Abstract
Traditional fermented foods harbor microbes that transform raw food components, improving their nutritional, shelf life, organoleptic, and health-promoting characteristics. Fermented foods are an important conduit of contact between bioactive components that act like antigens and the human body system. Versatile microbes in traditional fermented foods are associated with many health-promoting end-products, including dietary fatty acids and inherent fermenting microbial cells. Evidence shows that dietary fatty acid components regulate genes in a hormonally dependent manner, either directly via specific binding to nuclear receptors or indirectly by changing regulatory transcription factors. Fatty acids are implicated in anti-inflammatory, anti-obesogenic, immunoregulatory, cardioprotective, etc., activities. Challenges with scaling the production of traditional fermented foods stem from losing effective consortiums of microbial groups and the production of differential end-products. Industrialists scaling the production of traditional fermented foods must overcome safety and consistency challenges. They need to combine processes that lessen the advent of public health issues and introduce omics technologies that identify and maintain effective consortium groups, prune genes that code for toxic products, and inculcate microbes with additional beneficial characteristics. Incorporating omics in production will avail the benefits of traditional fermented foods to a larger population that craves them outside their native areas.
1. Introduction
Traditional fermented foods are veritable sources of health-promoting biomolecules, despite associated challenges. The health effects are made possible due to ubiquitous microbes that self-perpetuate by employing versatile metabolic activities to ferment suitable substrates. Fermentation compounds a complex milieu of active microorganisms, their metabolic activities, which they foster through a system of enzymes, and the raw materials or macromolecules they act upon to yield products of interest [1,2,3,4,5]. These critical by-products elicit significant benefits for gut microbes, the mucosal immune system, and other organs under the direct influence of the mucosal immune system [6]. Although most fermentation occurs without oxygen, acetic acid fermentation can go on aerobically. In biochemical terms, all processes that have organic compounds as electron donors are fermentative: whether raw food substrates are in man-made bio-processors, ingested diets in human intestines, or agro-wastes that clog our environments, microbial processes and metabolisms are effective enough in releasing encrypted and valuable components from transforming these macromolecules [7,8,9,10,11,12]. Figure 1A–C shows the transformation of essential macromolecules in foods into bioactive by-products with metabolic functions.
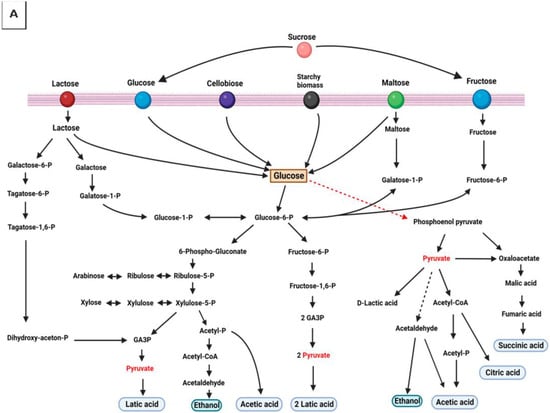
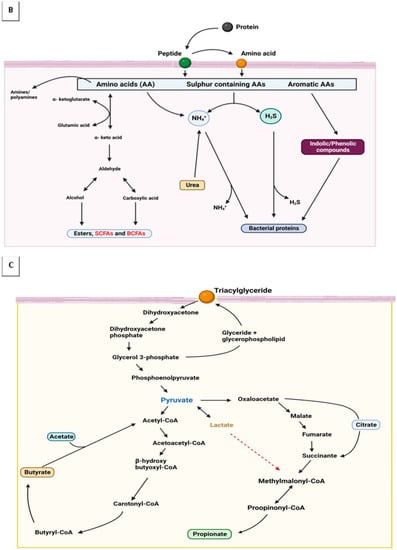
Figure 1.
Macromolecules are oxidized via hydrogen ions transfers forming intermediate products that are critical modulators of hosts’ immune responses. (A) Carbohydrate metabolism; (B) protein metabolism; and (C) glycerol metabolism.
Historically, the conversion of ethanol to acetic acid in vinegar production is the oldest known microbial transformation process. Similar well-documented processes were traced to the human hunter-gatherer transitional times about 15,000–20,000 years ago [10]. Many ancient Chinese reports found fermented rice, honey, and fruits in earthen pots dated 7000 BC [2,7,13]. In a nutshell, the discovery of fermentative processes might have occurred concurrently on every continent and might have helped sustain humankind and societies [10,14]. However, Louis Pasteur is said to have laid the foundations for the modern knowledge of fermentation and sterilization processes [13], which have contributed immensely to food safety and preservation. It is hard to propound an exhaustive traditional fermented food list. The reason is their diverse nature, which largely influences their territories of origin, various cultures/traditions, and the geography of the people [15]. Capable microbes transform well-arranged and energy-dense macromolecules, releasing simpler, biologically active molecules, and energy packs during fermentation by utilizing several catabolic pathways. Fermentation by-products are released into microbial extracellular spaces under stress conditions or in the exponential growth phase [4,16]. Both the scientific community and consumers are paying close attention to the beneficial influence fermented foods exert on the host’s health via the gut microbiome.
Fermented foods and associated biotechnologies have played important roles in poverty alleviation, malnutrition curation, food security, sustainable development, and economy boosting and have sparked multidisciplinary, permeating interests [4,17]. Fermented foods and concomitant microbes and metabolites modulate the hosts’ microbiome, leading scientists to consider this interaction a crucial health-influencing route with great potential in precision medicine. They complement human metabolic processes by expressing non-human encodable enzymes [3,17,18]. A cross-sectional study showed that early intake of fermented foods reduced the risk of childhood atopy [19,20]. Second, the gut harbors two-thirds of every migratory immune cell in the body—a colossal impact on the host’s health [6,20]. As an important fermentation by-product, fatty acids, in their correct quantity, permeate cellular biological functions, energy homeostasis, and physiological and immune responses [21,22,23] and increase the nutritional and organoleptic characteristics of fermented foods [24,25,26,27]; (see Table 1). Notwithstanding its numerous potentials, most traditional fermented foods are merely consumed in their native locality and bedeviled with inconsistent product outcomes. However, the globalization of the food markets has placed standardization and safety concerns with the scaled production of these health-beneficial foods. This review provides insight into the health effects of different fatty acids, and the challenges and regulatory issues surrounding commercializing traditional fermented foods.

Table 1.
Fatty acids from fermented foods and health benefits.
2. Characteristic and Physiological Influence of Dietary Fatty Acids
2.1. Fatty acid Characteristics
The transformation and subsequent absorption of appropriate quantities of dietary fatty acids or lipids by intestinal epithelial cells fundamentally drive energy balance and metabolic health. Dietary lipids are first absorbed by small intestinal epithelial cells in a multistep process before being released into circulation. As such, the small intestine epithelium serves as a crucial gatekeeper, managing the transit of dietary components into the body by coordinating diverse metabolic pathways. The pancreatic enzymes in the intestinal lumen break down macromolecules into simpler molecules, including free fatty acids and monoacylglycerols [34]. Dietary fatty acids are described according to their carbon chain length characteristics [35]. Long-chain fatty acids (LCFAs) are mostly isolated from dietary triglycerides [36] and have between 13 and 21 carbons [35,37,38]. Medium-chain fatty acids (MCFAs) are also gotten from dietary triglycerides [34] and have between 6 and 12 carbons [35]. In comparison, short-chain fatty acids (SCFAs) are primarily from dietary fiber fermentation [36] and have less than 6 carbons [39]. There are also dietary odd-chain saturated fatty acids (OCFAs) that are present at trace levels in dairy fat [40,41]; (see Table 2).

Table 2.
Health functions of some dietary fatty acids.
Dietary fatty acids are essential fermentation end-products and crucial energy biomolecules for many tissues. They are important biomarkers that modulate several physiological activities, including immune responses via gut microbiome [22,23,95,96]; (see Table 2). Traditional fermented foods are a good source of healthy fatty acids [97]. The health benefits of dietary fatty acids and fermented foods have been reviewed elsewhere [97,98,99]. Although conflicting results are touching on the health effects of fatty acids, our reports would mostly focus on the positive aspects. Moreover, due to the availability of data, this section will elucidate the health functions and properties of SCFAs. SCFAs are small molecular metabolites that play local and systemic roles in immune shaping, gut, and blood–brain barrier integrity, microglia maturation and function, and neuroinflammation prevention [39]. The major microbial transformers of SCFAs in the human gut belong to the phylum Firmicutes (Faecalibacterium prausnitzii and Clostridium leptum producing butyrate), phylum Actinobacteria (Bifidobacterium species producing acetate and lactate), and phylum Verrucomicrobia (producing propionate and acetate). Furthermore, in reports on specific butyrate synthase-related genes/enzymes, members of Actinobacteria, Bacteroidetes, Fusobacteria, Proteobacteria, Spirochaetes, and Thermotogae potentially transform SCFAs [100]. In general terms, studies show a reciprocal relationship between fatty acids’ metabolism and their health impact. They elicit their influence on several physiological processes such as the structure and function of the epithelial membrane, intracellular signaling pathways, transcription factor activity, gene expression, and the production of bioactive lipid mediators [38,96]. We shall elucidate these functions using the well-studied short-chain fatty acids (SCFAs).
2.2. SCFAs’ Functions and Signaling Patterns
SCFAs are the most studied fatty acids produced from non-digestible saccharides via intestinal bacterial fermentation—intestinal microbes maintain beneficial relationships at the tight junction between the mucosal epithelial cells (the primary physical barrier in the intestines). Interestingly, SCFA levels in liver and blood tissues are substantially lower than in the intestine. However, SCFAs and other fatty acid types are the primary intestinal epithelial cells’ fuel; they promote colonic epithelial cell proliferation, mucosal blood flow, and colonic motility [101]. SCFAs promote epithelial barrier function and stimulate epithelial cells through increased transepithelial electrical resistance (TER). Absorbed SCFAs are metabolized and trigger composite signaling pathways in the intestinal mucosa [101].
Butyrate is transported to intestinal epithelial cells by monocarboxylate transporter 1 (MCT1) and sodium-coupled monocarboxylate transporter 1 (SMCT1) [101]. Butyrate initiates the activation or repression of signaling pathways by binding to GPCRs and/or directly inhibiting HDACs [100]. Its activation reduces monolayer permeability while increasing TER during cell culture in an intestinal epithelial model comprised of either cultivated Caco-2 cell monolayers, T84 cells, rat small intestine cdx2-IEC cells, or small intestine porcine IPEC-J2 cells [100]. Acetate/GPR43 signaling mediates anti-inflammatory effects through NLRP3 inflammasome or cytokine/mediator regulation [100]. Trigger cascades affect immunological functions through cell surface G-protein coupled receptors (GPCRs) such as GPR41, GPR43, and GPR109A. Moreover, fatty acids act as ligands and activate anti-inflammatory signaling cascades [100].
Generally, fatty acids can activate HIF-1, STAT3, and SP1 or repress NF-κB transcription factors, increasing epithelial barrier function, antimicrobial peptides (AMPs) production, cell proliferation, and decreasing inflammation [100]. These effects are elicited by SCFAs’ actions on the caecal epithelial cells and not the caecal wall’s vascular or neurological system. However, SCFAs impact intestinal blood flow and generate smooth colon muscle contraction via the enteric nervous system [100,101]. Additional health functions are listed in Table 2. The importance of dietary fatty acids’ health outcomes was emphasized in a study that linked antibiotic use with reduced butyrate-producing bacteria proliferation [102]. Prolonged use of antibiotics lowers intracellular butyrate/PPAR signaling, raises iNOS and nitrate levels, and encourages the proliferation of Enterobacteriaceae in the intestine.
2.3. Fatty Acid Mediators: Gene Expression and Antimicrobial Activity
The gut microbe–fatty acid interactions support epithelial cell proliferation, regulate gene expression, increase TER, and promote the development of intestinal organoids and antimicrobial peptides (AMPs) [39,100]. Histone post-translational modifications (HPTMs) are key regulators of gene expression. Histone crotonylation connects chromatin to the gut microbiota, possibly through short-chain fatty acids and histone deacetylases [103]. Although organic acids promote colonic epithelial cell proliferation, studies on the transcription factor Foxo3 have revealed different effects of butyrate on intestinal stem/progenitor cells. They inhibit proliferation and delay wound repair [100]. Butyrate’s activation of STAT3 and SP1 transcription factors modulates epithelial barrier function through the induction of genes encoding tight-junction (TJ) components and protein reassembly [100].
Apart from activating toll-like receptors (TLRs) and modulating the NF-kB pathway, fatty acids, such as pentanoate and butyrate, also have anti-cancer activities [104,105]. They improve anti-tumor activity by increasing cytotoxic T lymphocytes (CTLs), related transcription factors (T-bet and Eomes), and chimeric antigen receptor (CAR) T cells via metabolic and epigenetic reprogramming [105]. Dietary SCFA metabolites attach to GPR43 to regulate the expression of AMPs that act as defense effectors. Some examples of AMPs are piscidins, cathelicidins, defensins, hepcidins, and high-density lipoproteins, which are modulated via the mTOR-STAT pathway [106,107]. Moreover, in peripheral tissues such as pancreatic cells, SCFAs increased the expression of CRAMP, which helps prevent the development of diabetes [106]. According to Zhang et al. [106], SCFAs increase oxygen consumption and activate HIF-1 signaling in macrophages by inhibiting HDAC. Furthermore, SCFA-induced HIF-1 can increase antimicrobial effector synthesis and bacterial clearance by macrophages. This novel mechanism could potentially contribute to bacterial clearance by macrophages.
3. Health Benefits of Dietary Fatty Acids
The production and consumption of fermented food is a potent strategy for addressing societal socioeconomic and health issues (including malnutrition, allergies, obesity, and aging) [20]. Fermentation processes regenerate NAD+ through redox reactions that result in pyruvate, its derivatives, and diverse other end-products. Pyruvate is a simple α-keto acid and major intermediate formed at the biochemical junction of glycolysis and the tricarboxylic acid cycle. Pyruvate precursors many valuable biomolecules [108,109]. Sugar, amino acids, and fatty acid-containing compounds are oxidized by transferring hydrogen ions from intermediate products to organic molecules such as pyruvate and acetyl CoA—which are final receptors for hydrogen ions [110]. The metabolism of these biomolecules results in flatus and fatty acids—critical modulators of hosts’ immune responses (see Figure 1A–C).
Traditional fermented foods all over the globe are believed to foster health through the functions and characteristics associated with their myriad components. They foster health via the activities of biosynthesized organic acids, modified nutrient molecules, gut microbiota, whole microbial cells or cell components, and activated immune cells [20]. The amount and type of dietary fatty acids affect the composition of membrane phospholipids and gene expression [111]. These biomolecules influence the biological and chemical barriers in the intestine, such as epithelial cells, tight junctions, and mucins, respectively [37], and act as signaling agents with known influences on membrane receptors (both on epithelial cells and immune cells) [48]. This means that dietary fatty acids (such as other food components) are not only nutrient compounds; they are packed with information and regulate genetic expressions. Experts believe that dietary fatty acids compete for common receptor molecules/mechanisms and either shut down the production of disease-causing genes or promote the expression of health-promoting genes [37]. Fatty acids act like hydrophobic hormones, binding to and activating nuclear receptors [111,112]. They regulate gene transcription via changes in the activity or abundance of nuclear receptors such as PPARs, LXR, HNF4α, TLR, SREBP, and RXR (see Figure 2).
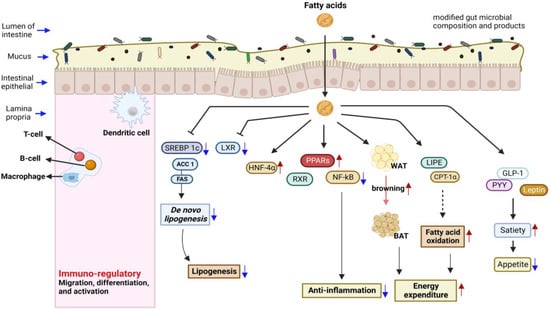
Figure 2.
Fatty acids regulate many health-promoting pathways and innate immune responses. They regulate gene transcription via changes in the activity or abundance of nuclear receptors.
Flavor-potentiating nucleotides and amino acids, which increase fermented foods’ attractiveness to consumers, are additional constituents that affect health [20]. Lastly, fermenting microbes in the food and host’s guts. Many ubiquitous and versatile microbial taxa elicit food spoilage or spontaneous fermentation processes by acting like chiral catalysts (high positional specificity and stereospecificity). They employ processes that add, remove, or modify functional groups of suitable macromolecules at specific sites [113]. Concomitant live microbes in fermented foods act as probiotics; they survive the gastric transit and reach the colon, where they exact their actions. Moreover, cellular components of inactivated microbes, such as peptidoglycan, surface proteins, exopolysaccharides, D-phenylacetic acid (by lactic acid bacteria: LAB), and lipoteichoic acids, elicit immune responses in many animal models [20]. Despite their transient nature, fermentation-associated microbes are metabolically active and aid in the synthesis of molecules from ingested food substances that modulate signaling effects via epithelial receptors [20]. Successions during fermentation eliminate most starters as substrates are transformed, harsh by-products are released, energy sources are used up, and the environment becomes unconducive [10]. Therefore, the characteristics and properties of finished products are determined by surviving microbes, including those in low concentrations [10]. However, not all fermented foods contain live microbes at the point of consumption—as some are deactivated [20].
4. Challenges with Traditional Fermented Foods
Scientists agree that microorganisms co-evolved with humans; however, studies are still exploring more profound levels of their influence on human health. For example, diets appreciably impact gut microbiome homeostasis. The gut microbiome is connected to the innate immune system, which virtually connects all-important body organs. Traditional fermented foods contain bioactive molecules that modulate the gut microbiome, systemic immune responses, and disease conditions. Fermentation outcomes can sometimes be mixed in desirability; acetic acid-producing bacteria such as Acetobacter, Gluconacetobacter, and Gluconobacter are valuable in vinegar production and microbial cellulose; however, their presence devalues wines through spoilage [114]. Studies also show that fatty acids bind and influence the expression of genes through the activation of receptors such as fatty acid binding proteins (FABPs) and peroxisome proliferator-activated receptors (PPARs) [36]. Haghikia and co-workers [22] showed that long-chain and short-chain fatty acids (fermentation by-products) had opposite modulating effects on T helper 1 cells. LCFAs upregulated the MAPK pathway via p38, enhanced the differentiation and proliferation, and impaired the intestinal sequestration of T helper 1 cells. In contrast, dietary SCFA suppressed the JNK1 and p38 pathways and expanded intestinal T-regulatory cells. This and many other studies show that fatty acids are energy sources and receptors for gene expression and systemic energy homeostasis regulation [36].
Natural fermentation processes (such as traditional food fermentation, intestinal diet-microbe interactions, and bio-geochemical cycling of agro or organic wastes) utilize diverse or mixed microbial cultures that inspire high productivity and functionality, resulting in the transformation of an extensive range of substrates. Co-evolution supports cross-feeding, co-metabolism, symbiotic growth, and increased microbial survival and diversity [11,115,116,117]. Traditional fermented foods contain microbial consortia, bioactive molecules, and specific anti-nutrient molecules (such as phytic acid) that could reduce the digestibility and bioavailability of proteins and carbohydrates [17]. Mixed cultures contrast axenic processes that utilize adapted or genetically engineered single-strain microbes to produce desired outcomes. For this purpose, axenic cultures are featured more in environmental remedial and commercially/scaled biotechnological processes that seek to achieve suitable substrate transformative results and less contamination [118].
Fermented foods have inherent signature microbes—both functional and non-functional. Traditional or subsistent production uses fermentation broths (or back-slopping) that contain autochthonous microbes to kick-start their processes; this is open to contamination and inconsistent outcomes. Another source of potentially beneficial or spoilage/pathogenic microbes is equipment-based organisms [119,120]—using the same vessel consistently facilitates the inoculation of raw food material from the previous batch. Fermentation processes create hostile eco-conditions that are unconducive to pathogenic microorganisms: appreciable proportions of organic acids (>100 mM), inhibitory growth substances (diacetyl, acetaldehydes, mycosin, and bacteriocins), salt, nitrite, antimicrobials, lowered water activity, and elimination of carbohydrate substrates [10,23,110]. However, in unhygienic conditions, hostile conditions may not be created fast enough to exclude toxin-producing and spore-forming microbes such as Escherichia coli, Listeria monocytogenes, Yersinia enterocolitica, Staphylococcus aureus, Bacillus cereus, Clostridium botulinum, etc. [121]. Industrialists will gather more sales clout if they label their products as having these signature characteristics, including the autochthonous microbes—as undefined and natural starter cultures possess high genetic diversity that might be responsible for product outcome [119,122]. Microbial fingerprints will not just allow consumers to distinguish similar products but also will ensure that consumers get the same thrill and benefits during and after consuming the said food. However, microbes are deactivated in a few fermented foods before consumption [20].
Traditional fermented foods are a significant source of protein, vitamins, minerals, and other vital nutrients [97]. However, the low or outright lack of modern biotechnology applications prevents clinically exploring their gut modulatory health function [10,17]. The most unexplored set of traditional foods is those from African and Asian countries, despite their high nutritional contents, from published data [10]. According to Oguntoyinbo [120] and De Filippis et al. [119], modernization and commercialization would eventually detect how traditional fermented products become mainstream commodities in the global market. Aspects such as safety, hygiene standards, and preservation of products’ characteristics would be of profound focus. According to Tamang et al. [10], Amplicon-based high-throughput sequencing and real-time quantitative PCR have been used to target different strains and species of microbes with high accuracy, elucidate their encoded metabolic pathways, and for quality control. As the demography of urban populations in Asia and Africa continues to grow, middle- and low-income earners will begin to demand standardized and well-packaged yet nutritious traditional-like fermented foods as part of their snacks or main diets.
5. Regulations and Perspectives
To commercialize traditional fermentation process(es), industrialists would have to beat the safety and consistency challenges. Additionally, other setbacks, including ethics and regulatory concerns, challenges with the applicability of research outputs due to the high cost of effective methods, the high cost of high-dimensional data analyses and interpretation, and low reproducibility [122,123], have to be overcome too. Many commercial industries that produce fermented food products apply good hygiene practice (GHP) and hazard analysis and critical control point (HACCP) systems to lessen the advent of public health issues. These systems must be applied to produce safe, uniquely improved products with bioavailable nutrients, an extended shelf life, and excellent sensory properties. These characteristics elicit eubiotic effects on the gut microbiome and high health-promoting functions [122,124]. Successful processes would have to factor in advanced and safe biotechnological processes that would preserve the peculiar characteristics of traditionally fermented foods and still retain the majority of those functional and non-functional microbes responsible for those characteristics [15,119,121,124].
Galimberti and team [122] suggest that applying multi-omics technologies when studying spontaneous microbial consortia, including their interaction in traditional fermented foods, helps preserve and predict unique microbial fingerprints, biotransformation processes that occur over time, and resultant health outcomes in humans. Multi-omics allows for the identification of capable microbes responsible for the production of targeted products. Obafemi and co-workers [17] suggest that researchers will have proper and in-depth profiles of active microbial consortia by utilizing high-throughput sequencing (HTS) techniques when studying African traditional fermented foods. HTS techniques reveal the taxonomy, diversity, functional potential, and gene expression patterns of capable microbes. Once these functional microbial genera are identified, regulations for labels could be formulated [20]. With improvements in several techniques, the cost and error setbacks can be reduced, allowing for near-predictive analysis and functions.
Advanced synthetic biology and controlled fermentation will not stripe the benefits of traditional biodiversity if done properly. It should enable the introduction of additional strains with known and advantageous functionalities [10]. However, designing and growing a selected community of starter cultures is not as simple as theocratized [119,125]. One will need a careful and informed orchestration of possible factors that could overshadow valuable traits. HTS application offers the potential to reconstruct draft and complete genomes directly from metagenomics reads, avoiding previous cultivation and isolation, overcoming the limitations of culture-based techniques, and allowing in situ strain monitoring [119]. Complex interactions that occur within microbial consortia in food environments can be elucidated with combined molecular approaches, increasing our understanding of how to exploit invaluable microbial resources, and ensure process efficiency as well as food quality and safety.
Metagenomics tools have been used to flag beneficial genes and harmful microbial traits, some of which code for flavor, alcohol production, antibiotic resistance, and mycotoxin production [20,126]. The rapidity and prediction accuracy help in forming safety policies, moving away from mere microbes’ searches to eliminating microbes with harmful products (toxins) and traits. For example, mycotoxin-producing Aspergillus and Penicillium have been successfully eradicated from cheese, koji, and other fermented food products [20]. However, as long as no complaints have been reported for certain of these traditionally fermented foods, the general public would continue to tolerate them up to their spoilage limit [14]. Because microbial cultures have transitioned from being part of food production to being viewed as food in itself [14], policies that ensure safe consumption and extended shelf life would be influenced by the type, culture, and origin of the food [127,128]. Therefore, regulations covering them as cultures for food production (ingredients), food additives, and foods would differ from the additional assessment level accorded novel or genetically modified microorganisms [14,127].
6. Conclusions
Although microbial growth and enzyme production in the food microenvironment can either result in spoilage or fermentation, the latter results in desirable attributes and end products. Traditional fermented foods render health to consumers through the actions of inherent probiotics or live microbes and other health-promoting components. Although there are no recommended levels, fermented foods laden with microbes and health-promoting components are good for gut health and the immune system—although only 26% of adults and 20% of children consume such foods. Recently, dietary recommendations suggested that we incorporate diverse sources of food components on our plates. Fermented foods are now seen as a good source of dietary fatty acids.
With the advent of concept foods that target select genes and pathways [129,130], traditional foods can act as templates for creating functional foods with multi-health and organoleptic functions. Fermented foods are popular due to associated health benefits: one-third of food consumed by humans is fermented types [130]. Studies show that although microorganisms are uniquely individual cells, they interact and act better in communal natural settings. Preserving these interactions could sustain the increasing demand for traditionally fermented foods—against those processed with axenic or single synthetic cultures, to initiate rapid processes and limit the emergence of spoilage or pathogenic strains. Bio-engineered consortia or inocula and their fermented by-products would benefit the consuming public more.
Author Contributions
Conceptualization: O.D.A. and H.Z.; writing—original draft preparation, Y.X., C.L.M., C.V.O., H.Z. and Y.Y.; writing—review and editing, Y.X., M.H., C.V.O., C.L.M., Y.Y., J.L., M.Z., B.L., H.Z., X.Y. and L.B.; visualization, M.H., C.V.O. and C.L.M.; supervision, O.D.A., H.Z., Y.Y. and K.Y. All authors made significant contributions to the development of all aspects of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable- because studies did not involve animal models or humans.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AA: arachidonic acid; ACCα: acetyl-CoA carboxylase α; AMPs: antimicrobial peptides; ApoCIII: apolipoprotein CIII; ATGL: adipose triglyceride lipase; BAT: brown adipose tissue; Caco-2 cells: human colon carcinoma cell line; CAR: chimeric antigen receptor; CCL5: C-C motif chemokine ligand 5; Cdx2-IEC cells: caudal type homeobox 2-intestinal cell line; cIAP2: cellular inhibitor of apoptosis protein-2; COX-2: cyclooxygenase-2; CRAMP: cathelicidin-related antimicrobial peptide; CTLs: cytotoxic T lymphocytes; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; FABP4/aP2: adipocyte fatty acid binding protein 4; FABPs: fatty acid binding proteins; FASN: fatty acid synthase; FAT: fatty acid translocase; FBG: fasting blood glucose; FGF15: fibroblast growth factor 15; FGFR4: FGF receptor 4; Foxo3: forkhead Box O3; FXR: farnesoid X receptor; GHP: good hygiene practice; GLUT4: glucose transporter 1; GPCRs: G-protein coupled receptors; GPR43: G protein-coupled receptor 43; H3Kac: histone 3 lysine acetylation; HACCP: hazard analysis and critical control point; HDACs: histone deacetylases; HFD: high-fat diet; HIF-1: hypoxia-inducible factor; HNF4α: hepatocyte nuclear factor 4α; HPA: heneicosapentaenoate; HPdLFs: human periodontal ligament fibroblasts; HPTMs: histone post-translational modifications; HTS: high-throughput sequencing; IL: interleukin; iNOS: inducible nitric oxide synthase; IPEC-J2 cells: intestinal porcine epithelial cell line-J2; JAK2: Janus kinase 2; JNK: c-Jun N-terminal kinase; LAB: lactic acid bacteria; LCFAs: long-chain fatty acids; LDL-c: low-density lipoprotein-cholesterols; LPL: lipoprotein lipase; LXR: liver X receptor; MAPK: mitogen-activated protein kinase; MCFAs: medium-chain fatty acids; MCP-1: monocyte chemotactic protein-1; MCT1: monocarboxylate transporter 1; mRNA: messenger ribonucleic acid; mTOR: mammalian target of rapamycin; mTORC1: mTOR complex 1; NAD+: nicotinamide adenine dinucleotide; NF-kB: nuclear factor kappa B; NLRP3: NOD-like receptor protein 3; OCFAs: odd-chain saturated fatty acids; PCR: polymerase chain reaction; PPARs: peroxisome proliferator-activated receptors; PUFA: polyunsaturated fatty acids; RXR: retinoid X receptor; SCFAs: short-chain fatty acids; SMCT1: sodium-coupled monocarboxylate transporter 1; SP1: specificity protein 1; SREBP: sterol regulatory element-binding proteins; STAT3: signal transducer and activator of transcription 3; TAG: triacylglycerol; TER: transepithelial electrical resistance; TG: triglycerides; TJ: tight junctions; TLR: toll-like receptors; TNF-α: tumor necrosis factor-alpha; VLDL-c: very low-density lipoprotein cholesterol; WAT: white adipose tissue; 5-LO: 5-lipoxygenase.
References
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Chilton, S.N.; Burton, J.P.; Reid, G. Inclusion of Fermented Foods in Food Guides around the World. Nutrients 2015, 7, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Feng, R.; Chen, L.; Chen, K. Fermentation Trip: Amazing Microbes, Amazing Metabolisms. Ann. Microbiol. 2018, 68, 717–729. [Google Scholar] [CrossRef]
- Mgbechidinma, C.L.; Adegoke, C.O.; Ogunbanwo, S.T. Lactic Acid Bacteria as Bioactive Potential Against Selected Resistance Candida Species and Pathogenic Bacteria. Int. J. Pharm. Biol. Sci. Arch. 2020, 8, 19–32. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ganapathy, V. Short, but Smart: SCFAs Train T Cells in the Gut to Fight Autoimmunity in the Brain. Immunity 2015, 43, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Etok, C.; Akan, O.; Adegoke, A. Bioremediation of Crude Oil Contaminated Soils Using Surfactants and Hydrocarbonoclastic Bacteria. Br. Microbiol. Res. J. 2015, 9, 1–6. [Google Scholar] [CrossRef]
- Mgbechidinma, C.L.; Zheng, G.; Baguya, E.B.; Zhou, H.; Okon, S.U.; Zhang, C. Fatty acid composition and nutritional analysis of waste crude fish oil obtained by optimized milder extraction methods. Environ Eng Res. 2023, 28, 22003. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Devi, M.K.; Kumar, P.S. Engineering Microbes for Enhancing the Degradation of Environmental Pollutants: A Detailed Review on Synthetic Biology. Environ. Res. 2022, 214, 113868. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Microbial Metabolic Pathways and the “fermented Plant Foods—Human Health” Axis. Foods 2021, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lensch, A.; Duwenig, E.; Dederer, H.G.; Kärenlampi, S.O.; Custers, R.; Borg, A.; Wyss, M. Recombinant DNA in Fermentation Products Is of No Regulatory Relevance. Food Control 2022, 141, 109170. [Google Scholar] [CrossRef]
- Vogel, R.F.; Hammes, W.P.; Habermeyer, M.; Engel, K.; Knorr, D.; Eisenbrand, G. Microbial Food Cultures–Opinion of the Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG). Mol. Nutr. Food Res. 2011, 55, 654–662. [Google Scholar] [CrossRef]
- Bell, V.; Ferrão, J.; Fernandes, T. Nutritional Guidelines and Fermented Food Frameworks. Foods 2017, 6, 65. [Google Scholar] [CrossRef]
- Nai, C.; Meyer, V. From Axenic to Mixed Cultures: Technological Advances Accelerating a Paradigm Shift in Microbiology. Trends Microbiol. 2018, 26, 538–554. [Google Scholar] [CrossRef]
- Obafemi, Y.D.; Oranusi, S.U.; Ajanaku, K.O.; Akinduti, P.A.; Leech, J.; Cotter, P.D. African Fermented Foods: Overview, Emerging Benefits, and Novel Approaches to Microbiome Profiling. NPJ Sci. Food 2022, 6, 15. [Google Scholar] [CrossRef]
- Olovo, C.; Udoekong, N.; Akan, O. Precision Nutrition, Diet and Gut-Microbiota in Obesity. J. Biotechnol. Bioresearch 2021, 2, 4. [Google Scholar]
- Hesselmar, B.; Hicke-roberts, A.; Wennergren, G. Allergy in Children in Hand Versus Machine Dishwashing. Pediatrics 2015, 135, e590-7. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Mallick, R.; Basak, S.; Duttaroy, A.K. Fatty Acids and Evolving Roles of Their Proteins in Neurological, Cardiovascular Disorders and Cancers. Prog. Lipid Res. 2021, 83, 101116. [Google Scholar] [CrossRef]
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef]
- Ferreri, C.; Masi, A.; Sansone, A.; Giacometti, G.; Larocca, A.V.; Menounou, G.; Scanferlato, R.; Tortorella, S.; Rota, D.; Conti, M.; et al. Fatty Acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Suryanarayana, L.C.; Chandrashekara, K.A.; Krishnan, P.; Kush, A.; Ravikumar, P. Lactobacillus Plantarum Mediated Fermentation of Psidium Guajava, L. Fruit Extract. J. Biosci. Bioeng. 2015, 119, 430–432. [Google Scholar] [CrossRef]
- Jia, R.; Chen, H.; Chen, H.; Ding, W. Effects of Fermentation with Lactobacillus Rhamnosus GG on Product Quality and Fatty Acids of Goat Milk Yogurt. J. Dairy Sci. 2016, 99, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Yang, Y.; Wang, Z.; Lawrence, P.; Worobo, R.W.; Brenna, J.T. High Levels of Branched Chain Fatty Acids in Nātto and Other Asian Fermented Foods. Food Chem. 2019, 286, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Guo, W.; Huang, Z.; Tang, X.; Zhang, Q.; Yang, B.; Zhao, J.; Cui, S.; Zhang, H. Production of Conjugated Fatty Acids in Probiotic-Fermented Walnut Milk with the Addition of Lipase. LWT 2022, 172, 114204. [Google Scholar] [CrossRef]
- Erbaş, M.; Kemal Uslu, M.; Ozgun Erbaş, M.; Certel, M. Effects of Fermentation and Storage on the Organic and Fatty Acid Contents of Tarhana, a Turkish Fermented Cereal Food. J. Food Compos. Anal. 2006, 19, 294–301. [Google Scholar] [CrossRef]
- Asarat, M.; Apostolopoulos, V.; Vasiljevic, T.; Donkor, O. Short-Chain Fatty Acids Produced by Synbiotic Mixtures in Skim Milk Differentially Regulate Proliferation and Cytokine Production in Peripheral Blood Mononuclear Cells. Int. J. Food Sci. Nutr. 2015, 66, 755–765. [Google Scholar] [CrossRef]
- Annunziata, G.; Tenore, G.C.; Ciampaglia, R.; Schisano, C.; Narciso, V.; Maisto, M.; Novellino, E. Short-Time Lactic-Acid Fermentation Improves the Nutraceutical Value of Black Tea Beverage. In Proceedings of the CHIMALI 2018, Italian Food Chemistry Congress, Camerino, Italy, 24–27 September 2018; p. 1. [Google Scholar]
- Uțoiu, E.; Matei, F.; Toma, A.; Diguță, C.F.; Ștefan, L.M.; Mănoiu, S.; Vrăjmașu, V.V.; Moraru, I.; Oancea, A.; Israel-Roming, F.; et al. Bee Collected Pollen with Enhanced Health Benefits, Produced by Fermentation with a Kombucha Consortium. Nutrients 2018, 10, 1365. [Google Scholar] [CrossRef]
- Hu, R.; Zeng, F.; Wu, L.; Wan, X.; Chen, Y.; Zhang, J.; Liu, B. Fermented Carrot Juice Attenuates Type 2 Diabetes by Mediating Gut Microbiota in Rats. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The Chemical Profiling of Fatty Acids during the Brewing Process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Rawls, J.F. Feeling the Burn: Intestinal Epithelial Cells Modify Their Lipid Metabolism in Response to Bacterial Fermentation Products. Cell Host Microbe 2020, 27, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Roopashree, P.G.; Shetty, S.S.; Suchetha Kumari, N. Effect of Medium Chain Fatty Acid in Human Health and Disease. J. Funct. Foods 2021, 87, 104724. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Vasu, R.; Zhang, H. The Role of Long-Chain Fatty Acids in Inflammatory Bowel Disease. Mediators Inflamm. 2019, 2019, 8495913. [Google Scholar] [CrossRef]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Marrocco, F.; Delli Carpini, M.; Garofalo, S.; Giampaoli, O.; De Felice, E.; Di Castro, M.A.; Maggi, L.; Scavizzi, F.; Raspa, M.; Marini, F.; et al. Short-Chain Fatty Acids Promote the Effect of Environmental Signals on the Gut Microbiome and Metabolome in Mice. Commun. Biol. 2022, 5, 517. [Google Scholar] [CrossRef]
- Venn-Watson, S.K.; Butterworth, C.N. Broader and Safer Clinically-Relevant Activities of Pentadecanoic Acid Compared to Omega-3: Evaluation of an Emerging Essential Fatty Acid across Twelve Primary Human Cell-Based Disease Systems. PLoS ONE 2022, 17, e0268778. [Google Scholar] [CrossRef]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Hornemann, S.; Petzke, K.J.; Schulze, M.B.; Pfeiffer, A.F.H.; Klaus, S. Odd-Chain Fatty Acids as a Biomarker for Dietary Fiber Intake: A Novel Pathway for Endogenous Production from Propionate. Am. J. Clin. Nutr. 2017, 105, 1544–1551. [Google Scholar] [CrossRef]
- Miguel, A.S.; Salgado, M.T.; Rodriguez, M.S.M.; Pachon, J.; Martin, M.A.S.; Lobatoa, P.C.; Pastor, M.R. Role of Butyric Acid in Food and Intestinal Health. Immunol. Infect. 2018, 1, 1–5. [Google Scholar]
- Stachowska, E.; Wisniewska, M.; Dziezyc, A.; Bohatyrewicz, A. Could the Use of Butyric Acid Have a Positive Effect on Microbiota and Treatment of Type 2 Diabetes? Eur. Rev. Med. Pharm. Sci. 2021, 25, 4570–4578. [Google Scholar]
- Coppola, S.; Avagliano, C.; Calignano, A.; Berni Canani, R. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef] [PubMed]
- Tengeler, A.C.; Gart, E.; Wiesmann, M.; Arnoldussen, I.A.C.; van Duyvenvoorde, W.; Hoogstad, M.; Dederen, P.J.; Verweij, V.; Geenen, B.; Kozicz, T.; et al. Propionic Acid and Not Caproic Acid, Attenuates Nonalcoholic Steatohepatitis and Improves (Cerebro) Vascular Functions in Obese Ldlr−/−. Leiden Mice. FASEB J. 2020, 34, 9575–9593. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Baskaran, S.A.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Anticarcinogenic Properties of Medium Chain Fatty Acids on Human Colorectal, Skin and Breast Cancer Cells in Vitro. Int. J. Mol. Sci. 2015, 16, 5014–5027. [Google Scholar] [CrossRef] [PubMed]
- Lemarié, F.; Beauchamp, E.; Legrand, P.; Rioux, V. Revisiting the Metabolism and Physiological Functions of Caprylic Acid (C8:0) with Special Focus on Ghrelin Octanoylation. Biochimie 2016, 120, 40–48. [Google Scholar] [CrossRef]
- Hismiogullari, S.E.; Elyurek, E.; Hismiogullari, A.A.; Sahin, F.; Basalan, M.; Yenice, S. Effects of Caproic and Caprylic Acids on Microbial Growth and Cytotoxicity. J. Anim. Vet. Adv. 2008, 7, 731–735. [Google Scholar]
- Jain, S.; Rai, R.; Singh, D.; Vohora, D. Octanoic Acid a Major Component of Widely Consumed Medium-Chain Triglyceride Ketogenic Diet Is Detrimental to Bone. Sci. Rep. 2021, 11, 7003. [Google Scholar] [CrossRef]
- Shoji, H.; Kunugi, H.; Miyakawa, T. Acute and Chronic Effects of Oral Administration of a Medium-Chain Fatty Acid, Capric Acid, on Locomotor Activity and Anxiety-like and Depression-Related Behaviors in Adult Male C57BL/6J Mice. Neuropsychopharmacol. Reports 2022, 42, 59–69. [Google Scholar] [CrossRef]
- Mett, J.; Müller, U. The Medium-Chain Fatty Acid Decanoic Acid Reduces Oxidative Stress Levels in Neuroblastoma Cells. Sci. Rep. 2021, 11, 6135. [Google Scholar] [CrossRef]
- Damiano, F.; De Benedetto, G.E.; Longo, S.; Giannotti, L.; Fico, D.; Siculella, L.; Giudetti, A.M. Decanoic Acid and Not Octanoic Acid Stimulates Fatty Acid Synthesis in U87MG Glioblastoma Cells: A Metabolomics Study. Front. Neurosci. 2020, 14, 783. [Google Scholar] [CrossRef]
- Warren, E.C.; Dooves, S.; Lugarà, E.; Damstra-Oddy, J.; Schaf, J.; Heine, V.M.; Walker, M.C.; Williams, R.S.B. Decanoic Acid Inhibits MTORC1 Activity Independent of Glucose and Insulin Signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 23617–23625. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Zhang, J.; Xu, B.; Tegomo, A.F.; Sagada, G.; Zheng, L.; Wang, L.; Id, Q.S. Effect of Dietary Supplementation of Lauric Acid on Growth Performance, Antioxidative Capacity, Intestinal Development and Gut Microbiota on Black Sea Bream (Acanthopagrus Schlegelii). PLoS ONE 2022, 17, e0262427. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Chen, J.W.; Rathod, J.; Jiang, Y.Z.; Tsai, P.J.; Hung, Y.P.; Ko, W.C.; Paredes-Sabja, D.; Huang, I.H. Lauric Acid Is an Inhibitor of Clostridium Difficile Growth in Vitro and Reduces Inflammation in a Mouse Infection Model. Front. Microbiol. 2018, 8, 2635. [Google Scholar] [CrossRef]
- Uday-Kumar, D.; Christopher, V.; Sobarani, D.; Nagendra-Sastry, Y. Lauric Acid as Potential Natural Product in the Treatment of Cardiovascular Disease: A Review. J. Bioanal. Biomed. 2014, 6, 37–39. [Google Scholar] [CrossRef]
- Grünert, S.C.; Wendel, U.; Lindner, M.; Leichsenring, M.; Schwab, K.O.; Vockley, J.; Lehnert, W.; Ensenauer, R. Clinical and Neurocognitive Outcome in Symptomatic Isovaleric Acidemia. Orphanet J. Rare Dis. 2012, 7, 9. [Google Scholar] [CrossRef]
- Szymanska, E.; Jezela-Stanek, A.; Bogdanska, A.; Rokicki, D.; Emczynska-Seliga, E.E.; Pajdowska, M.; Ciara, E.; Tylki-Szymanska, A. Long Term Follow-up of Polish Patients with Isovaleric Aciduria. Clinical and Molecular Delineation of Isovaleric Aciduria. Diagnostics 2020, 10, 738. [Google Scholar] [CrossRef]
- Netto Cândido, T.L.; da Silva, L.E.; Cândido, F.G.; Valente, F.X.; da Silva, J.S.; Gomes Lopes, D.R.; do Carmo Gouveia Peluzio, M.; Mantovani, H.C.; de Cássia Gonçalves Alfenas, R. Effect of the Ingestion of Vegetable Oils Associated with Energy-Restricted Normofat Diet on Intestinal Microbiota and Permeability in Overweight Women. Food Res. Int. 2021, 139, 109951. [Google Scholar] [CrossRef]
- Cho, K.M.; Kim, Y.S.; Lee, M.; Lee, H.Y.; Bae, Y.S. Isovaleric Acid Ameliorates Ovariectomy-Induced Osteoporosis by Inhibiting Osteoclast Differentiation. J. Cell. Mol. Med. 2021, 25, 4287–4297. [Google Scholar] [CrossRef]
- Lee, S.Y.; Rhee, M.S.; Dougherty, R.H.; Kang, D.H. Antagonistic Effect of Acetic Acid and Salt for Inactivating Escherichia Coli O157: H7 in Cucumber Puree. J. Appl. Microbiol. 2010, 108, 1361–1368. [Google Scholar] [CrossRef]
- Valdes, D.S.; So, D.; Gill, P.A.; Kellow, N.J. Effect of Dietary Acetic Acid Supplementation. J. Acad. Nutr. Diet. 2021, 121, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fan, X.; Lu, Y.; Chen, D.; Zhao, Y.; Qi, K. Dietary Acetic Acid Suppress High-Fat Diet-Induced Obesity in Mice by Altering Taurine Conjugated Bile Acids Metabolism. Curr. Res. Food Sci. 2022, 5, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Meng, L.; Ai, D.; Hou, N.; Li, H.; Shuai, X.; Peng, X. Acetic Acid Alleviates the Inflammatory Response and Liver Injury in Septic Mice by Increasing the Expression of TRIM40. Exp. Ther. Med. 2019, 17, 2789–2798. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological Effects of Propionic Acid in Humans; Metabolism, Potential Applications and Underlying Mechanisms. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Linker, R.A.; Gold, R.; Haghikia, A.; Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080. [Google Scholar] [CrossRef]
- Adler, G.K.; Hornik, E.S.; Murray, G.; Bhandari, S.; Yadav, Y.; Heydarpour, M.; Basu, R.; Garg, R.; Tirosh, A. Acute Effects of the Food Preservative Propionic Acid on Glucose Metabolism in Humans. BMJ Open Diabetes Res. Care 2021, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Al-lahham, S.; Roelofsen, H.; Rezaee, F.; Weening, D.; Hoek, A.; Vonk, R. Propionic Acid Affects Immune Status and Metabolism in Adipose Tissue from Overweight Subjects. Eur. J. Clin. Investig. 2012, 42, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Rioux, V.; Catheline, D.; Bouriel, M.; Legrand, P. Dietary Myristic Acid at Physiologically Relevant Levels Increases the Tissue Content of C20: 5 n-3 and C20: 3 n-6 in the Rat. Reprod. Nutr. Dev. 2005, 45, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.-S.; Shu, G.; Xie, Q.-P.; Zhu, X.-T.; Gao, P.; Zhou, G.-X.; Wang, S.; Wang, L.-N.; Xi, Q.Y.; Zhang, Y.-L.; et al. Myristic Acid (MA) Promotes Adipogenic Gene Expression and the Differentiation of Porcine Intramuscular Adipocyte Precursor Cells. J. Integr. Agric. 2014, 13, 2488–2499. [Google Scholar] [CrossRef]
- Olivieri, O.; Speziali, G.; Castagna, A.; Pattini, P.; Udali, S.; Pizzolo, F.; Liesinger, L.; Gindlhuber, J.; Tomin, T.; Schittmayer, M.; et al. The Positive Association between Plasma Myristic Acid and ApoCIII Concentrations in Cardiovascular Disease Patients Is Supported by the Effects of Myristic Acid in HepG2 Cells. J. Nutr. 2020, 150, 2707–2715. [Google Scholar] [CrossRef]
- de Souza, J.; Strieder-Barboza, C.; Contreras, G.A.; Lock, A.L. Effects of Timing of Palmitic Acid Supplementation during Early Lactation on Nutrient Digestibility, Energy Balance, and Metabolism of Dairy Cows. J. Dairy Sci. 2019, 102, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-delgado, J.; Barroso, E.; Vázquez-carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhong, Y.; Zhou, S.; Li, Q.Q. Palmitic Acid-Induced Apoptosis in Pancreatic β-Cells Is Increased by Liver X Receptor Agonist and Attenuated by Eicosapentaenoate. In Vivo 2011, 25, 711–718. [Google Scholar] [PubMed]
- Senyilmaz-Tiebe, D.; Pfaff, D.H.; Virtue, S.; Schwarz, K.V.; Fleming, T.; Altamura, S.; Muckenthaler, M.U.; Okun, J.G.; Vidal-puig, A.; Nawroth, P.; et al. Dietary Stearic Acid Regulates Mitochondria in Vivo in Humans. Nat. Commun. 2018, 9, 3129. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhao, X.; Siegal, G.P.; Desmond, R.; Hardy, R.W. Dietary Stearic Acid Leads to a Reduction of Visceral Adipose Tissue in Athymic Nude Mice. PLoS ONE 2014, 9, e104083. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nill, K.; Takechi-Haraya, Y.; Playford, M.P.; Nguyen, D.; Yu, Z.; Pryor, M.; Tang, J.; Rojulpote, K.V.; Mehta, N.N.; et al. Differential Effect of Dietary Supplementation with a Soybean Oil Enriched in Oleic Acid versus Linoleic Acid on Plasma Lipids and Atherosclerosis in LDLR-Deficient Mice. Int. J. Mol. Sci. 2022, 23, 8385. [Google Scholar] [CrossRef]
- Carrillo, C.; Cavia, M.M.; Alonso-Torre, S. Role of Oleic Acid in Immune System: Mechanism of Action, a Review. Nutr. Hosp. 2012, 27, 978–990. [Google Scholar] [CrossRef]
- Schuldt, L.; Brandenstein, K.; Jacobs, C.; Symmank, J. Oleic Acid-Related Anti-Inflammatory Effects in Force-Stressed PdL Fibroblasts Are Mediated by H3 Lysine Acetylation Associated with Altered IL10 Expression. Epigenetics 2022, 17, 1892–1904. [Google Scholar] [CrossRef]
- Teres, S.; Barcelo-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escriba, P.V. Oleic Acid Content Is Responsible for the Reduction in Blood Pressure Induced by Olive Oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Epidemiology and Prevention Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef]
- Zhao, J.V.; Schooling, M.C. Effect of Linoleic Acid on Ischemic Heart Disease and Its Risk Factors: A Mendelian Randomization Study. BMC Med. 2019, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.Y.; Lemaitre, R.N.; King, I.B.; Song, X.; Psaty, B.M.; Siscovick, D.S.; Mozaffarian, D. And Total and Cause-Specific Mortality. Circulation 2014, 130, 1245–1253. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio: Health Implications. Nutrition-Sante 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Bemelmans, W.J.E.; Muskiet, F.A.J.; Feskens, E.J.M.; Vries, J.H.M.; Broer, J.; May, J.F.; Jong, B.M. Associations of Alpha-Linolenic Acid and Linoleic Acid with Risk Factors for Coronary Heart Disease. Eur. J. Clin. Nutr. 2000, 54, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Kwon, H.H.; Hong, J.S.; Yoon, J.Y.; Park, M.S.; Jang, M.Y.; Suh, D.H. Effect of Dietary Supplementation with Omega-3 Fatty Acid and Gamma-Linolenic Acid on Acne Vulgaris: A Randomised, Double- Blind, Controlled Trial. Investig. Rep. 2014, 94, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Gara-Ali, M.; Zili, F.; Hosni, K.; Ben Ouada, H.; Ben-Mahrez, K. Lipophilic Extracts of the Thermophilic Cyanobacterium Leptolyngbya Sp. and Chlorophyte Graesiella Sp. and Their Potential Use as Food and Anticancer Agents. Algal Res. 2021, 60, 102511. [Google Scholar] [CrossRef]
- Romero, L.O.; Caires, R.; Nickolls, A.R.; Chesler, A.T.; Cordero-Morales, J.F.; Vásquez, V. A Dietary Fatty Acid Counteracts Neuronal Mechanical Sensitization. Nat. Commun. 2020, 11, 2997. [Google Scholar] [CrossRef] [PubMed]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of Dietary Odd-Chain Saturated Fatty Acid Pentadecanoic Acid Parallels Broad Associated Health Benefits in Humans: Could It Be Essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Bao-To, N.; Nguyen, Y.T.-K.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Pentadecanoic Acid, an Odd-Chain Fatty Acid, Suppresses the Stemness of MCF-7/SC Human Breast Cancer Stem-Like Cells through JAK2/STAT3 Signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef]
- Araujo, B.C.; Skrzynska, A.K.; Marques, V.H.; Tinajero, A.; Rio-Zaragoza, O.B.; Viana, M.T.; Mata-Sotres, J.A. Dietary Arachidonic Acid (20:4n-6) Levels and Its Effect on Growth Performance, Fatty Acid Profile, Gene Expression for Lipid Metabolism, and Health Status of Juvenile California Yellowtail (Seriiola Dorsalis). Fishes 2022, 7, 18. [Google Scholar] [CrossRef]
- Sueyasu, T.; Morita, S.; Tokuda, H.; Kaneda, Y.; Rogi, T.; Shibata, H. Dietary Arachidonic Acid Improves Age-Related Excessive Enhancement of the Stress Response. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2110–2119. [Google Scholar]
- Sambra, V.; Echeverria, F.; Valenzuela, A.; Chouinard-Watkins, R.; Valenzuela, R. Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle. Nutrients 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Chen, C.; Fu, J.; Zhu, L.; Shu, J.; Jin, M.; Wang, Y.; Zong, X. Dietary Fatty Acids in Gut Health: Absorption, Metabolism and Function. Anim. Nutr. 2021, 7, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Ita, R.E.; Egong, E.J.; Udofia, L.E.; Mgbechidinma, C.L.; Akan, O.D. Metaproteomics Insights into Fermented Fish and Vegetable Products and Associated Microbes. Food Chem. Mol. Sci. 2021, 3, 100045. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Kang, S.A.; Kim, H. Probiotics and Their Fermented Food Products Are Beneficial for Health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Running, C.A.; Mattes, R.D. Different Oral Sensitivities to and Sensations of Short-, Medium-, and Long-Chain Fatty Acids in Humans. Am. J. Physiol. Gastrointestine Liver Physiol. 2014, 307, 381–389. [Google Scholar] [CrossRef]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Ota, S.; Sakuraba, H. Uptake and Advanced Therapy of Butyrate in Inflammatory Bowel Disease. Immuno 2022, 2, 692–702. [Google Scholar]
- Byndloss, M.X.; Olsan, E.E.; Rivera-chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-Activated PPAR-γ-Signaling Inhibits Dysbiotic Enterobacteriaceae Expansion Mariana. Science 2017, 357, 570–575. [Google Scholar] [CrossRef]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota Derived Short Chain Fatty Acids Promote Histone Crotonylation in the Colon through Histone Deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial Short-Chain Fatty Acids Modulate CD8+ T Cell Responses and Improve Adoptive Immunotherapy for Cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Liu, M.; Lan, Y.; Sun, H.; Mai, K.; Wan, M. Short-Chain Fatty Acids Promote Intracellular Bactericidal Activity in Head Kidney Macrophages from Turbot (Scophthalmus maximus L.) via Hypoxia Inducible Factor-1α. Front. Immunol. 2020, 11, 615536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 Mediates Microbiota Metabolite SCFA Regulation of Antimicrobial Peptide Expression in Intestinal Epithelial Cells via Activation of MTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef]
- Zang, J.; Yu, D.; Zhang, P.; Xu, Y.; Xia, W. The Key Enzymes and Flavor Precursors Involved in Formation of Characteristic Flavor Compounds of Low-Salt Fermented Common Carp (Cyprinus carpio L.). LWT 2022, 154, 112806. [Google Scholar] [CrossRef]
- Maleki, N.; Eiteman, M.A. Recent Progress in the Microbial Production of Pyruvic Acid. Fermentation 2017, 3, 8. [Google Scholar] [CrossRef]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2021, 10, 69. [Google Scholar]
- Pegorier, J.-P.; May, C.L.; Girard, J. Control of Gene Expression by Fatty Acids. J. Nutr. 2004, 134, 2444S–2449S. [Google Scholar] [CrossRef]
- Bravo-Ruiz, I.; Medina, M.Á.; Martínez-Poveda, B. From Food to Genes: Transcriptional Regulation of Metabolism by Lipids and Carbohydrates. Nutrients 2021, 13, 1513. [Google Scholar] [CrossRef] [PubMed]
- Cano-flores, A.; Gómez, J.; Escalona-Torres, I.S.; Velasco-Bejarano, B. Microorganisms as Biocatalysts and Enzyme Sources. In Microorganisms; Blumenberg, M., Shaaban, M., Elgaml, A., Eds.; IntechOpen: London, UK, 2020; pp. 1–33. [Google Scholar]
- Raspor, P.; Goranovic, D. Biotechnological Applications of Acetic Acid Bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124. [Google Scholar] [CrossRef]
- Gravel, D.; Bell, T.; Barbera, C.; Bouvier, T.; Pommier, T.; Venail, P.; Mouquet, N. Experimental Niche Evolution Alters the Strength of the Diversity–Productivity Relationship. Nature 2011, 469, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Rev. Adv. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Mgbechidinma, C.L.; Akan, O.D.; Zhang, C.; Huang, M.; Linus, N.; Zhu, H.; Wakil, S.M. Integration of Green Economy Concepts for Sustainable Biosurfactant Production—A Review. Bioresour. Technol. 2022, 364, 128021. [Google Scholar] [CrossRef] [PubMed]
- Borchert, E.; Hammerschmidt, K.; Hentschel, U.; Deines, P. Enhancing Microbial Pollutant Degradation by Integrating Eco-Evolutionary Principles with Environmental Biotechnology. Trends Microbiol. 2021, 29, 908–918. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Recent Past, Present, and Future of the Food Microbiome. Annu. Rev. Food Sci. Technol. 2018, 9, 589–608. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A. Safety Challenges Associated with Traditional Foods of West Africa. Food Rev. Int. 2014, 30, 338–358. [Google Scholar] [CrossRef]
- Rossi, L.P.R.; Almeida, R.C.C.; Lopes, L.S.; Figueiredo, A.C.L.; Ramos, M.P.P.; Almeida, P.F. Occurrence of Listeria spp. in Brazilian Fresh Sausage and Control of Listeria Monocytogenes Using Bacteriophage P100. Food Control 2011, 22, 954–958. [Google Scholar] [CrossRef]
- Galimberti, A.; Bruno, A.; Agostinetto, G.; Casiraghi, M.; Guzzetti, L.; Labra, M. Fermented Food Products in the Era of Globalization: Tradition Meets Biotechnology Innovations. Curr. Opin. Biotechnol. 2021, 70, 36–41. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Alfredo, M.; Milagro, F. Holistic Integration of Omics Tools for Precision Nutrition in Health and Disease. Nutrients 2022, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Arias-Sánchez, F.I.; Vessman, B.; Mitri, S. Artificially Selecting Microbial Communities: If We Can Breed Dogs, Why Not Microbiomes? PLoS Biol. 2019, 17, e3000356. [Google Scholar] [CrossRef]
- Srinivas, M.; Sullivan, O.O.; Cotter, P.D.; Van Sinderen, D.; Kenny, J.G. The Application of Metagenomics to Study Microbial Communities and Develop Desirable Traits in Fermented Foods. Foods 2022, 11, 3297. [Google Scholar] [CrossRef]
- Laulund, S.; Wind, A.; Derkx, P.M.F.; Zuliani, V. Regulatory and Safety Requirements for Food Cultures. Microorganisms 2017, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Gómez-Sala, B.; O’Connor, E.M.; Kenny, J.G.; Cotter, P.D. Global Regulatory Frameworks for Fermented Foods: A Review. Front. Nutr. 2022, 9, 902642. [Google Scholar] [CrossRef]
- Hu, Z.; Li, M.; Cao, Y.; Akan, O.D.; Guo, T.; Luo, F. Targeting AMPK Signaling by Dietary Polyphenols in Cancer Prevention. Mol. Nutr. Food Res. 2022, 66, e2100732. [Google Scholar] [CrossRef]
- Akan, O.D.; Qin, D.; Guo, T.; Lin, Q.; Luo, F. Sirtfoods: New Concept Foods, Functions, and Mechanisms. Foods 2022, 11, 2955. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).