei, B.; Cai, Z.; Pan, X. Comparison of bamboo green, timber and yellow in sulfite, sulfuric acid and sodium hydroxide pretreatments for enzymatic saccharification. Bioresour. Technol. 2014, 151, 91–99. [Google Scholar] [CrossRef]
- Wang, Q.Q.; He, Z.; Zhu, Z.; Zhang, Y.-H.P.; Ni, Y.; Luo, X.L.; Zhu, J.Y. Evaluations of cellulose accessibilities of lignocelluloses by solute exclusion and protein adsorption techniques. Biotechnol. Bioeng. 2012, 109, 381–389. [Google Scholar] [PubMed]
- Cannella, D.; Hsieh, C.-w.C.; Felby, C.; Jørgensen, H. Production and effect of aldonic acids during enzymatic hydrolysis of lignocellulose at high dry matter content. Biotechnol. Biofuels 2012, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Du, S.-k.; Su, X.; Yang, W.; Wang, Y.; Kuang, M.; Ma, L.; Fang, D.; Zhou, D. Enzymatic saccharification of high pressure assist-alkali pretreated cotton stalk and structural characterization. Carbohydr. Polym. 2016, 140, 279–286. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Li, M.; Cao, S.; Meng, X.; Studer, M.; Wyman, C.E.; Ragauskas, A.J.; Pu, Y. The effect of liquid hot water pretreatment on the chemical–structural alteration and the reduced recalcitrance in poplar. Biotechnol. Biofuels 2017, 10, 327. [Google Scholar] [CrossRef]
- Yu, G.; Yano, S.; Inoue, H.; Inoue, S.; Endo, T.; Sawayama, S. Pretreatment of rice straw by a hot-compressed water process for enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2010, 160, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Pu, Y.; Yang, B.; Ragauskas, A.; Wyman, C.E. Comparison of microwaves to fluidized sand baths for heating tubular reactors for hydrothermal and dilute acid batch pretreatment of corn stover. Bioresour. Technol. 2011, 102, 5952–5961. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Arantes, V.; Saddler, J.N.; Ferraz, A.; Milagres, A.M.F. Limitation of cellulose accessibility and unproductive binding of cellulases by pretreated sugarcane bagasse lignin. Biotechnol. Biofuels 2017, 10, 176. [Google Scholar] [CrossRef]
- Hu, J.; Arantes, V.; Saddler, J.N. The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: Is it an additive or synergistic effect? Biotechnol. Biofuels 2011, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Li, Y.; Wan, C. Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour. Technol. 2011, 102, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sun, J.; Zhang, J.; Tu, Y.; Bao, J. High titer L-lactic acid production from corn stover with minimum wastewater generation and techno-economic evaluation based on Aspen plus modeling. Bioresour. Technol. 2015, 198, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Aljundi, I.H.; Belovich, J.M.; Talu, O. Adsorption of lactic acid from fermentation broth and aqueous solutions on Zeolite molecular sieves. Chem. Eng. Sci. 2005, 60, 5004–5009. [Google Scholar] [CrossRef]
- Din, N.A.S.; Lim, S.J.; Maskat, M.Y.; Mutalib, S.A.; Zaini, N.A.M. Lactic acid separation and recovery from fermentation broth by ion-exchange resin: A review. Bioresour. Bioprocess. 2021, 8, 31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).