Traditional and New Microorganisms in Lactic Acid Fermentation of Food
Abstract
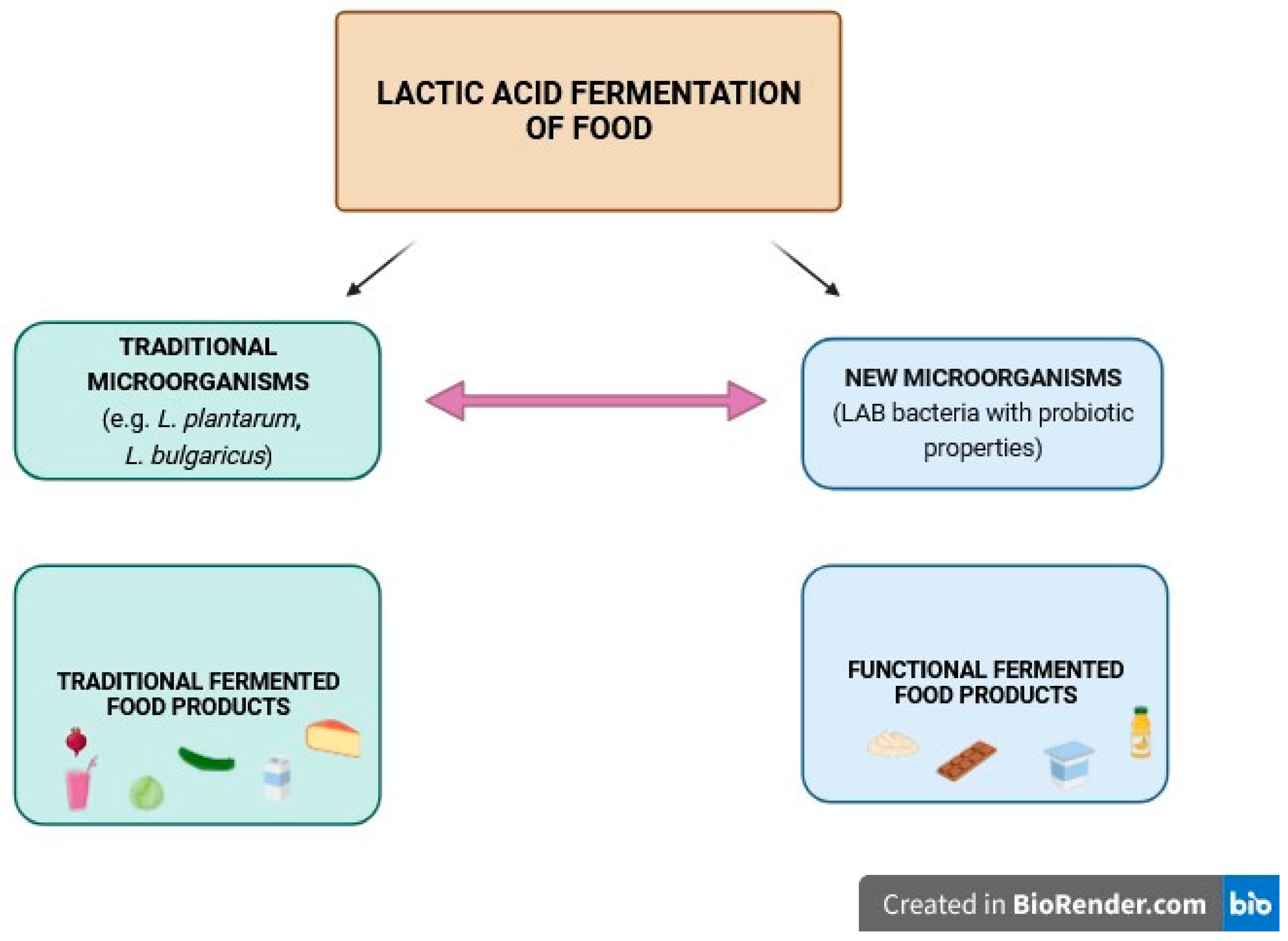
1. Introduction
2. The Historical Overview of Lactic Acid Fermentation in Food Production
3. The Traditional Application of Lactic Acid Fermentation in Food Production
3.1. Dairy Products
3.2. Fermented Plant-Origin Foods
3.3. Bakery Products
- (a)
- Lactic acid fermentation is a key component in the production of sourdough-based bread [62]. In this process, a mixture of flour and water is left to ferment, allowing wild yeast and LAB, naturally present in the environment, to thrive. These microorganisms metabolize the carbohydrates in the dough, producing lactic acid and acetic acid. This acidification of the dough imparts a distinct tangy flavor to sourdough bread and contributes to its unique texture and extended shelf life.
- (b)
- pH Control: Lactic acid fermentation is utilized to control the pH levels in bakery products. By adding lactic acid or using starter cultures with specific LAB strains, bakeries can adjust the acidity of the dough. This helps to improve the texture, flavor, and overall quality of the final product.
- (c)
- Staling Prevention: Lactic acid can also help in preventing staling (the process where bread becomes dry and less palatable) in bakery products. The staling process, in general, explains the mechanism of bread aging and begins immediately after baking [63,64]. When the thermal energy input is stopped, phase transition processes occur, changing the texture of the bread. The recrystallization of amylose within the first few hours after baking has a favorable impact on the solidification of the crumb structure, whereas amylopectin, the second principal macromolecule accounting for the starch percentage in wheat, crystallizes over a longer period of days.
- (d)
- Flavor improving: Lactic acid produced during fermentation contributes to the flavor profile of bakery products. Flavor composition in fermented wheat flour foods depends on some factors, such as fermentation process, cooking procedure, fat oxidation, and also where fermentation by sourdough-associated microbiota plays an important role in geographical indication of cereal [65,66] goods.It imparts a mild tangy taste, which can be desirable in various bread varieties, such as bagels, pretzels, and some types of rolls.
- (e)
- Improving food safety: Lactic acid, along with acetic acid produced during fermentation, has antimicrobial properties. It helps in preserving the freshness of baked goods and inhibiting the growth of harmful microorganisms, extending the shelf life of products [67].
3.4. Meat and Fish Products
3.5. Oriented Fermented Food Products
4. Probiotics as Novel Microorganisms in Lactic Acid Fermentation of Food
- Providing conditions in the food matrix that will guarantee the viability of probiotics, i.e., their growth and/or survival during food processing and storage, and at the same time maintaining a beneficial effect on health;
- Ensuring adequate sensory properties of the product;
- Ensuring a sufficiently high number of probiotic bacteria. The probiotic features and health benefits conferred are known to be LAB-strain-specific [146]. Probiotic food products must have a high concentration of microorganism cells (≥106 colony-forming units (CFU mL−1), or between 108 and 1011 colony-forming units (CFU) per day), to have the required positive impact [147]. Such a high number of bacterial cells should persist throughout the shelf life of food. Probiotic cells must survive the passage through the gastrointestinal tract, reach the colon in sufficient numbers, and, finally, adhere to, and colonize the gut epithelium [148,149,150].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaggia, F.; Di Gioia, D.; Baffoni, L.; Biavati, B. The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends Food Sci. Technol. 2011, 22, S58–S66. [Google Scholar] [CrossRef]
- Kołożyn-Krajewska, D.; Dolatowski, Z.J. Probiotic meat products and human nutrition. Process. Biochem. 2012, 47, 1761–1772. [Google Scholar] [CrossRef]
- Karbowiak, M.; Zielińska, D. Postbiotyki—Właściwości, zastosowanie i wpływ na zdrowie człowiek/ Postbiotics—Properties, application and impact on human health. Żywność Nauka Technol. Jakosc/Food Sci. Technol. Qual. 2020, 27, 22–37. [Google Scholar] [CrossRef]
- Kołożyn-Krajewska, D.; Dolatowski, Z.J. Probiotics in fermented meat products. Acta Sci. Pol. Technol. Aliment. 2009, 8, 61–76. [Google Scholar]
- Niakousari, M.; Razmjooei, M.; Nejadmansouri, M.; Barba, F.J.; Marszałek, K.; Koubaa, M. Current Developments in Industrial Fermentation Processes. In Fermentation Processes: Emerging and Conventional Technologies; Koubaa, M., Barba, F.J., Rooh-Inejad, S., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 23–96. [Google Scholar]
- Tamang, J.P. Diversity of fermented foods. In Fermented Foods and Beverages of the World, 1st ed.; Tamang, J.P., Kailasapathy, K., Eds.; CRC Press: New York, NY, USA, 2010; pp. 41–84. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Rosenberg, H.; Zhao, G.; Lengyel, D. Nadel Fermented beverage and food storage in 13,000 y-old stone mortars at Raqefet Cave, Israel: Investigating Natufian ritual feasting. J. Archaeol. Sci. Rep. 2021, 21, 783–793. [Google Scholar]
- Wang, J.; Jiang, L.; Sun, H. Early evidence for beer drinking in a 9000-year-old platform mound in southern China. PLoS ONE 2021, 16, e0255833. [Google Scholar] [CrossRef]
- Salque, M.; Bogucki, P.I.; Pyzel, J.; Sobkowiak-Tabaka, I.; Grygiel, R.; Szmyt, M.; Evershed, R.P. Earliest evidence for cheese making in the sixth millennium bc in northern Europe. Nature 2013, 493, 522–525. [Google Scholar] [CrossRef]
- El-Gendy, S.M.; Shaker, M. Fermented foods of Egypt and the Middle East. J. Food Prot. 1983, 46, 358–367. [Google Scholar] [CrossRef]
- Leroy, F.; Geyzen, A.; Janssens, M.; De Vuyst, L.; Scholliers, P. Meat fermentation at the crossroads of innovation and tradition: A historical outlook. Trends Food Sci. Technol. 2013, 31, 130–137. [Google Scholar] [CrossRef]
- Xiong, T.; Guan, Q.; Song, S.; Hao, M.; Xie, M. Dynamic changes of lactic acid bacteria flora during Chinese sauerkraut fermentation. Food Control 2012, 26, 178–181. [Google Scholar] [CrossRef]
- Peñas, E.; Martinez-Villaluenga, C.; Frias, J. Chapter 24—Sauerkraut: Production, Composition, and Health Benefits. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 557–576. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Jin, Y.-I.; Jeong, J.-C.; Chang, Y.H.; Lee, Y.; Jeong, Y.; Kim, M. Probiotic characteristics of Bacillus strains isolated from Korean traditional soy sauce. LWT Food Sci. Technol. 2017, 79, 518–524. [Google Scholar] [CrossRef]
- Chang, H.C. Healthy and safe Korean traditional fermented foods: Kimchi and chongkukjang. J. Ethn. Foods 2018, 5, 161–166. [Google Scholar] [CrossRef]
- De Grijs, R. Plague of the Sea, and the Spoyle of Mariners—A Brief History of Fermented Cabbage as Antiscorbutic—Hektoen International. 2021. Available online: https://hekint.org/2021/06/17/plague-of-the-sea-and-the-spoyle-of-mariners-a-brief-history-of-fermented-cabbage-as-antiscorbutic/ (accessed on 10 October 2023).
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Baty, C.; Marché, L.; Chuat, V.; Picard, O.; Lortal, S.; Valence, F. Lactofermentation of vegetables: An ancient method of preservation matching new trends. Trends Food Sci. Technol. 2023, 139, 104112. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Oliveira, K.; de Oliveira, M.E. Influence of lactic acid bacteria metabolites on physical and chemical food properties. Curr. Opin. Food Sci. 2023, 49, 100981. [Google Scholar] [CrossRef]
- Holzapfel, W. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2001, 75, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Budzyńska, A.; Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Andrzejewska, M.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Two Faces of Fermented Foods—The Benefits and Threats of Its Consumption. Front. Microbiol. 2022, 13, 845166. [Google Scholar] [CrossRef] [PubMed]
- Limosowtin, G.K.Y.; Powell, I.B.; Parente, E. Types of starters. In Dairy Starter Cultures; Cogan, T.M., Accolas, J.P., Eds.; Wiley-VCH: New York, NY, USA, 1996; pp. 101–130. [Google Scholar]
- Bernardeau, M.; Vernoux, J.P.; Henridubernet, S.; Guéguen, M. Safety assessment of dairy microorganisms: The Lactobacillus genus. Int. J. Food Microbiol. 2008, 126, 126278285. [Google Scholar] [CrossRef] [PubMed]
- Montet, D.; Ray, R.C.; Zakhia-Rozis, N. Lactic Acid Fermentation of Vegetables and Fruits. In Microorganisms and Fermentation of Traditional Foods; CRC Press: Boca Raton, FL, USA, 2014; pp. 118–150. [Google Scholar]
- Mojka, K. Charakterystyka mlecznych napojów fermentowanych. Probl. Hig. Epidemiol. 2013, 94, 722–729. [Google Scholar]
- Bassi, D.; Puglisi, E.; Cocconcelli, P.S. Comparing natural and selected starter cultures in meat and cheese fermentations. Curr. Opin. Food Sci. 2015, 2, 118–122. [Google Scholar] [CrossRef]
- Abarquero, D.; Renes, E.; Fresno, J.M.; Tornadijo, M.E. Study of exopolysaccharides from lactic acid bacteria and their industrial applications: A review. Int. J. Food Sci. Technol. 2022, 57, 16–26. [Google Scholar] [CrossRef]
- Hadjimbei, E.; Botsaris, G.; Chrysostomou, S. Beneficial Effects of Yoghurts and Probiotic Fermented Milks and Their Functional Food Potential. Foods 2022, 11, 2691. [Google Scholar] [CrossRef]
- Lick, S.; Drescher, K.; Heller, K.J. Survival of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in the Terminal Ileum of Fistulated Göttingen Minipigs. Appl. Environ. Microbiol. 2001, 67, 4137–4143. [Google Scholar] [CrossRef][Green Version]
- Pothuraju, R.; Yenuganti, V.R.; Hussain, S.A.; Sharma, M. Chapter 29—Fermented Milk in Protection Against Inflammatory Mechanisms in Obesity. In Immunity and Inflammation in Health and Disease; İçinde, S., Chatterjee, W., Jungraithmayr, D.B., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 389–401. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Joseph, R.; Bachhawat, A.K. Yeasts: Production and Commercial Uses. In Encyclopedia of Food Microbiology, 2nd ed.; İçinde, C.A., Batt, M.L.T., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 823–830. [Google Scholar] [CrossRef]
- Kesenkaş, H.; Gürsoy, O.; Özbaş, H. Chapter 14—Kefir. In Fermented Foods in Health and Disease Prevention; Frias, İ.J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 339–361. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Selective and differential enumerations of Lactobacillus delbrueckii subsp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium spp. in yoghurt—A review. Int. J. Food Microbiol. 2011, 149, 194–208. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Fox, P.F. Cheese—Overview. In Encyclopedia of Dairy Sciences, 2nd ed.; John, W.F., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 533–543. ISBN 9780123744074. [Google Scholar]
- Łepecka, A.; Okoń, A.; Szymański, P.; Zielińska, D.; Kajak-Siemaszko, K.; Jaworska, D.; Neffe-Skocińska, K.; Sionek, B.; Trząskowska, M.; Kołożyn-Krajewska, D.; et al. The Use of Unique, Environmental Lactic Acid Bacteria Strains in the Traditional Production of Organic Cheeses from Unpasteurized Cow’s Milk. Molecules 2022, 27, 1097. [Google Scholar] [CrossRef]
- Nalepa, B.; Markiewicz, L.H. Microbiological Biodiversity of Regional Cow, Goat and Ewe Milk Cheeses Produced in Poland and Antibiotic Resistance of Lactic Acid Bacteria Isolated from Them. Animals 2022, 13, 168. [Google Scholar] [CrossRef]
- Beresford, T.P.; Fitzsimons, N.A.; Brennan, N.L.; Cogan, T.M. Recent advances in cheese microbiology. Int. Dairy J. 2001, 11, 259–274. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Mancini, L.; Fox, P.F. Pros and cons for using non-starter lactic acid bacteria (NSLAB) as secondary/adjunct starters for cheese ripening. Trends Food Sci. Technol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.; Díaz-Castro, J.; López-Aliaga, I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Ramos, C.L.; Bressani, A.P.; Batista, N.N.; Martinez, S.J.; Dias, D.R.; Schwan, R.F. Indigenous fermented foods: Nutritional and safety aspects. Curr. Opin. Food Sci. 2023, 53, 101075. [Google Scholar] [CrossRef]
- Gänzle, M.G. Food fermentations for improved digestibility of plant foods—An essential ex situ digestion step in agricultural societies? Curr. Opin. Food Sci. 2020, 32, 124–132. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef]
- Wuyts, S.; Van Beeck, W.; Allonsius, C.N.; Broek, M.F.v.D.; Lebeer, S. Applications of plant-based fermented foods and their microbes. Curr. Opin. Biotechnol. 2020, 61, 45–52. [Google Scholar] [CrossRef]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic Fermented Fruit or Vegetable Juices: Past, Present and Future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Beganović, J.; Pavunc, A.L.; Gjuračić, K.; Špoljarec, M.; Šušković, J.; Kos, B. Improved Sauerkraut Production with Probiotic Strain Lactobacillus plantarum L4 and Leuconostoc mesenteroides LMG 7954. J. Food Sci. 2011, 76, M124–M129. [Google Scholar] [CrossRef]
- Heperkan, D. Microbiota of table olive fermentations and criteria of selection for their use as starters. Front. Microbiol. 2013, 4, 143. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Rajendram, R.; Caggia, C. Lactic Acid Bacteria in Table Olive Fermentations in Olives and Olive Oil in Health and Disease; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Singapore, 2010; pp. 369–376. [Google Scholar]
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control 2013, 31, 539–545. [Google Scholar] [CrossRef]
- Chavan, R.S.; Chavan, S.R. Sourdough Technology-A Traditional Way for Wholesome Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 10, 169–182. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2020, 108, 71–83. [Google Scholar] [CrossRef]
- Oshiro, M.; Tanaka, M.; Zendo, T.; Nakayama, J. Impact of pH on succession of sourdough lactic acid bacteria communities and their fermentation properties. Biosci. Microbiota Food Health 2020, 39, 152–159. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Qu, J.; Wang, J. Bacterial diversity in traditional Jiaozi and sourdough revealed by high-throughput sequencing of 16S rRNA amplicons. LWT-Food Sci. Technol. 2017, 81, 319–325. [Google Scholar] [CrossRef]
- Liu, T.J.; Li, Y.; Chen, J.C.; Sadiq, F.A.; Zhang, G.H.; Li, Y.; He, G.Q. Prevalence and diversity of lactic acid bacteria in Chinese traditional sourdough revealed by culture dependent and pyrosequencing approaches. LWT-Food Sci. Technol. 2016, 68, 91–97. [Google Scholar] [CrossRef]
- Palla, M.; Cristani, C.; Giovannetti, M.; Agnolucci, M. Identification and characterization of lactic acid bacteria and yeasts of PDO Tuscan bread sourdough by culture dependent and independent methods. Int. J. Food Microbiol. 2017, 250, 19–26. [Google Scholar] [CrossRef]
- Ravyts, F.; De Vuyst, L. Prevalence and impact of single-strain starter cultures of lactic acid bacteria on metabolite formation in sourdough. Food Microbiol. 2011, 28, 1129–1139. [Google Scholar] [CrossRef]
- Gänzle, M.; Ripari, V. Composition and function of sourdough microbiota: From ecological theory to bread quality. Int. J. Food Microbiol. 2016, 239, 19–25. [Google Scholar] [CrossRef]
- Comasio, A.; Verce, M.; Van Kerrebroeck, S.; De Vuyst, L. Diverse microbial composition of dourdoughs from different origins. Front. Microbiol. 2020, 11, 1212. [Google Scholar] [CrossRef]
- Nionelli, L.; Rizzello, C.G. Sourdough-Based Biotechnologies for the Production of Gluten-Free Foods. Foods 2016, 5, 65. [Google Scholar] [CrossRef]
- Le-Bail, A.; Boumali, K.; Jury, V.; Ben-Aissa, F.; Zuniga, R. Impact of the baking kinetics on staling rate and mechanical prop-erties of bread crumb and degassed bread crumb. J. Cereal Sci. 2009, 50, 235–240. [Google Scholar] [CrossRef]
- Fadda, C.; Sanguinetti, A.M.; Del Caro, A.; Collar, C.; Piga, A. Bread Staling: Updating the View. Compr. Rev. Food Sci. Food Saf. 2014, 13, 473–492. [Google Scholar] [CrossRef]
- Pétel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Graça, C.; Edelmann, M.; Raymundo, A.; Sousa, I.; Coda, R.; Sontag-Strohm, T.; Huang, X. Yoghurt as a starter in sourdough fermentation to improve the technological and functional properties of sourdough-wheat bread. J. Funct. Foods 2022, 88, 104877. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Mortazavi, S.A.; Abedfar, A. Application of the selected antifungal LAB isolate as a protective starter culture in pan whole-wheat sourdough bread. Food Control 2019, 95, 298–307. [Google Scholar] [CrossRef]
- Forstova, V.; Belkova, B.; Riddellova, K.; Vaclavik, L.; Prihoda, J.; Hajslova, J. Acrylamide formation in traditional Czech leavened wheat-rye breads and wheat rolls. Food Control 2014, 38, 221–226. [Google Scholar] [CrossRef]
- Nachi, I.; Fhoula, I.; Smida, I.; Ben Taher, I.; Chouaibi, M.; Jaunbergs, J.; Bartkevics, V.; Hassouna, M. Assessment of lactic acid bacteria application for the reduction of acrylamide formation in bread. LWT-Food Sci. Technol. 2018, 92, 435–441. [Google Scholar] [CrossRef]
- Esfahani, B.N.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Reduction of acrylamide in whole-wheat bread by combining lactobacilli and yeast fermentation. Food Addit. Contam. Part A 2017, 34, 1904–1914. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Siepmann, F.B.; Ripari, V.; Waszczynskyj, N.; Spier, M.R. Overview of Sourdough Technology: From Production to Marketing. Food Bioprocess Technol. 2018, 11, 242–270. [Google Scholar] [CrossRef]
- Ahlberg, S.H.; Joutsjoki, V.; Korhonen, H.J. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food Microbiol. 2015, 207, 87–102. [Google Scholar] [CrossRef]
- Bangar, S.P.; Sharma, N.; Kumar, M.; Ozogul, F.; Purewal, S.S.; Trif, M. Recent developments in applications of lactic acid bacteria against mycotoxin production and fungal contamination. Food Biosci. 2021, 44, 101444. [Google Scholar] [CrossRef]
- Limbad, M.; Maddox, N.; Hamid, N.; Kantono, K. Sensory and Physicochemical Characterization of Sourdough Bread Prepared with a Coconut Water Kefir Starter. Foods 2020, 9, 1165. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Leroy, F. Microbial ecology and process technology of sourdough fermentation. Adv. Appl. Microbiol. 2017, 100, 49–160. [Google Scholar] [CrossRef]
- Kaveh, S.; Hashemi, S.M.B.; Abedi, E.; Amiri, M.J.; Conte, F.L. Bio-Preservation of Meat and Fermented Meat Products by Lactic Acid Bacteria Strains and Their Antibacterial Metabolites. Sustainability 2023, 15, 10154. [Google Scholar] [CrossRef]
- Rantsiou, K.; Cocolin, L. Fermented Meat Products. In Molecular Techniques in the Microbial Ecology of Fermented; Cocolin, L., Ercolini, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; p. 92. ISBN 978-0-387-74519-0/978-0-387-74520-6. [Google Scholar]
- da Costa, W.K.A.; de Souza, G.T.; Brandão, L.R.; de Lima, R.C.; Garcia, E.F.; dos Santos Lima, M.; de Souza, E.L.; Saarela, M.; Magnani, M. Exploiting antagonistic activity of fruit-derived Lactobacillus to control pathogenic bacteria in fresh cheese and chicken meat. Food Res. Int. 2018, 108, 172–182. [Google Scholar] [CrossRef]
- Dos Santos Cruxen, C.E.; Funck, G.D.; Haubert, L.; da Silva Dannenberg, G.; de Lima Marques, J.; Chaves, F.C.; da Silva, W.P.; Fiorentini, Â.M. Selection of native bacterial starter culture in the production of fermented meat sausages: Application potential, safety aspects, and emerging technologies. Food Res. Int. 2019, 122, 371–382. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Lücke, F.-K. Quality improvement and fermentation control in meat products. In Advances in Fermented Foods and Beverages. Improving Quality, Technologies and Health Benefit; Holzapfel, W., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 357–376. [Google Scholar] [CrossRef]
- Van Reckem, E.; Geeraerts, W.; Charmpi, C.; Van der Veken, D.; De Vuyst, L.; Leroy, F. Exploring the Link Between the Geographical Origin of European Fermented Foods and the Diversity of Their Bacterial Communities: The Case of Fermented Meats. Front. Microbiol. 2019, 10, 2302. [Google Scholar] [CrossRef]
- Ojha, K.S.; Kerry, J.P.; Duffy, G.; Beresford, T.; Tiwari, B.K. Technological advances for enhancing quality and safety of fermented meat products. Trends Food Sci. Technol. 2015, 44, 105–116. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Wang, D.; Gao, F.; Zhang, K.; Tian, J.; Jin, Y. Research update on the impact of lactic acid bacteria on the substance metabolism, flavor, and quality characteristics of fermented meat products. Foods 2022, 11, 2090. [Google Scholar] [CrossRef]
- Pennacchia, C.; Vaughan, E.; Villani, F. Potential probiotic Lactobacillus strains from fermented sausages: Further investigations on their probiotic properties. Meat Sci. 2006, 73, 90–101. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [Google Scholar]
- Zang, J.; Xu, Y.; Xia, W.; Regenstein, J.M. Quality, functionality, and microbiology of fermented fish: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1228–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Q.; Li, L.; Chen, S.; Zhao, Y.; Li, C.; Xiang, H.; Wu, Y.; Sun-Waterhouse, D. Transforming the fermented fish landscape: Microbiota enable novel, safe, flavorful, and healthy products for modern consumers. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3560–3601. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, S.-A.; Jo, Y.M.; Seo, H.; Kim, G.Y.; Cheon, S.W.; Yang, S.H.; Jeon, C.O.; Han, N.S. Probiotic potential of Tetragenococcus halophilus EFEL7002 isolated from Korean soy Meju. BMC Microbiol. 2022, 22, 149. [Google Scholar] [CrossRef] [PubMed]
- Belleggia, L.; Osimani, A. Fermented fish and fermented fish-based products, an ever-growing source of microbial diversity: A literature review. Food Res. Int. 2023, 172, 113112. [Google Scholar] [CrossRef] [PubMed]
- Mannaa, M.; Han, G.; Seo, Y.-S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Reale, A. A Holistic Review on Euro-Asian Lactic Acid Bacteria Fermented Cereals and Vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Baubekova, A.; Akhmetsadykova, S.; Faye, B. Traditional dairy fermented products in Central Asia. Int. Dairy J. 2023, 137, 105514. [Google Scholar] [CrossRef]
- Rodzi, N.A.R.M.; Lee, L.K. Traditional fermented foods as vehicle of non-dairy probiotics: Perspectives in South East Asia countries. Food Res. Int. 2021, 150, 110814. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Ruddle, K.; Rosma, A.; Singh, A.; Ann, A.; Raj, A.; Gupta, A.; Kumar, A.; Thilakaratne, B.M.K.; Neopany, B.; Angchok, D.; et al. Indigenous Fermented Foods in Fermented Meat Products, Fish and Fish Products, Alkaline Fermented Foods, Tea, and Other Related Products; CRC Press: New York, NY, USA, 2015; pp. 645–713. [Google Scholar]
- Kömürcü, T.C.; Bilgiçli, N. Effect of ancient wheat flours and fermentation types on tarhana properties. Food Biosci. 2022, 50, 101982. [Google Scholar] [CrossRef]
- Temiz, H.; Tarakçı, Z. Composition of volatile aromatic compounds and minerals of tarhana enriched with cherry laurel (Laurocerasus officinalis). J. Food Sci. Technol. 2017, 54, 735–742. [Google Scholar] [CrossRef]
- Şimşek, Ö.S.; Çon, A.H. Comparison of lactic acid bacteria diversity during the fermentation of Tarhana produced at home and on a commercial scale. Food Sci. Biotechnol. 2017, 26, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Arici, M.; Daglioglu, O. Boza: A lactic acid fermented cereal beverage as a traditional Turkish food. Food Rev. Int. 2002, 18, 39–48. [Google Scholar] [CrossRef]
- Bozdemir, M.; Gümüş, T.; Kamer, D.D.A. Technological and beneficial features of lactic acid bacteria isolated from Boza A cereal-based fermented beverage. Food Biotechnol. 2022, 36, 209–233. [Google Scholar] [CrossRef]
- Heperkan, D.; Daskaya-Dikmen, C.; Bayram, B. Evaluation of lactic acid bacterial strains of boza for their exopolysaccharide and enzyme production as a potential adjunct culture. Process. Biochem. 2014, 49, 1587–1594. [Google Scholar] [CrossRef]
- Ilango, S.; Antony, U. Probiotic microorganisms from non-dairy traditional fermented foods. Trends Food Sci. Technol. 2021, 118, 617–638. [Google Scholar] [CrossRef]
- Ray, R.; El Sheikha, A.; Kumar, S. Oriental Fermented Functional (Probiotic) Foods. In Microorganisms and Fermentation of Traditional Foods; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Chao, S.-H.; Tomii, Y.; Watanabe, K.; Tsai, Y.-C. Diversity of lactic acid bacteria in fermented brines used to make stinky tofu. Int. J. Food Microbiol. 2008, 123, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lan, G.; Tian, X.; He, L.; Li, C.; Zeng, X.; Wang, X. Effect of Fermentation Parameters on Natto and Its Thrombolytic Property. Foods 2021, 10, 2547. [Google Scholar] [CrossRef] [PubMed]
- Kłosowski, G.; Mikulski, D.; Pielech-Przybylska, K. Pyrazines Biosynthesis by Bacillus Strains Isolated from Natto Fermented Soybean. Biomolecules 2021, 11, 1736. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the microbial community of Japanese koji and miso: A review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.Y.; Huang, X.; Liu, Z.; Chua, J.-Y.; Liu, S.-Q. Evaluating the effect of lactic acid bacterial fermentation on salted soy whey for development of a potential novel soy sauce-like condiment. Curr. Res. Food Sci. 2022, 5, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Rani, R.P. Fermented Fruits and Vegetables of Asia: A Potential Source of Probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Paramithiotis, S.; Shin, H.-S. Kimchi and Other Widely Consumed Traditional Fermented Foods of Korea: A Review. Front. Microbiol. 2016, 7, 1493. [Google Scholar] [CrossRef]
- Cha, J.; Kim, Y.B.; Park, S.-E.; Lee, S.H.; Roh, S.W.; Son, H.-S.; Whon, T.W. Does kimchi deserve the status of a probiotic food? Crit. Rev. Food Sci. Nutr. 2023, 1–14. [Google Scholar] [CrossRef]
- Dharaneedharan, S.; Heo, M.-S. Korean Traditional fermented foods-a potential resource of beneficial microorganisms and their applications. J. Life Sci. 2016, 26, 496–502. [Google Scholar] [CrossRef]
- Choi, I.H.; Noh, J.S.; Han, J.-S.; Kim, H.J.; Han, E.-S.; Song, Y.O.; Barghi, M.; Shin, E.-S.; Son, M.-H.; Choi, S.-D.; et al. Kimchi, a Fermented Vegetable, Improves Serum Lipid Profiles in Healthy Young Adults: Randomized Clinical Trial. J. Med. Food 2013, 16, 223–229. [Google Scholar] [CrossRef]
- Wang, J.; Aziz, T.; Bai, R.; Zhang, X.; Shahzad, M.; Sameeh, M.Y.; Khan, A.A.; Dablool, A.S.; Zhu, Y. Dynamic change of bacterial diversity, metabolic pathways, and flavor during ripening of the Chinese fermented sausage. Front. Microbiol. 2022, 13, 990606. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Zhao, H. Unraveling microbial community diversity and succession of Chinese Sichuan sausages during spontaneous fermentation by high-throughput sequencing. J. Food Sci. Technol. 2019, 56, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, T.; Kiyohara, M.; Matsui, H.; Yamamoto, K.; Kondo, T.; Katayama, T.; Kumagai, H. Pyrosequencing survey of the microbial diversity of ‘narezushi’, an archetype of modern Japanese sushi. Lett. Appl. Microbiol. 2011, 53, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Kuda, T.; An, C.; Takahashi, H.; Kimura, B. Radical scavenging capacities of saba-narezushi, Japanese fermented chub mackerel, and its lactic acid bacteria. Lwt-Food Sci. Technol. 2012, 47, 25–30. [Google Scholar] [CrossRef]
- Kiyohara, M.; Koyanagi, T.; Matsui, H.; Yamamoto, K.; Take, H.; Katsuyama, Y.; Tsuji, A.; Miyamae, H.; Kondo, T.; Nakamura, S.; et al. Changes in Microbiota Population during Fermentation of Narezushi as Revealed by Pyrosequencing Analysis. Biosci. Biotechnol. Biochem. 2012, 76, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kuda, T.; An, C.; Kanno, T.; Takahashi, H.; Kimura, B. Inhibitory effects of Leuconostoc mesenteroides 1RM3 isolated from narezushi, a fermented fish with rice, on Listeria monocytogenes infection to Caco-2 cells and A/J mice. Anaerobe 2011, 18, 19–24. [Google Scholar] [CrossRef]
- Dai, Z.; Li, Y.; Wu, J.; Zhao, Q. Diversity of Lactic Acid Bacteria during Fermentation of a Traditional Chinese Fish Product, Chouguiyu (Stinky Mandarinfish). J. Food Sci. 2013, 78, M1778–M1783. [Google Scholar] [CrossRef]
- Mani-López, E.; Enrique Palou López-Malo, A. Chapter 8—Biopreservatives as Agents to Prevent Food Spoilage. In Handbook of Food Bioengineering, Microbial Contamination and Food Degradation; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 235–270. ISBN 9780128115152. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Vieira, A.T.; Teixeira, M.M.; Martins, F.S. The Role of Probiotics and Prebiotics in Inducing Gut Immunity. Front. Immunol. 2013, 4, 445. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Chapter 1.2—Immunity in the Gut: Mechanisms and Functions. In Viral Gastroenteritis; Lennart Svensson, U., Desselberger, H.B., Greenberg, M.K.E., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 23–46. [Google Scholar]
- Elshaghabee, E.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Dittel, B.N. Interrelatedness between dysbiosis in the gut microbiota due to immunodeficiency and disease penetrance of colitis. Immunology 2015, 146, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and Inputs From Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters During Food-Animal Production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Trindade, D.P.d.A.; Barbosa, J.P.; Martins, E.M.F.; Tette, P.A.S. Isolation and identification of lactic acid bacteria in fruit processing residues from the Brazilian Cerrado and its probiotic potential. Food Biosci. 2022, 48, 101739. [Google Scholar] [CrossRef]
- Zielińska, D.; Kolożyn-Krajewska, D. Food-Origin Lactic Acid Bacteria May Exhibit Probiotic Properties: Review. BioMed Res. Int. 2018, 2018, 5063185. [Google Scholar] [CrossRef] [PubMed]
- Domingos-Lopes, M.; Lamosa, P.; Stanton, C.; Ross, R.; Silva, C. Isolation and characterization of an exopolysaccha-ride-producing Leuconostoc citreum strain from artisanal cheese. Lett. Appl. Microbiol. 2018, 67, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Konkit, M.; Kim, M. Activities of amylase, proteinase, and lipase enzymes from Lactococcus chungangensis and its application in dairy products. J. Dairy Sci. 2016, 99, 4999–5007. [Google Scholar] [CrossRef]
- Sáez, G.; Hébert, E.; Saavedra, L.; Zárate, G. Molecular identification and technological characterization of lactic acid bacteria isolated from fermented kidney beans flours (Phaseolus vulgaris L. and P. coccineus) in northwestern Argentina. Food Res. Int. 2017, 102, 605–615. [Google Scholar] [CrossRef]
- Altieri, C.; Ciuffreda, E.; Di Maggio, B.; Sinigaglia, M. Lactic acid bacteria as starter cultures. In Starter Cultures in Food Production; Speranza, B., Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2017. [Google Scholar] [CrossRef]
- Laranjo, M.; Elias, M.; Fraqueza, M.J. The Use of Starter Cultures in Traditional Meat Products. J. Food Qual. 2017, 2017, 9546026. [Google Scholar] [CrossRef]
- Multari, S.; Carafa, I.; Barp, L.; Caruso, M.; Licciardello, C.; Larcher, R.; Tuohy, K.; Martens, S. Effects of Lactobacillus spp. on the phytochemical composition of juices from two varieties of Citrus sinensis L. Osbeck: ‘Tarocco’ and ‘Washington navel’. LWT 2020, 125, 109205. [Google Scholar] [CrossRef]
- Genevois, C.; Pieniazek, F.; Messina, V.; Flores, S.; Pla, M.d.E. Bioconversion of pumpkin by-products in novel supplements supporting Lactobacillus casei. LWT 2019, 105, 23–29. [Google Scholar] [CrossRef]
- Barbaccia, P.; Francesca, N.; Di Gerlando, R.; Busetta, G.; Moschetti, G.; Gaglio, R.; Settanni, L. Biodiversity and dairy traits of indigenous milk lactic acid bacteria grown in presence of the main grape polyphenols. FEMS Microbiol. Lett. 2020, 367, fnaa066. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.A.C.; Bedani, R.; LeBlanc, J.G.; Saad, S.M.I. Passion fruit by-product and fructooligosaccharides stimulate the growth and folate production by starter and probiotic cultures in fermented soymilk. Int. J. Food Microbiol. 2017, 261, 35–41. [Google Scholar] [CrossRef]
- Grattepanche, F.; Lacroix, C. 13—Production of viable probiotic cells. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; McNeil, B., Archer, D., Giavasis, I., Harvey, L., Eds.; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Putta, S.; Yarla, N.S.; Lakkappa, D.B.; Imandi, S.B.; Malla, R.R.; Chaitanya, A.K.; Chari, B.P.V.; Saka, S.; Vechalapu, R.R.; Kamal, M.A.; et al. Chapter 2—Probiotics: Supplements, Food, Pharmaceutical Industry. In Therapeutic, Probiotic, and Unconventional Foods; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Jampaphaeng, K.; Cocolin, L.; Maneerat, S. Selection and evaluation of functional characteristics of autochthonous lactic acid bacteria isolated from traditional fermented stinky bean (Sataw-Dong). Ann. Microbiol. 2016, 67, 25–36. [Google Scholar] [CrossRef]
- Uriot, O.; Denis SJuniua, M.; Roussel, Y.; Dary-Mourot, A.; Blanquet-Diot, S. Streptococcus thermophilus: From yogurt starter to a new promising probiotic candidate? J. Funct. Food 2017, 37, 74–89. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Fragkos, K.C.; Scott, S.M.; Whelan, K. The effect of probiotics on functional constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1075–1084. [Google Scholar] [CrossRef]
- Krawęcka, A.; Libera, J.; Latoch, A. The Use of the Probiotic Lactiplantibacillus plantarum 299v in the Technology of Non-Dairy Ice Cream Based on Avocado. Foods 2021, 10, 2492. [Google Scholar] [CrossRef]
- Aspri, M.; Leni, G.; Galaverna, G.; Papademas, P. Bioactive properties of fermented donkey milk, before and after in vitro sim-ulated gastrointestinal digestion. Food Chem. 2018, 268, 476–484. [Google Scholar] [CrossRef]
- Grumet, L.; Tromp, Y.; Stiegelbauer, V. The Development of High-Quality Multispecies Probiotic Formulations: From Bench to Market. Nutrients 2020, 12, 2453. [Google Scholar] [CrossRef]
- Kim, K.-T.; Yang, S.J.; Paik, H.-D. Probiotic properties of novel probiotic Levilactobacillus brevis KU15147 isolated from radish kimchi and its antioxidant and immune-enhancing activities. Food Sci. Biotechnol. 2021, 30, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Łepecka, A.; Szymański, P.; Okoń, A.; Zielińska, D. Antioxidant activity of environmental lactic acid bacteria strains isolated from organic raw fermented meat products. LWT 2023, 174, 114440. [Google Scholar] [CrossRef]
- Ołdak, A.; Zielińska, D.; Łepecka, A.; Długosz, E.; Kołożyn-Krajewska, D. Lactobacillus plantarum Strains Isolated from Polish Regional Cheeses Exhibit Anti-Staphylococcal Activity and Selected Probiotic Properties. Probiotics Antimicrob. Proteins 2020, 12, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Christensen, I.B.; Vedel, C.; Clausen, M.-L.; Kjærulff, S.; Agner, T.; Nielsen, D.S. Targeted Screening of Lactic Acid Bacteria With Antibacterial Activity Toward Staphylococcus aureus Clonal Complex Type 1 Associated With Atopic Dermatitis. Front. Microbiol. 2021, 12, 733847. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sionek, B.; Szydłowska, A.; Küçükgöz, K.; Kołożyn-Krajewska, D. Traditional and New Microorganisms in Lactic Acid Fermentation of Food. Fermentation 2023, 9, 1019. https://doi.org/10.3390/fermentation9121019
Sionek B, Szydłowska A, Küçükgöz K, Kołożyn-Krajewska D. Traditional and New Microorganisms in Lactic Acid Fermentation of Food. Fermentation. 2023; 9(12):1019. https://doi.org/10.3390/fermentation9121019
Chicago/Turabian StyleSionek, Barbara, Aleksandra Szydłowska, Kübra Küçükgöz, and Danuta Kołożyn-Krajewska. 2023. "Traditional and New Microorganisms in Lactic Acid Fermentation of Food" Fermentation 9, no. 12: 1019. https://doi.org/10.3390/fermentation9121019
APA StyleSionek, B., Szydłowska, A., Küçükgöz, K., & Kołożyn-Krajewska, D. (2023). Traditional and New Microorganisms in Lactic Acid Fermentation of Food. Fermentation, 9(12), 1019. https://doi.org/10.3390/fermentation9121019






