Alleviating Effect of Lactiplantibacillus plantarum NXU0011 Fermented Wolfberry on Ulcerative Colitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of LP+Ly
2.2. Animal Experimental Design
2.3. Disease Activity Index (DAI)
2.4. Histological Assessment
2.5. RNA Isolation and qRT-PCR
2.6. Immunohistochemistry Staining
2.7. DNA Sequencing and Gut Microbiota Analysis
2.8. Plasma Metabolites Analysis via Untargeted Metabolomics
2.9. Statistical and Bioinformatics Analysis
3. Results
3.1. Protective Effect of LP+Ly on DSS-induced Colitis in Mice
3.2. Effect of LP+Ly on Cytokine Concentrations in the Colon
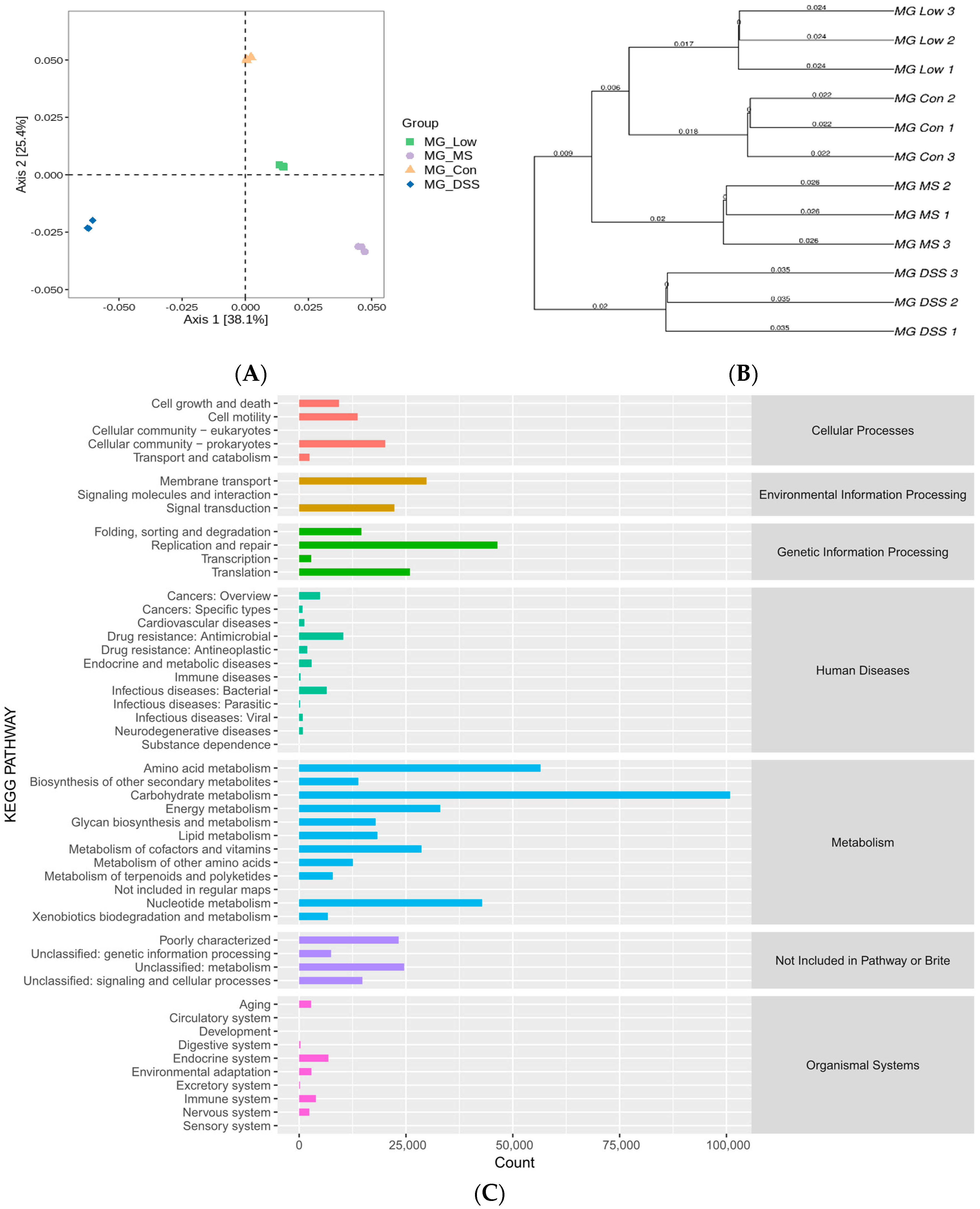
3.3. LP+Ly and the Structure of the Gut Microbiota
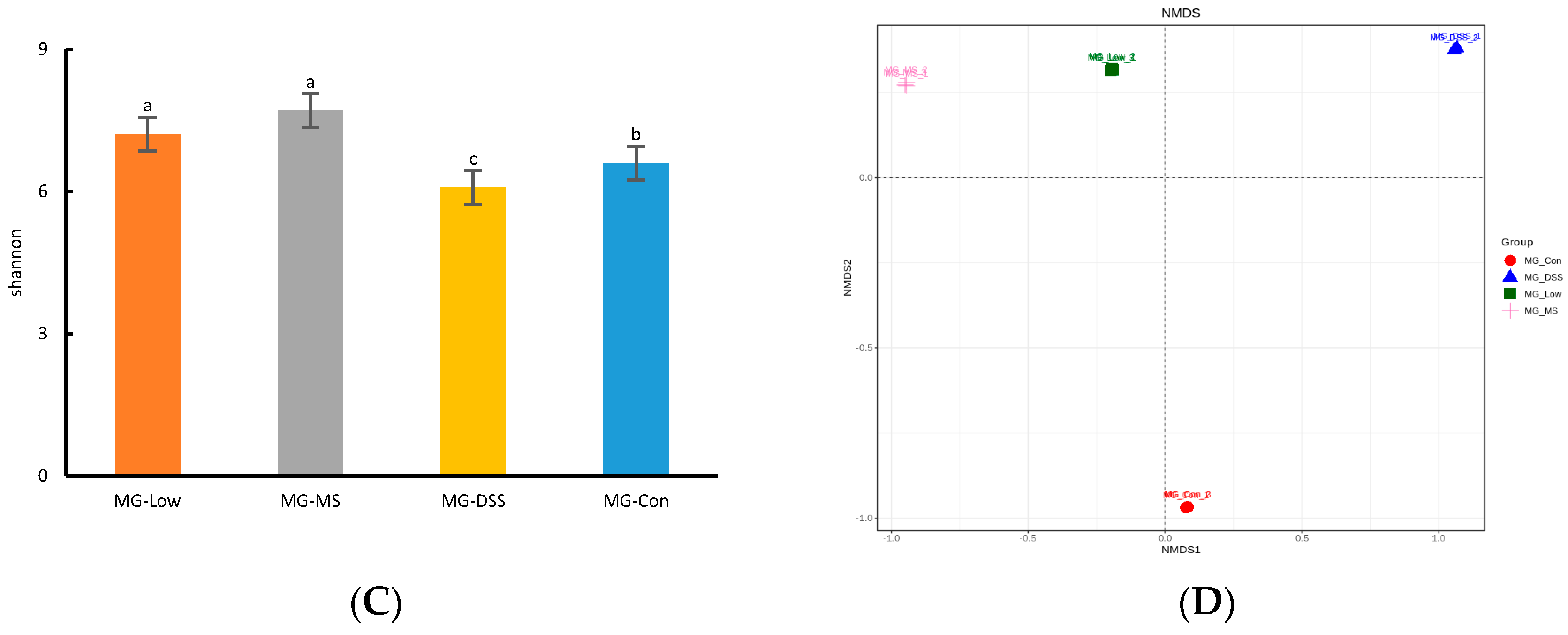
3.3.1. Analysis of Intestinal Flora Diversity in Mice Following LP+Ly Intervention
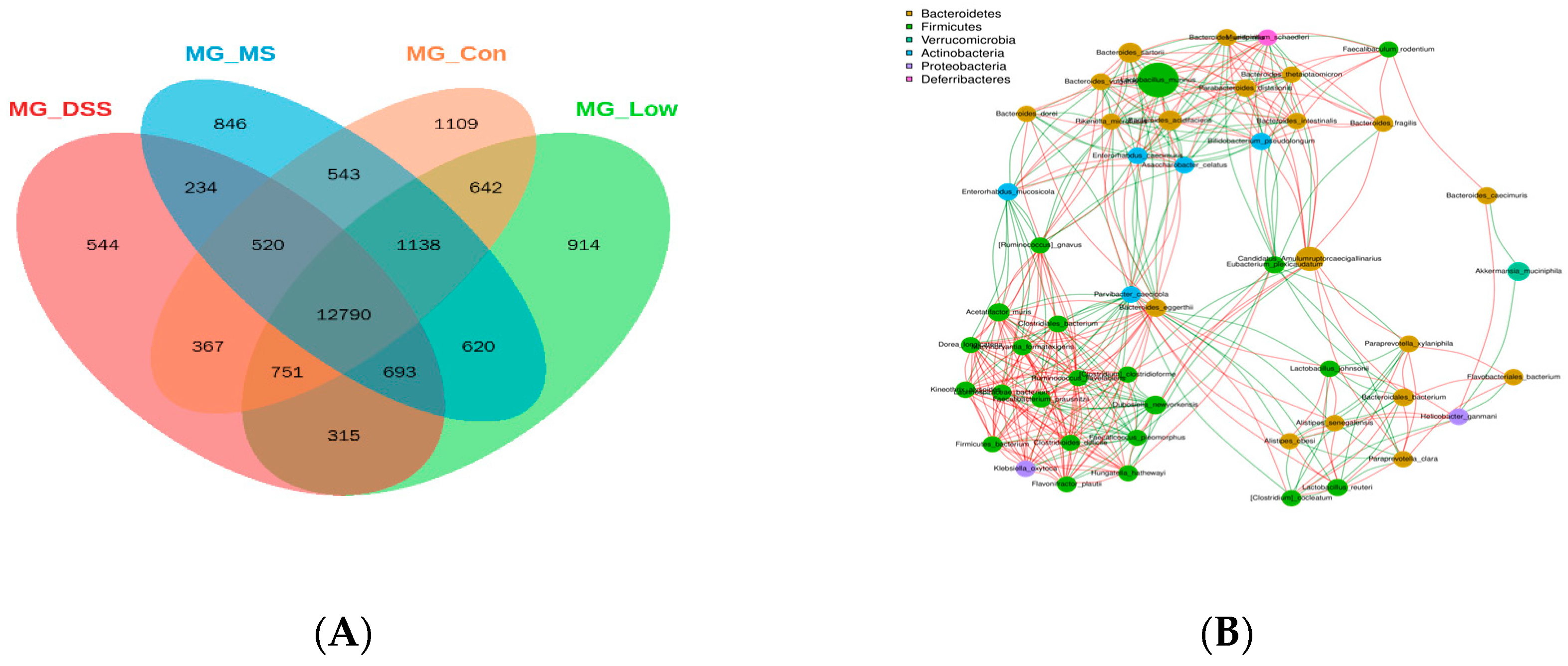
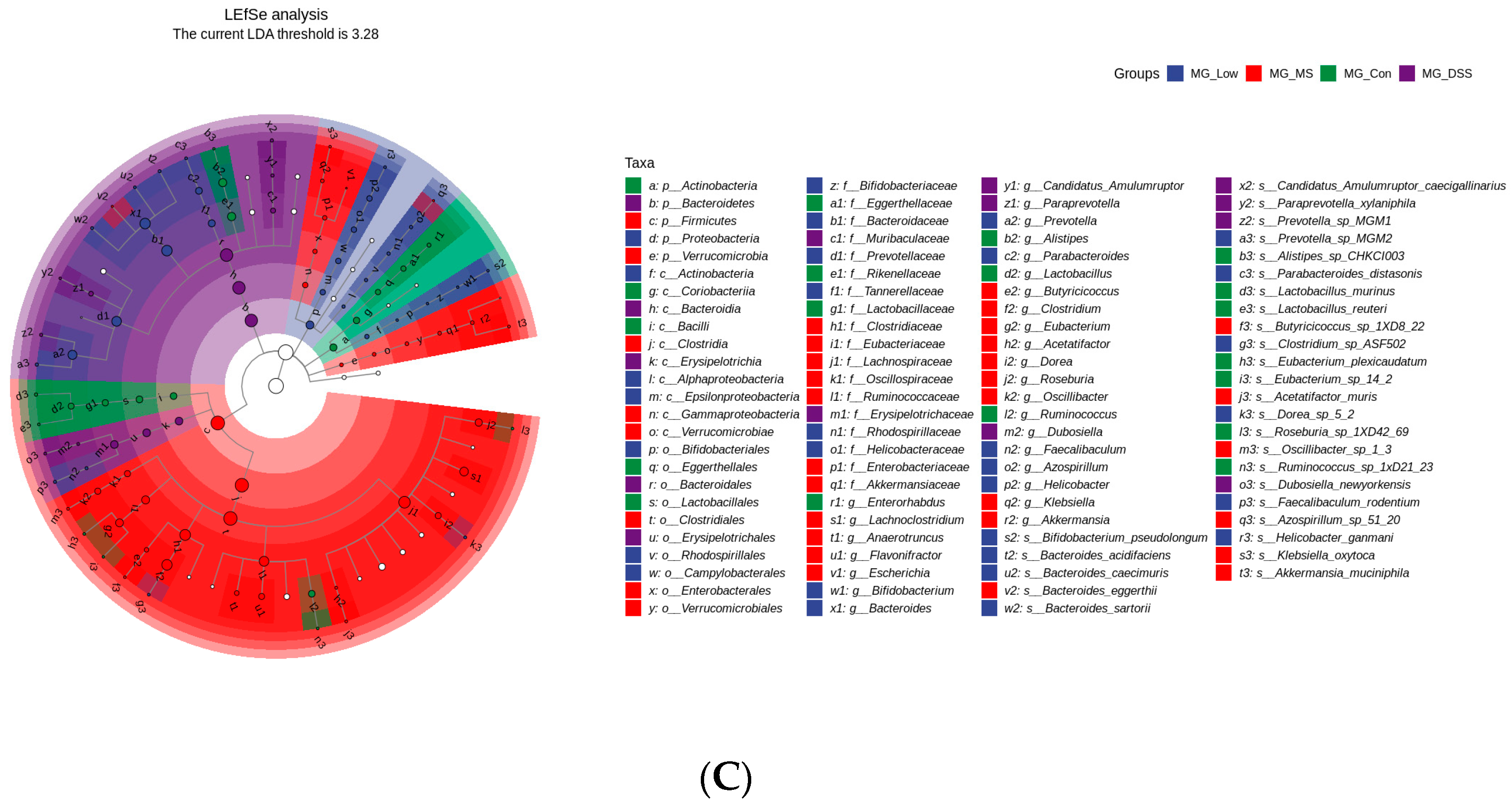
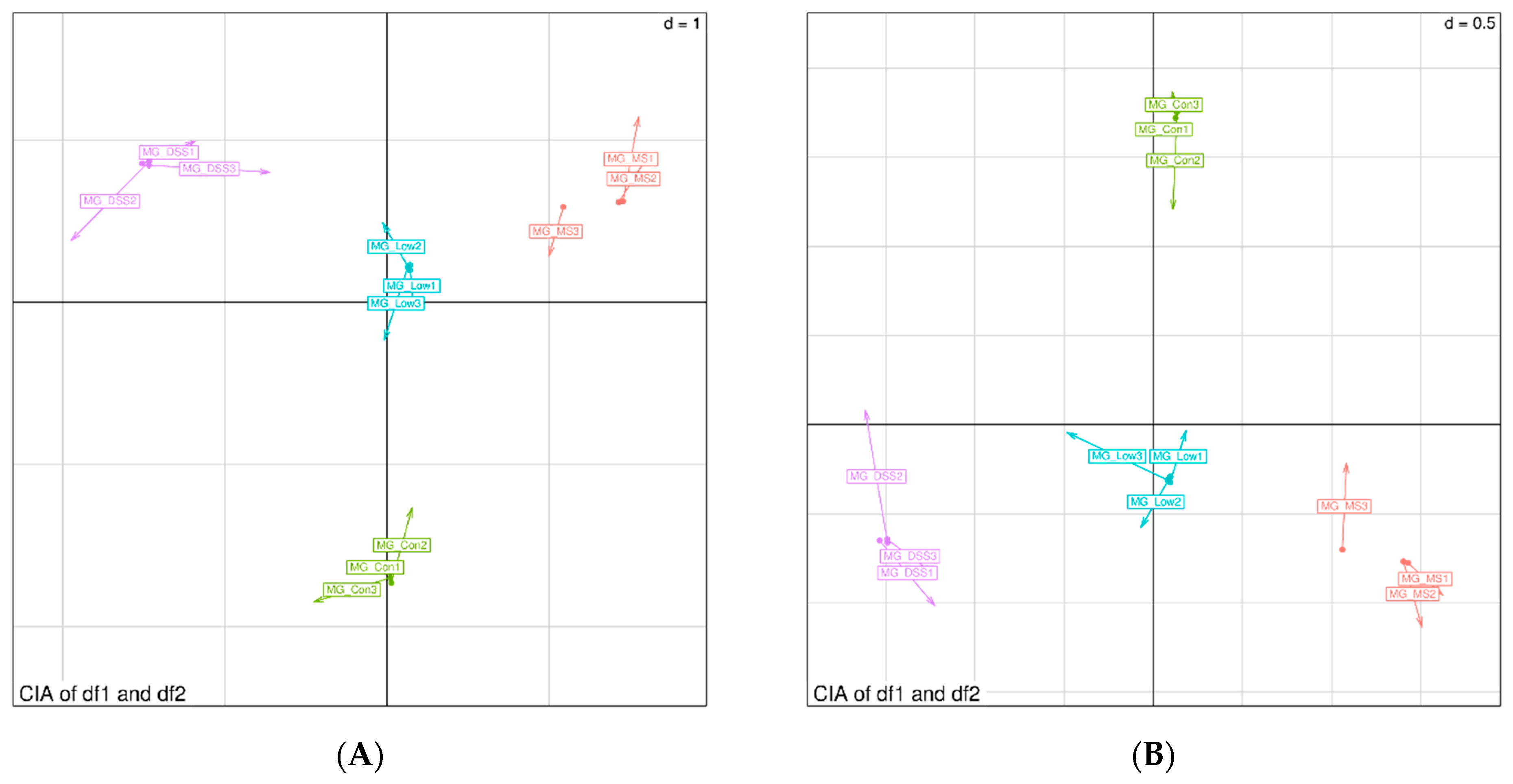
3.3.2. Impact of LP+Ly on the Structure of Gut Microbiota
3.3.3. Analysis of the Effect of LP+Ly on the Functional Level of Intestinal Flora in Mice
3.4. Impact of LP+Ly on the Metabolome of Serum Samples
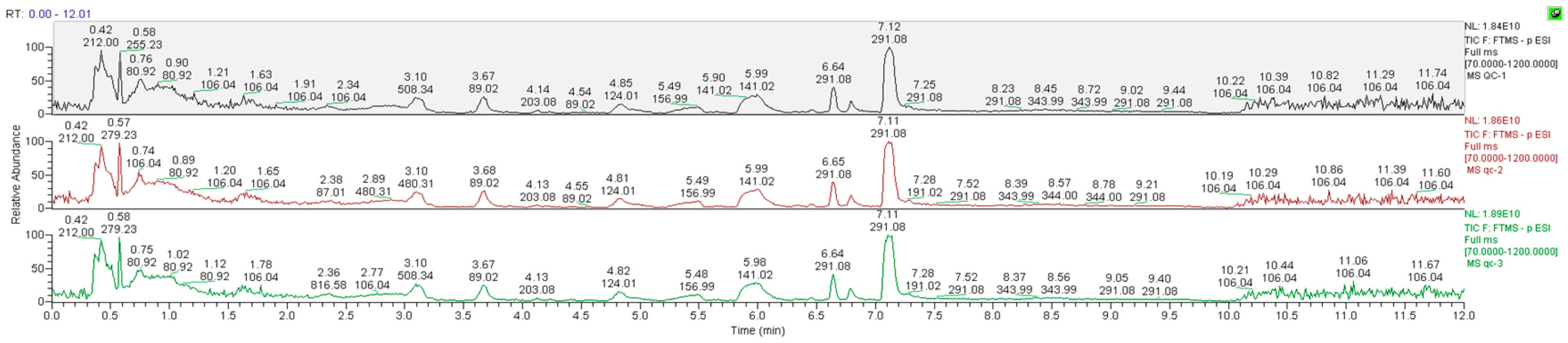
3.4.1. Data Quality Control (QC) Analysis
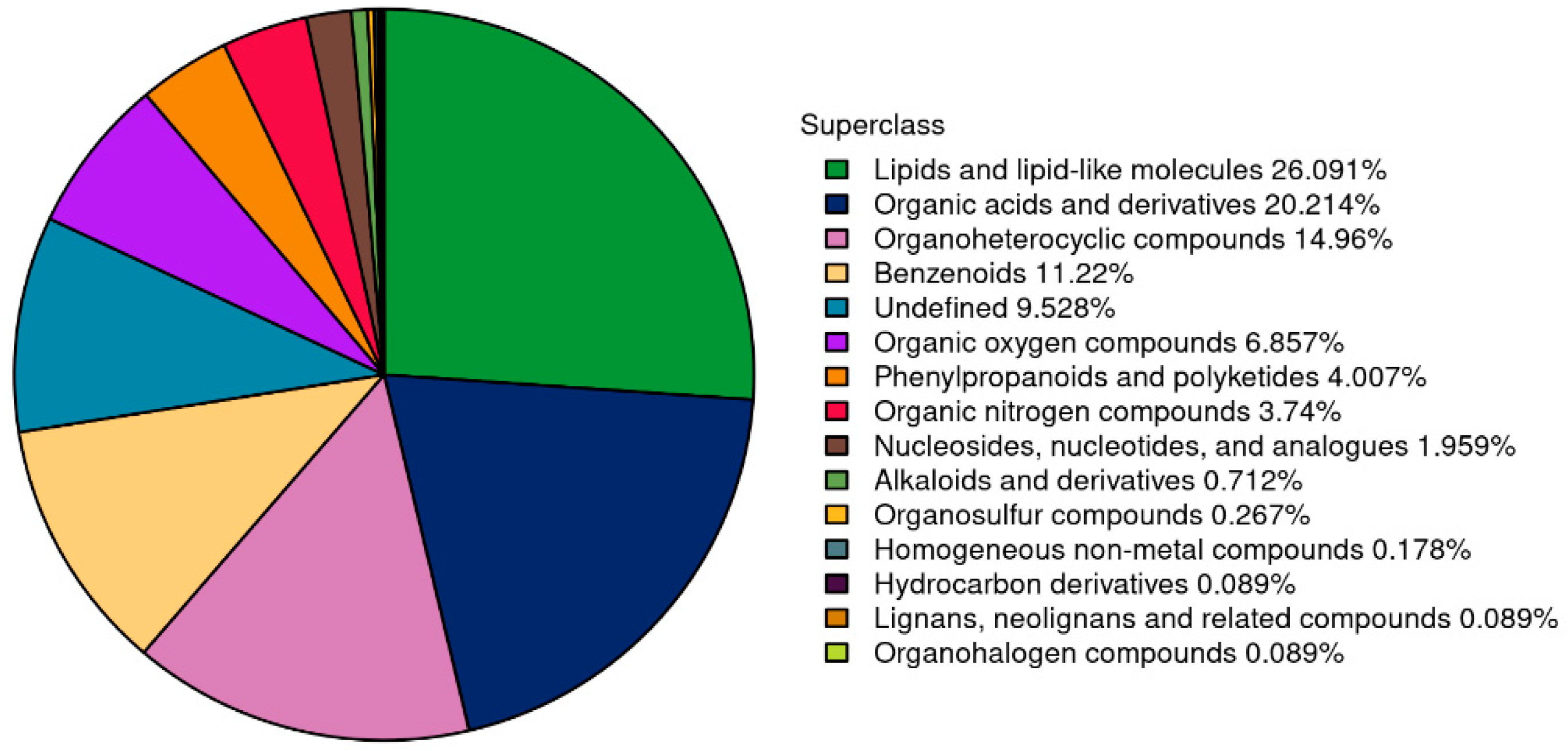
3.4.2. Summary of Plasma Metabolite Identification Results
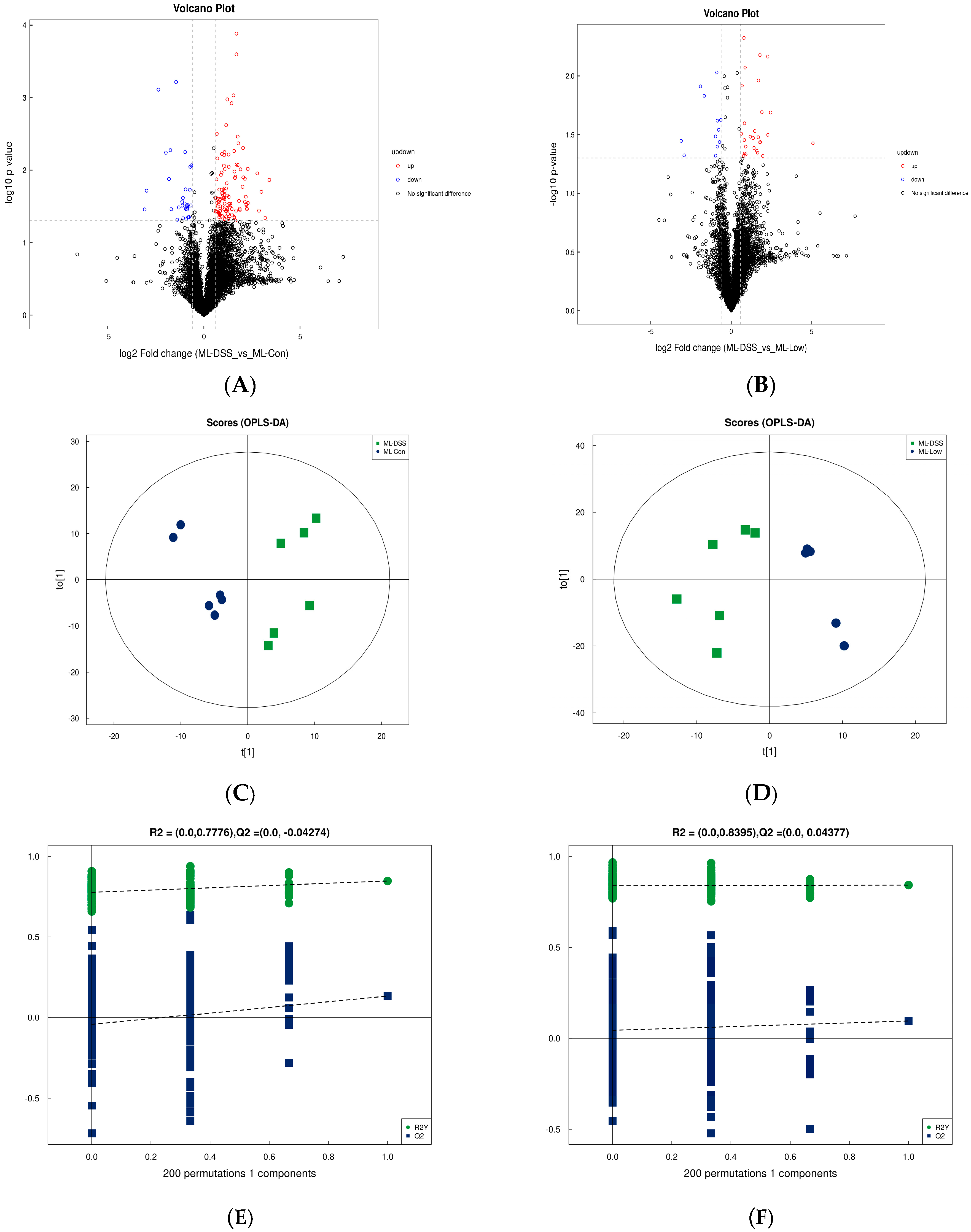
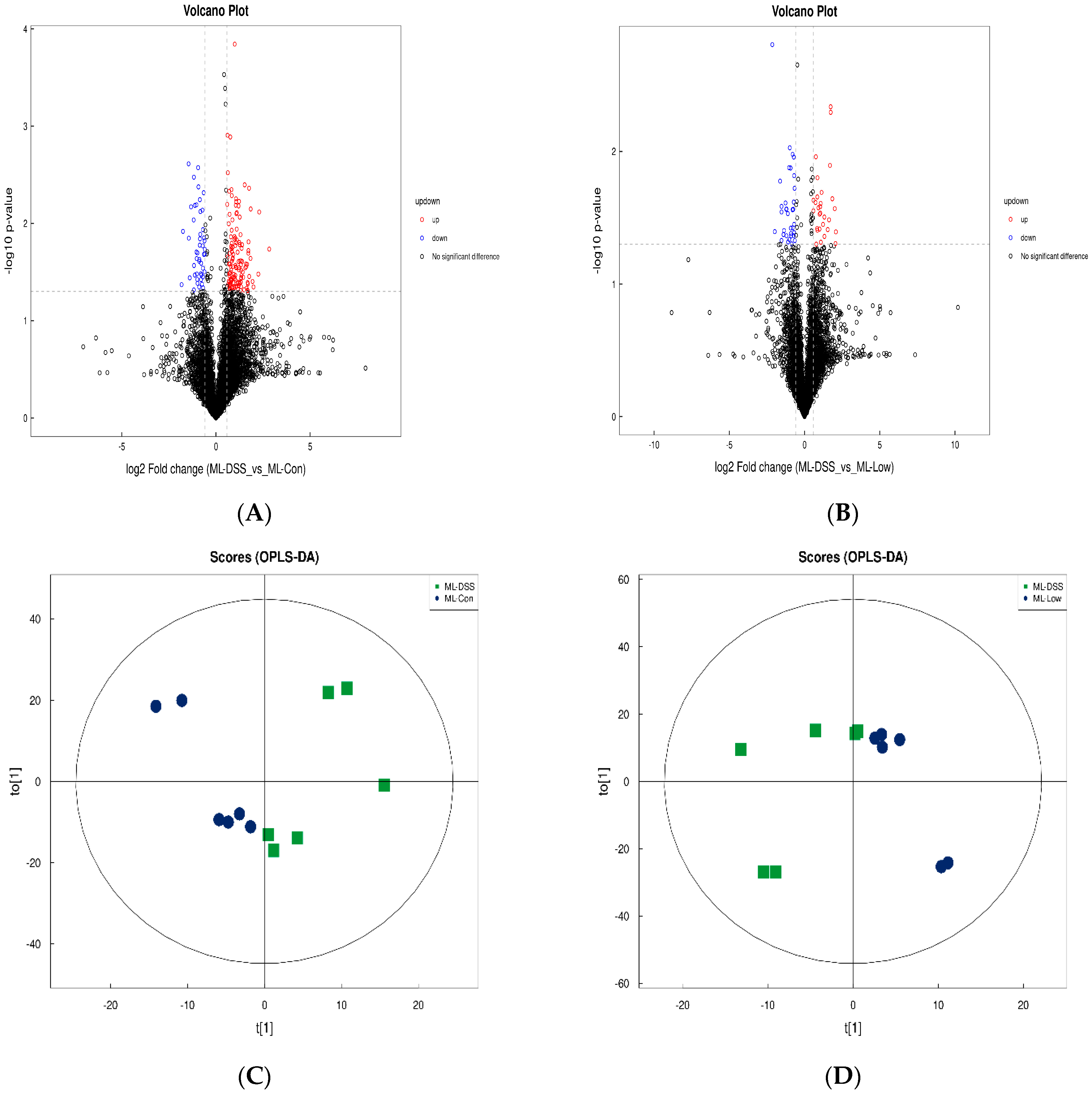
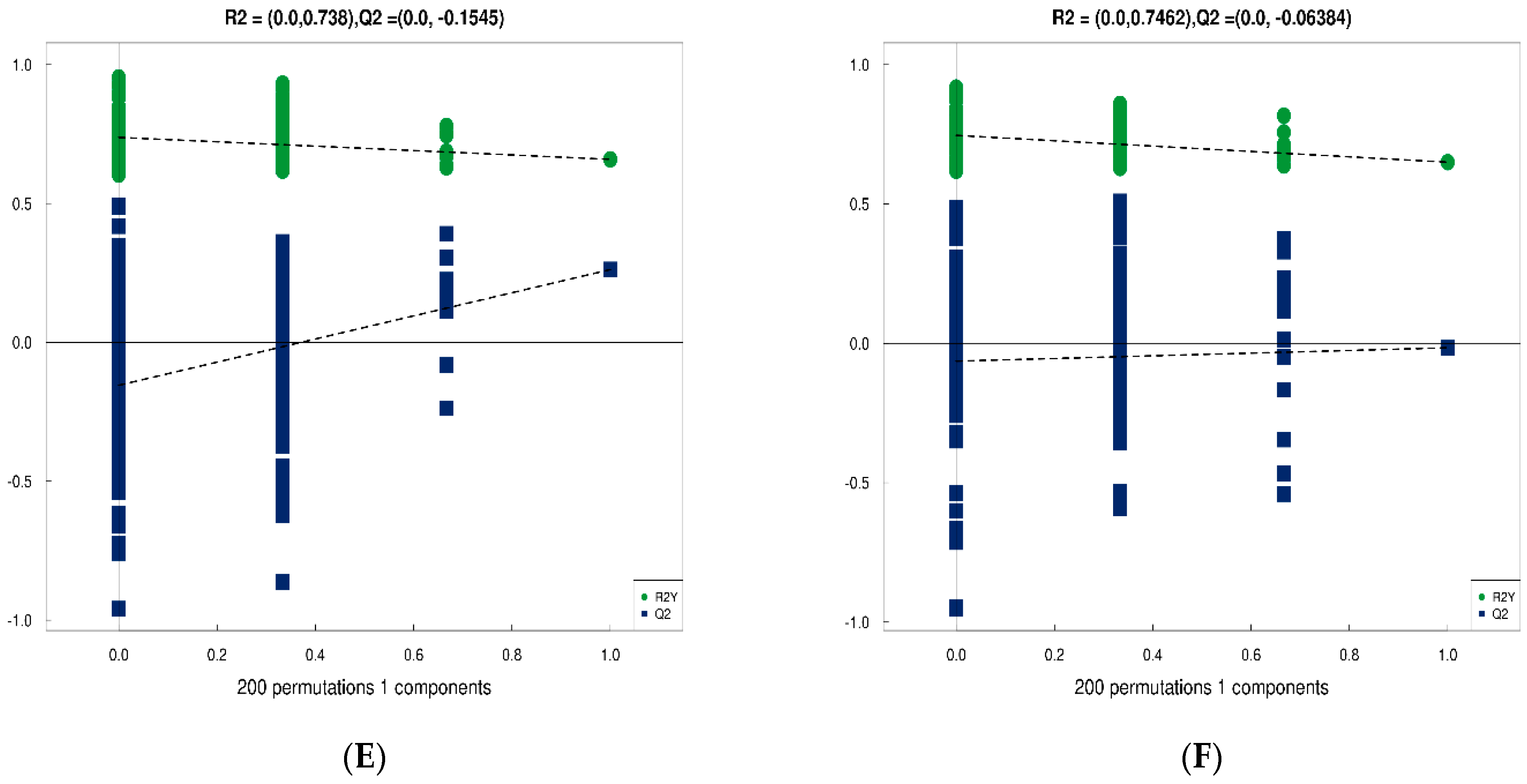
3.4.3. Analysis of Differences between Groups of Plasma Metabolites and Permutation Test
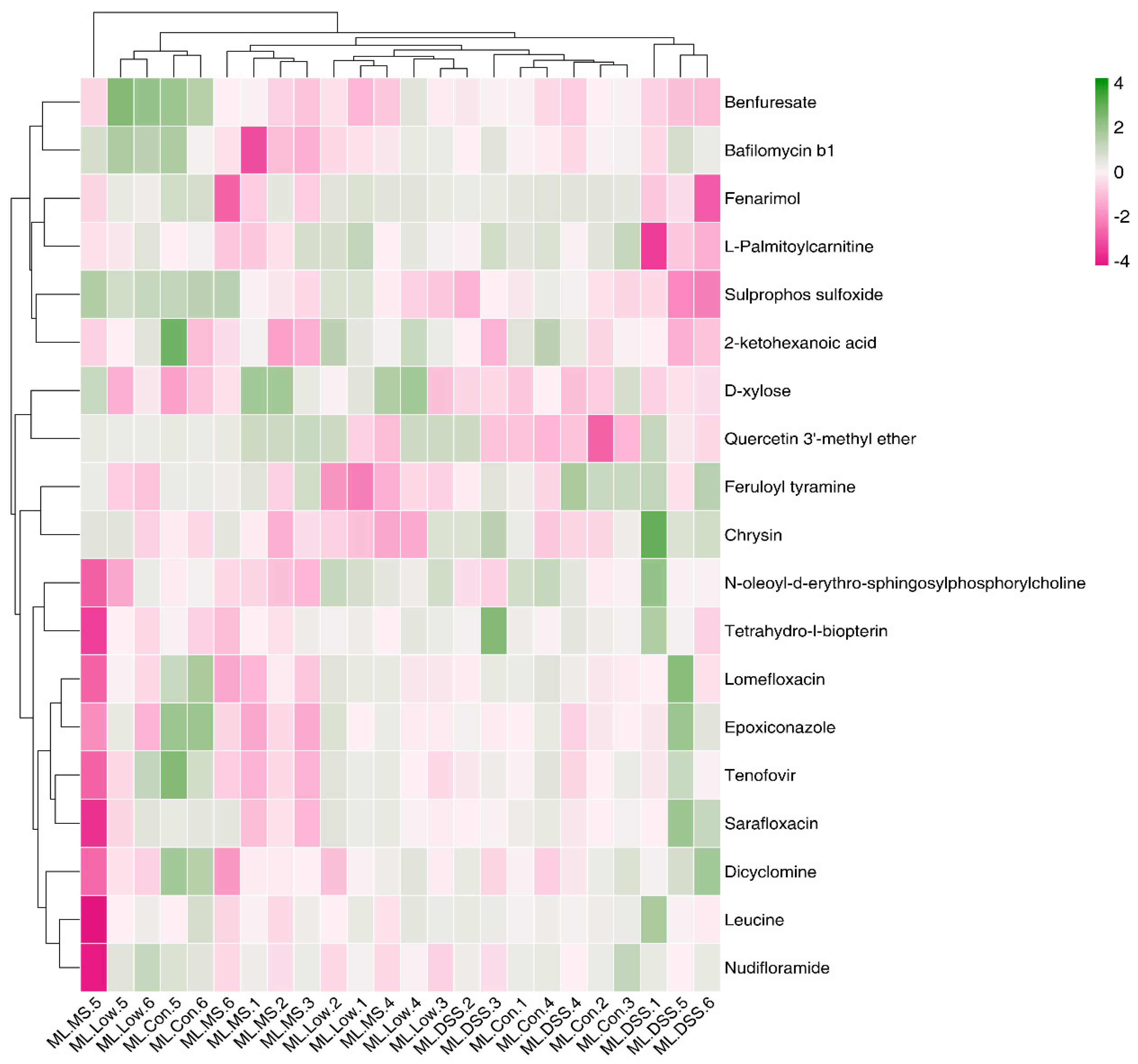
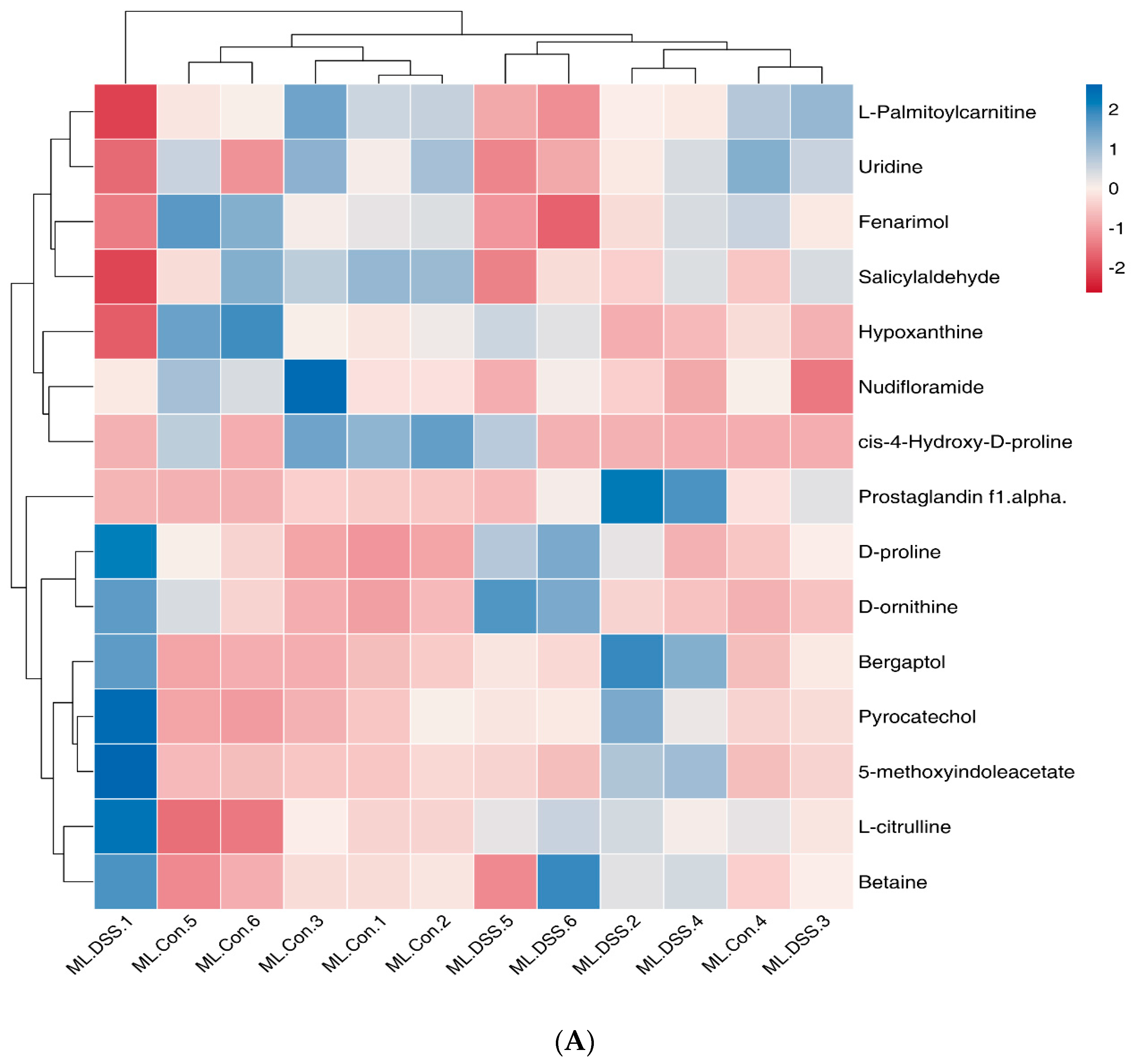
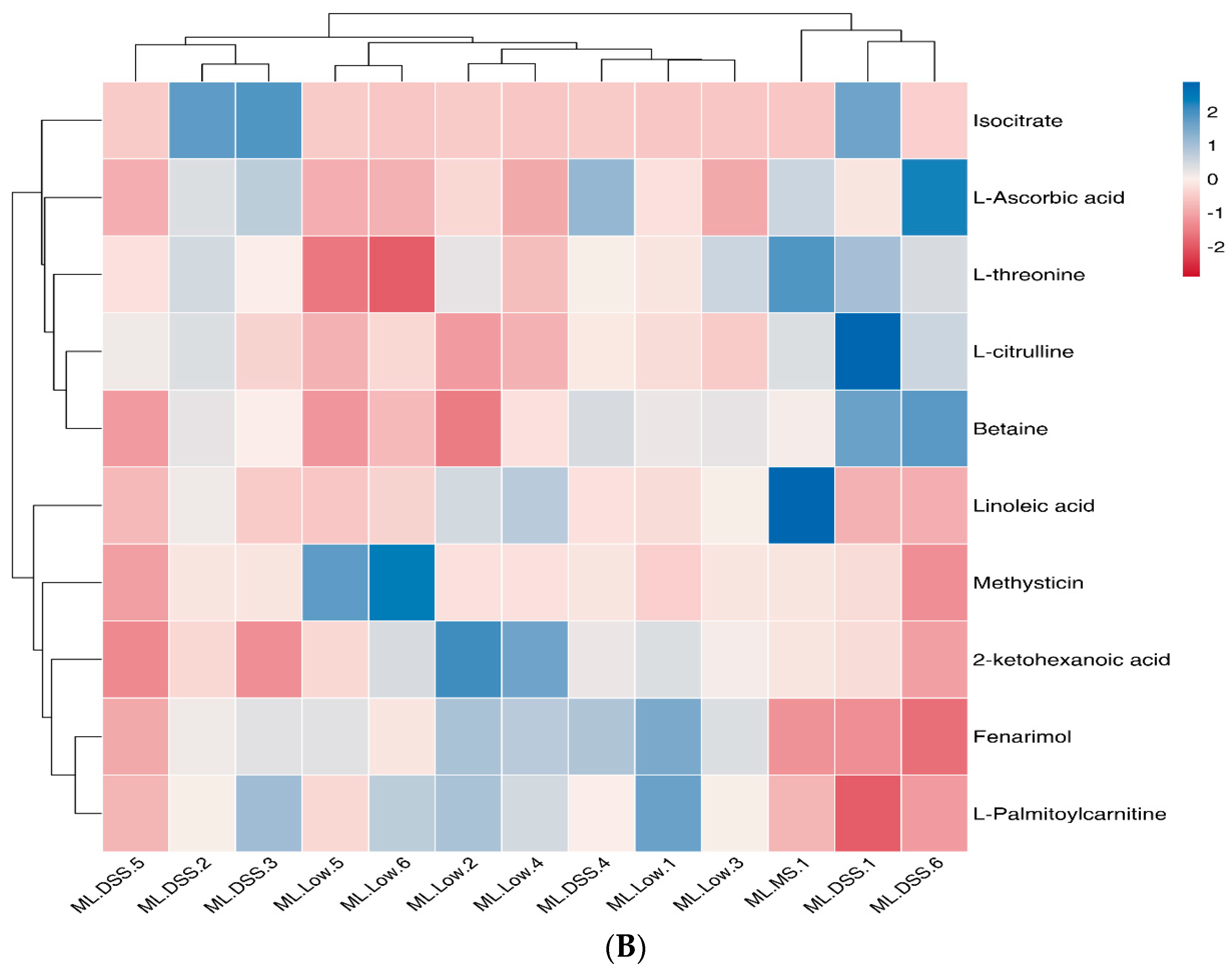
3.4.4. Screening of Differential Metabolites
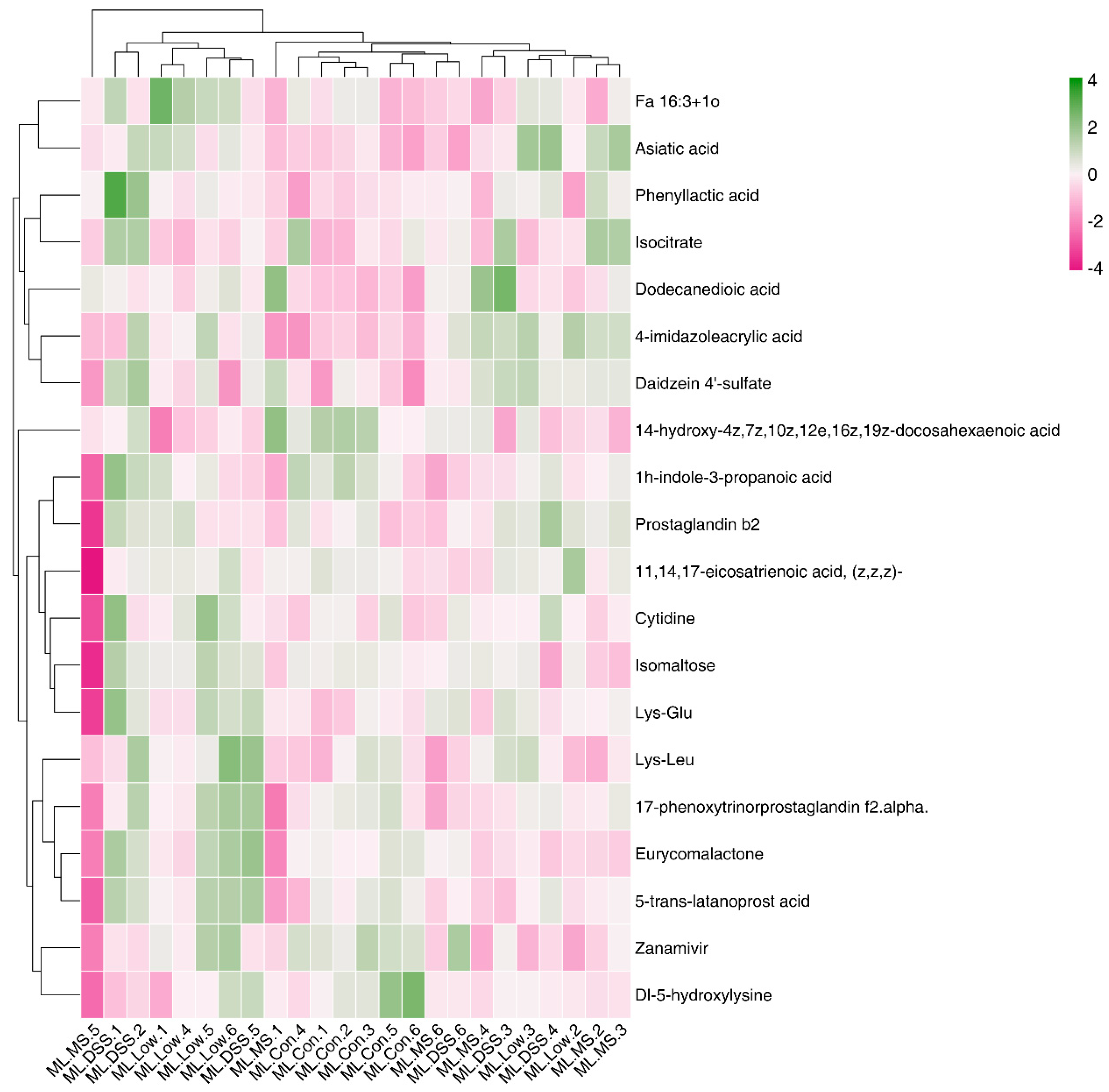
3.4.5. Cluster Analysis of Differential Metabolites
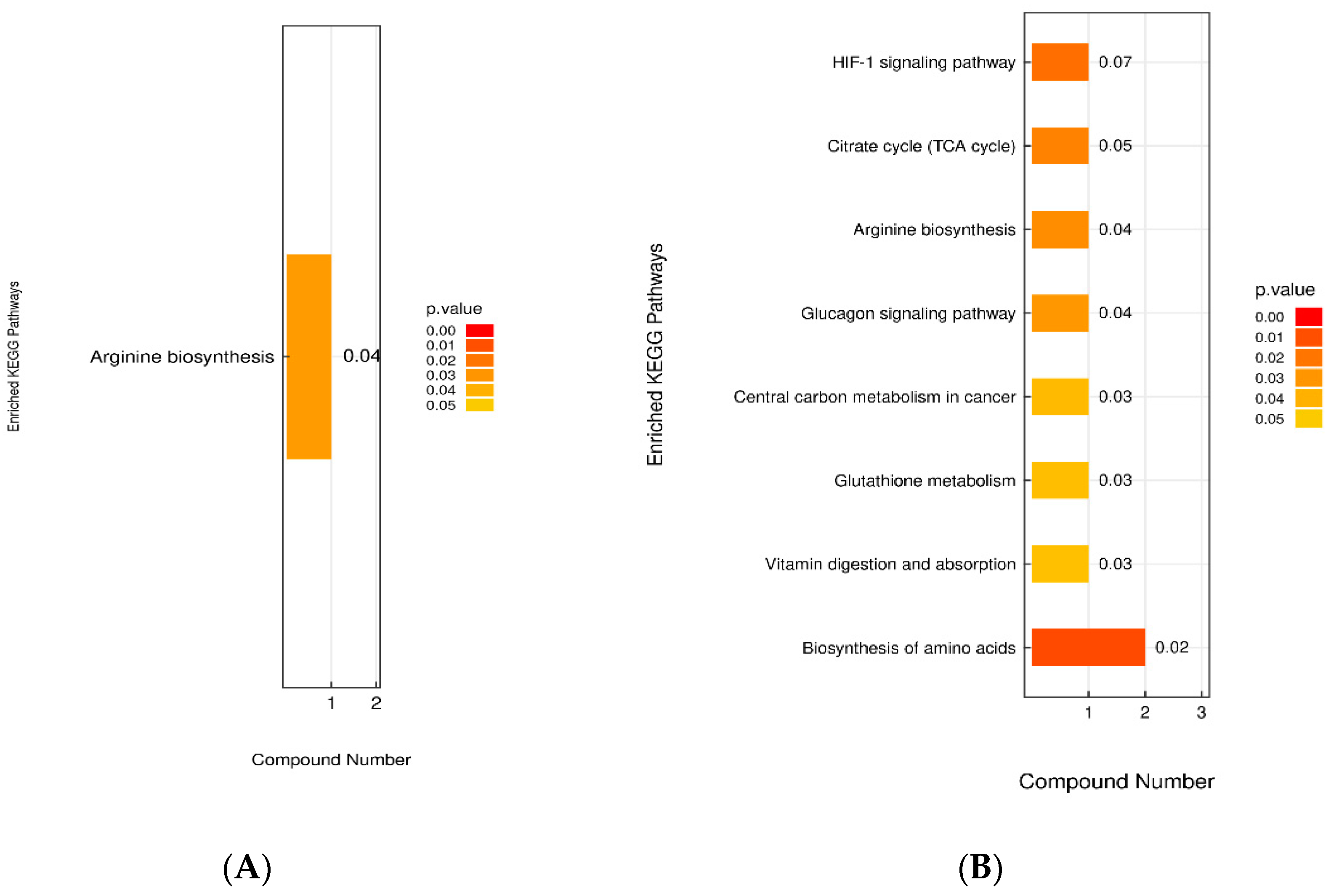
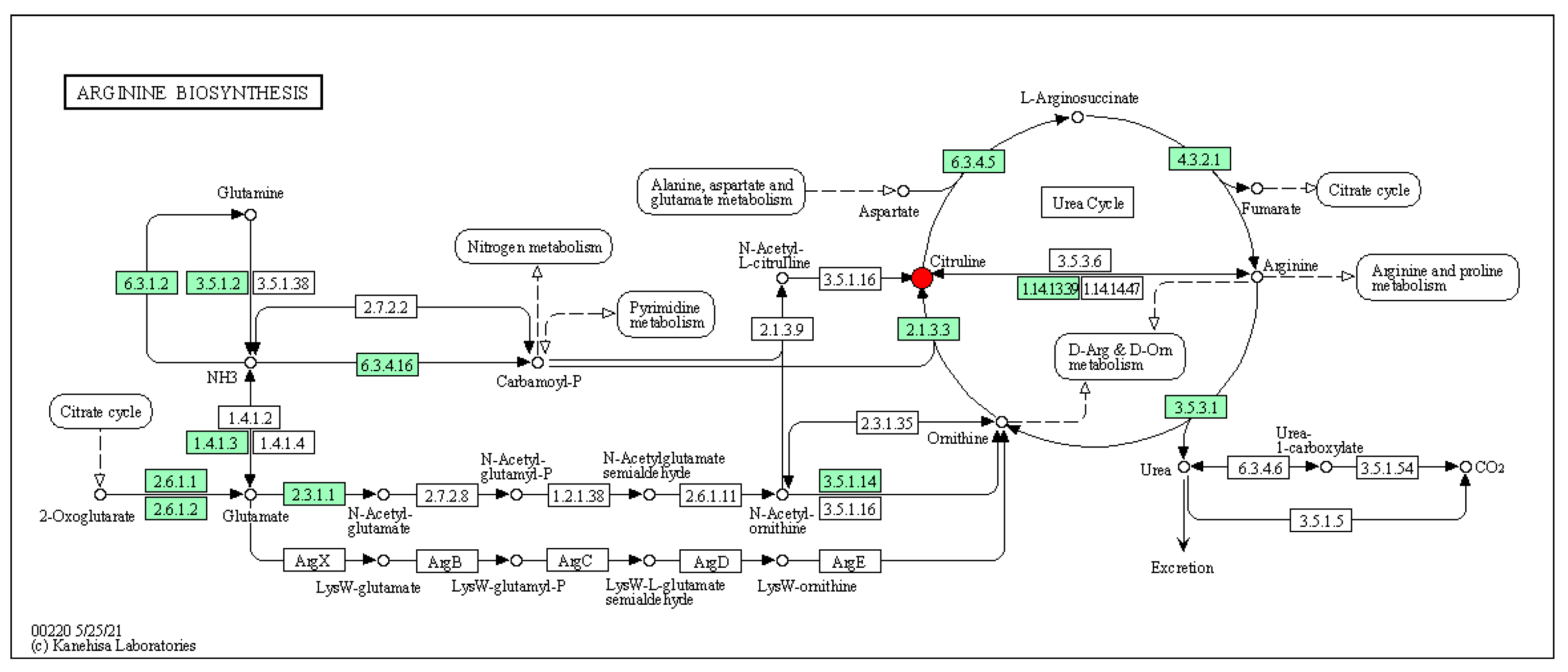
3.4.6. Analysis of the KEGG Metabolic Pathway with Differential Metabolites
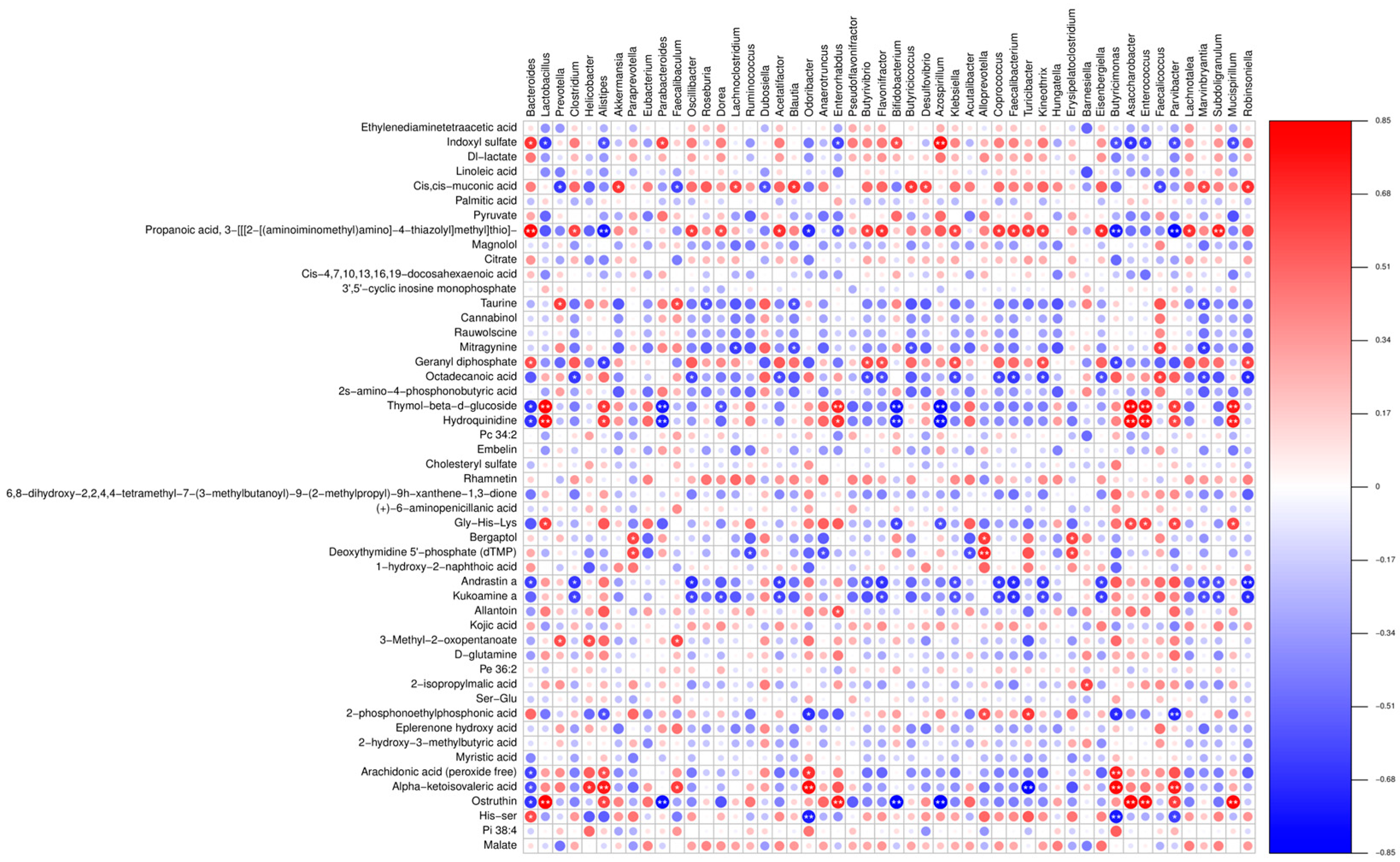
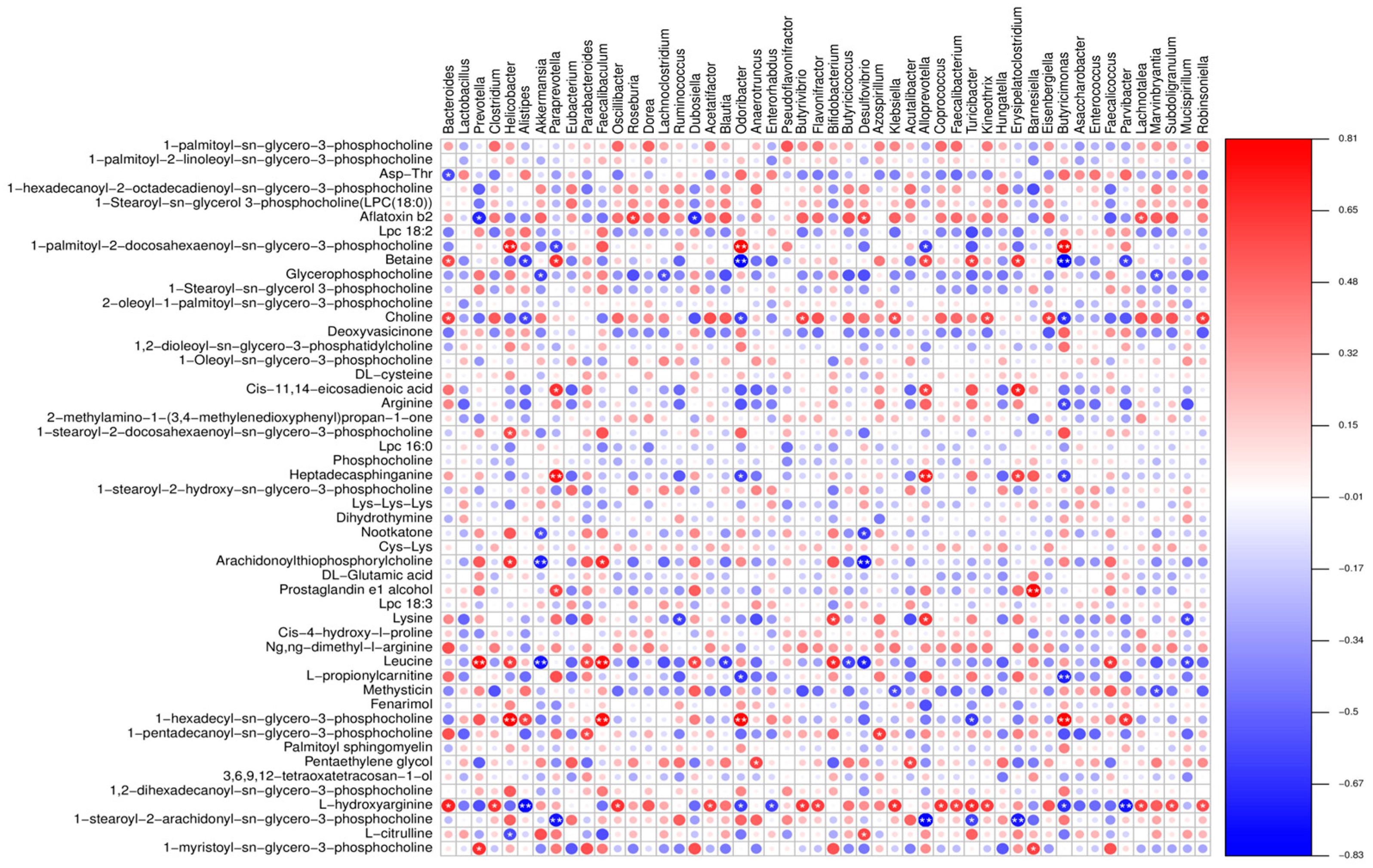
3.5. Combined Resonance Analysis of Intestinal Flora and Metabolites
4. Analysis and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Scoring/Points | Weight Loss/% | Stool Characteristics | Rectal Bleeding (Occult Blood/Gross Blood in Stool) |
|---|---|---|---|
| 0 | 0 | Normal | Occult blood |
| 1 | 1–5 | Stool consistency: Soft but formed | Occult blood |
| 2 | 6–10 | Stool consistency: Soft | Visible blood traces |
| 3 | 11–18 | Stool consistency: Very soft and loose | Visible, significant blood |
| 4 | >18 | Watery diarrhea | Profuse bleeding |
| Reagent | 20 μL System | Final Concentration |
|---|---|---|
| 2X SYBR Green Mix | 10 μL | 1× |
| RT Product | 2 μL | |
| Bulge-LoopTM miRNA Forward Primer (5M) | 0.8 μL | 200 nM |
| Bulge-LoopTM Reverse Primer (5M) | 0.8 μL | 200 nM |
| RNase-free H2O | To 20 μL |
| rt (s) | Name | VIP | FC | p-Value | m/z | Adduct |
|---|---|---|---|---|---|---|
| 510.32 | 2-ketohexanoic acid | 1.61 | 0.53 | 0.0133 | 131.08 | [M+H]+ |
| 407.39 | L-citrulline | 1.98 | 1.33 | 0.0332 | 198.08 | [M+Na]+ |
| 476.16 | Fenarimol | 4.00 | 0.55 | 0.0498 | 331.05 | [M+H]+ |
| 169.08 | L-Ascorbic acid | 2.10 | 5.44 | 0.021 | 197.01 | (M+Na-2H)− |
| 584.04 | Isocitrate | 3.28 | 33.75 | 0.038 | 173.01 | (M-H2O-H)− |
Appendix B
References
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Barbaro, M.R.; Ventura, M.; Barbara, G. Pre- and probiotic overview. Curr. Opin. Pharmacol. 2018, 43, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Sotoodehnejadnematalahi, F.; Amiri, M.M.; Pourshafie, M.R.; Rohani, M. Prophylactic vs. Therapeutic Effect of Probiotics on the Inflammation Mediated by the NF-κB Pathway in Inflammatory Bowel Conditions. Biomedicines 2023, 11, 1675. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-M.; Chien, M.-Y.; Wang, L.-Y.; Chuang, C.-H.; Chen, C.-H. Goji Ferment Ameliorated Acetaminophen-Induced Liver Injury in vitro and in vivo. Probiotics Antimicrob. Proteins 2022, 15, 1102–1112. [Google Scholar] [CrossRef]
- Hooper, K.M.; Barlow, P.G.; Stevens, C.; Henderson, P. Inflammatory Bowel Disease Drugs: A Focus on Autophagy. J. Crohns Colitis 2017, 11, 118–127. [Google Scholar] [CrossRef]
- Dai, Z.F.; Ma, X.Y.; Yang, R.; Wang, H.; Xu, D.; Yang, J.; Guo, X.; Meng, S.; Xu, R.; Li, Y.; et al. Intestinal flora alterations in patients with ulcerative colitis and their association with inflammation. Exp. Ther. Med. 2021, 22, 1322. [Google Scholar] [CrossRef]
- Bergemalm, D.; Andersson, E.; Hultdin, J.; Eriksson, C.; Rush, S.T.; Kalla, R.; Adams, A.T.; Keita, Å.V.; D’Amato, M.; Gomollon, F.; et al. Dominique, Systemic Inflammation in Preclinical Ulcerative Colitis. Gastroenterology 2021, 161, 1526–1539.e9. [Google Scholar] [CrossRef]
- Liu, D.; Galvanin, F.; Yu, Y. Formulation Screening and Freeze-Drying Process Optimization of Ginkgolide B Lyophilized Powder for Injection. AAPS PharmSciTech 2018, 19, 541–550. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, Y.; Stanton, C.; Ross, R.P.; Wang, Z.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Dose-response efficacy and mechanisms of orally administered CLA-producing Bifidobacterium breve CCFM683 on DSS-induced colitis in mice. J. Funct. Foods 2020, 75, 104245. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.C.H.F.; Le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from Different Geographic Origins Decreases Intestinal Inflammation and Bacteroides spp. Populations in a Model of DSS-Induced Colitis. Mol. Nutr. Food Res. 2018, 62, 1800080. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Facchin, S.; Patuzzi, I.; Ford, A.C.; Massimi, D.; Valle, G.; Sattin, E.; Simionati, B.; Bertazzo, E.; Zingone, F.; et al. A specific microbiota signature is associated to various degrees of ulcerative colitis as assessed by a machine learning approach. Gut Microbes 2022, 14, 2028366. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Santoru, M.L.; Piras, C.; Murgia, A.; Palmas, V.; Camboni, T.; Liggi, S.; Ibba, I.; Lai, M.A.; Orrù, S.; Blois, S.; et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci. Rep. 2017, 7, 9523. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, K.; Gao, P.; Pu, Q.; Lin, P.; Qin, S.; Xie, N.; Hur, J.; Li, C.; Huang, C.; et al. Microbial and genetic-based framework identifies drug targets in inflammatory bowel disease. Theranostics 2021, 11, 7491–7506. [Google Scholar] [CrossRef]
- Wilson, K.T. Dietary Arginine Regulates Severity of Experimental Colitis and Affects the Colonic Microbiome. Front. Cell. Infect. Microbiol. 2019, 9, 66. [Google Scholar]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef]
- Polytarchou, C.; Hommes, D.W.; Palumbo, T.; Hatziapostolou, M.; Koutsioumpa, M.; Koukos, G.; Van Der Meulen-de Jong, A.E.; Oikonomopoulos, A.; Van Deen, W.K.; Vorvis, C.; et al. MicroRNA214 Is Associated With Progression of Ulcerative Colitis, and Inhibition Reduces Development of Colitis and Colitis-Associated Cancer in Mice. Gastroenterology 2015, 149, 981–992.e11. [Google Scholar] [CrossRef]
- Imhann, F.; Vila, A.V.; Bonder, M.J.; Fu, J.; Gevers, D.; Visschedijk, M.C.; Spekhorst, L.M.; Alberts, R.; Franke, L.; Van Dullemen, H.M.; et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018, 67, 108–119. [Google Scholar] [CrossRef]
- Farraye, F.A.; Odze, R.D.; Eaden, J.; Itzkowitz, S.H. AGA Technical Review on the Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. Gastroenterology 2010, 138, 746–774.e4. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, G.; Yang, Q.; Ye, J.; Cai, X.; Tsering, P.; Cheng, X.; Hu, C.; Zhang, S.; Cao, P. Gut microbiota drives the attenuation of dextran sulphate sodium-induced colitis by Huangqin decoction. Oncotarget 2017, 8, 48863–48874. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xue, Y.; Du, M.; Zhu, M.-J. Preventive effects of Goji berry on dextran-sulfate-sodium-induced colitis in mice. J. Nutr. Biochem. 2017, 40, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. The Gut Microbiome and Metabolic Health. Curr. Nutr. Rep. 2017, 6, 16–23. [Google Scholar] [CrossRef]
- Castaño-Rodríguez, N.; Kaakoush, N.O.; Lee, W.S.; Mitchell, H.M. Dual role of Helicobacter and Campylobacter species in IBD: A systematic review and meta-analysis. Gut 2017, 66, 235–249. [Google Scholar] [CrossRef]
- Yap, T.W.-C.; Gan, H.-M.; Lee, Y.-P.; Leow, A.H.-R.; Azmi, A.N.; Francois, F.; Perez-Perez, G.I.; Loke, M.-F.; Goh, K.-L.; Vadivelu, J. Helicobacter pylori Eradication Causes Perturbation of the Human Gut Microbiome in Young Adults. PLoS ONE 2016, 11, e0151893. [Google Scholar] [CrossRef]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Kumari, R. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. 2013, 19, 3404. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Andoh, A.; Sugimoto, M. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J. Cell. Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef]
- Derwa, Y.; Gracie, D.J.; Hamlin, P.J.; Ford, A.C. Systematic review with meta-analysis: The efficacy of probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 389–400. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions and Signaling Pathways of Amino Acids in Intestinal Inflammation. BioMed Res. Int. 2018, 2018, 9171905. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.H.; Tong, G.; Xiao, K.; Jiao, L.F.; Ke, Y.L.; Hu, C.H. L -Cysteine protects intestinal integrity, attenuates intestinal inflammation and oxidant stress, and modulates NF-κB and Nrf2 pathways in weaned piglets after LPS challenge. Innate Immun. 2016, 22, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Lletjós, S.; Beaumont, M.; Tomé, D.; Benamouzig, R.; Blachier, F.; Lan, A. Dietary Protein and Amino Acid Supplementation in Inflammatory Bowel Disease Course: What Impact on the Colonic Mucosa? Nutrients 2017, 9, 310. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Wu, M.; Liu, G.; Yang, G.; Xion, Y.; Su, D.; Wu, L.; Li, T.; Chen, S.; et al. Serum Amino Acids Profile and the Beneficial Effects of L-Arginine or L-Glutamine Supplementation in Dextran Sulfate Sodium Colitis. PLoS ONE 2014, 9, e88335. [Google Scholar] [CrossRef]
- Al-Drees, A.; Khalil, M.S. Histological and immunohistochemical effects of L-arginine and silymarin on TNBS-induced inflammatory bowel disease in rats. Histol. Histopathol. 2016, 31, 1259–1270. [Google Scholar] [CrossRef]
- Andrade, M.E.R.; Santos, R.D.G.C.D.; Soares, A.D.N.; Costa, K.A.; Fernandes, S.O.A.; De Souza, C.M.; Cassali, G.D.; De Souza, A.L.; Faria, A.M.C.; Cardoso, V.N. Pretreatment and Treatment With L-Arginine Attenuate Weight Loss and Bacterial Translocation in Dextran Sulfate Sodium Colitis. J. Parenter. Enter. Nutr. 2016, 40, 1131–1139. [Google Scholar] [CrossRef]
- Coburn, L.A.; Gong, X.; Singh, K.; Asim, M.; Scull, B.P.; Allaman, M.M.; Williams, C.S.; Rosen, M.J.; Washington, M.K.; Barry, D.P.; et al. L-arginine Supplementation Improves Responses to Injury and Inflammation in Dextran Sulfate Sodium Colitis. PLoS ONE 2012, 7, e33546. [Google Scholar] [CrossRef]
- Shin, S.; Jeong, H.M.; Chung, S.E.; Kim, T.H.; Thapa, S.K.; Lee, D.Y.; Song, C.H.; Lim, J.Y.; Cho, S.-M.; Nam, K.-Y.; et al. Simultaneous analysis of acetylcarnitine, proline, hydroxyproline, citrulline, and arginine as potential plasma biomarkers to evaluate NSAIDs-induced gastric injury by liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 165, 101–111. [Google Scholar] [CrossRef]
- Hugenholtz, F.; De Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell. Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef]
- Singh, V.; Yeoh, B.S.; Chassaing, B.; Xiao, X.; Saha, P.; Olvera, R.A.; Lapek, J.D.; Zhang, L.; Wang, W.-B.; Hao, S.; et al. Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer. Cell 2018, 175, 679–694.e22. [Google Scholar] [CrossRef] [PubMed]

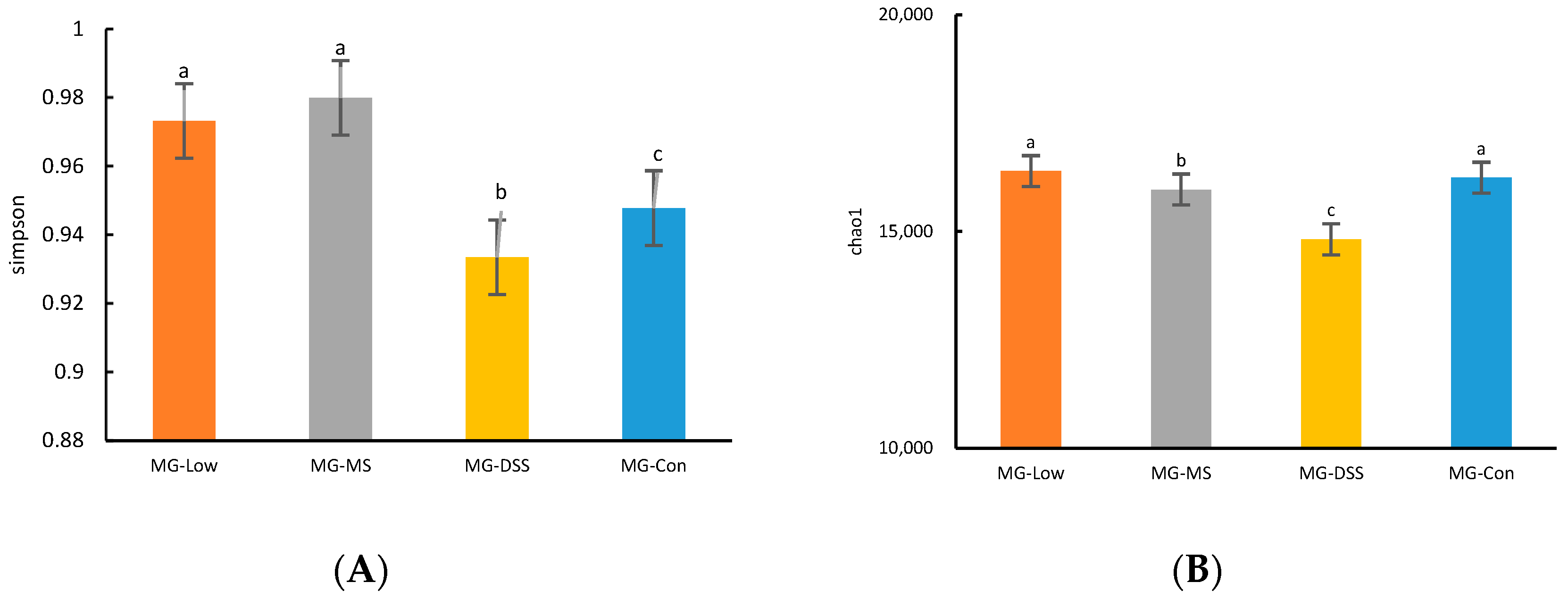

| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| miR-214 | CGCTTTACAGCAGGCACAGA | TAAGGTTCATCACGACAGGTICAC |
| GAPDH | AAGGTCGGAGTCACCGGATT | CTGGAAGATGGTGATGGGATT |
| Group | Number of Samples (Cases) | miR-214 | IL-6 | P-STAT3 |
|---|---|---|---|---|
| Con | 6 | 1.147 ± 0.768 | 0.8007 ± 0.3948 | 0.9345 ± 0.9819 |
| DSS | 6 | 3.623 ± 1.128 ## | 6.2169 ± 1.8290 ## | 17.3432 ± 5.2125 ## |
| MS | 6 | 1.197 ± 0.686 * | 4.9281 ± 2.3704 | 12.4073 ± 3.3159 |
| High | 6 | 1.450 ± 1.329 * | 2.8525 ± 0.2716 ** | 5.8161 ± 2.3814 ** |
| Low | 6 | 2.580 ± 0.548 | 4.6217 ± 1.2715 | 5.0924 ± 1.8529 ** |
| rt (s) | Name | VIP | FC | p-Value | m/z | Adduct |
|---|---|---|---|---|---|---|
| 476.16 | Fenarimol | 5.74 | 0.44 | 0.009 | 331.05 | [M+H]+ |
| 407.39 | L-citrulline | 2.34 | 1.46 | 0.033 | 198.08 | [M+Na]+ |
| 447.33 | Asp-Thr | 19.54 | 0.74 | 0.033 | 235.09 | [M+H]+ |
| 86.17 | Nudifloramide | 1.07 | 0.72 | 0.035 | 153.07 | [M+H]+ |
| 23.96 | Bergaptol | 6.86 | 2.44 | 0.006 | 201.02 | [M-H]− |
| 24.08 | 2-phosphonoethylphosphonic acid | 6.14 | 3.06 | 0.013 | 188.99 | [M-H]− |
| 331.83 | D-proline | 1.52 | 1.95 | 0.022 | 114.06 | [M-H]− |
| 23.94 | Pyrocatechol | 1.43 | 2.60 | 0.029 | 109.03 | [M-H]− |
| 25.02 | Daidzein 4′-sulfate | 1.03 | 4.61 | 0.030 | 333.01 | [M-H]− |
| 37.76 | 14-hydroxy-4z,7z,10z,12e,16z,19z-docosahexaenoic acid | 1.13 | 0.47 | 0.046 | 343.23 | [M-H]− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, M.; Ji, Q.; Guo, G.; Zhang, H.; Wang, Y.; Zhai, R.; Pan, L. Alleviating Effect of Lactiplantibacillus plantarum NXU0011 Fermented Wolfberry on Ulcerative Colitis in Mice. Fermentation 2023, 9, 971. https://doi.org/10.3390/fermentation9110971
Nie M, Ji Q, Guo G, Zhang H, Wang Y, Zhai R, Pan L. Alleviating Effect of Lactiplantibacillus plantarum NXU0011 Fermented Wolfberry on Ulcerative Colitis in Mice. Fermentation. 2023; 9(11):971. https://doi.org/10.3390/fermentation9110971
Chicago/Turabian StyleNie, Mingxia, Quan Ji, Gang Guo, Haiyan Zhang, Yanhong Wang, Ru Zhai, and Lin Pan. 2023. "Alleviating Effect of Lactiplantibacillus plantarum NXU0011 Fermented Wolfberry on Ulcerative Colitis in Mice" Fermentation 9, no. 11: 971. https://doi.org/10.3390/fermentation9110971
APA StyleNie, M., Ji, Q., Guo, G., Zhang, H., Wang, Y., Zhai, R., & Pan, L. (2023). Alleviating Effect of Lactiplantibacillus plantarum NXU0011 Fermented Wolfberry on Ulcerative Colitis in Mice. Fermentation, 9(11), 971. https://doi.org/10.3390/fermentation9110971






