Dynamic Changes and Correlation Analysis of Polysaccharide Content and Color Parameters in Glycyrrhiza Stems and Leaves during Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Samples
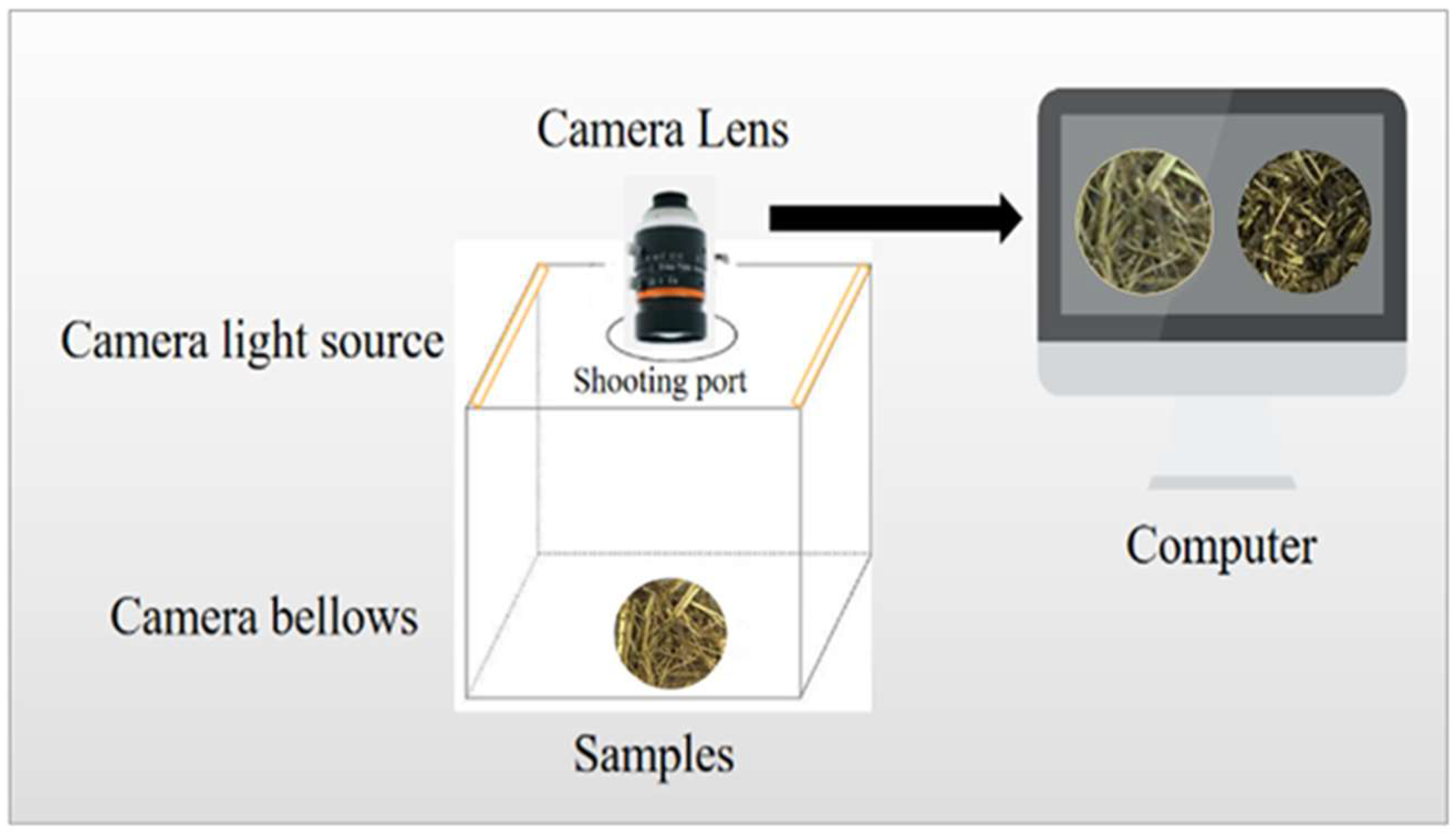
2.2. Construction of Computer Vision System and Image Collection
2.3. Chemical Analysis
2.4. Microbiological Analysis
2.5. Statistical Analysis
3. Results and Discussion
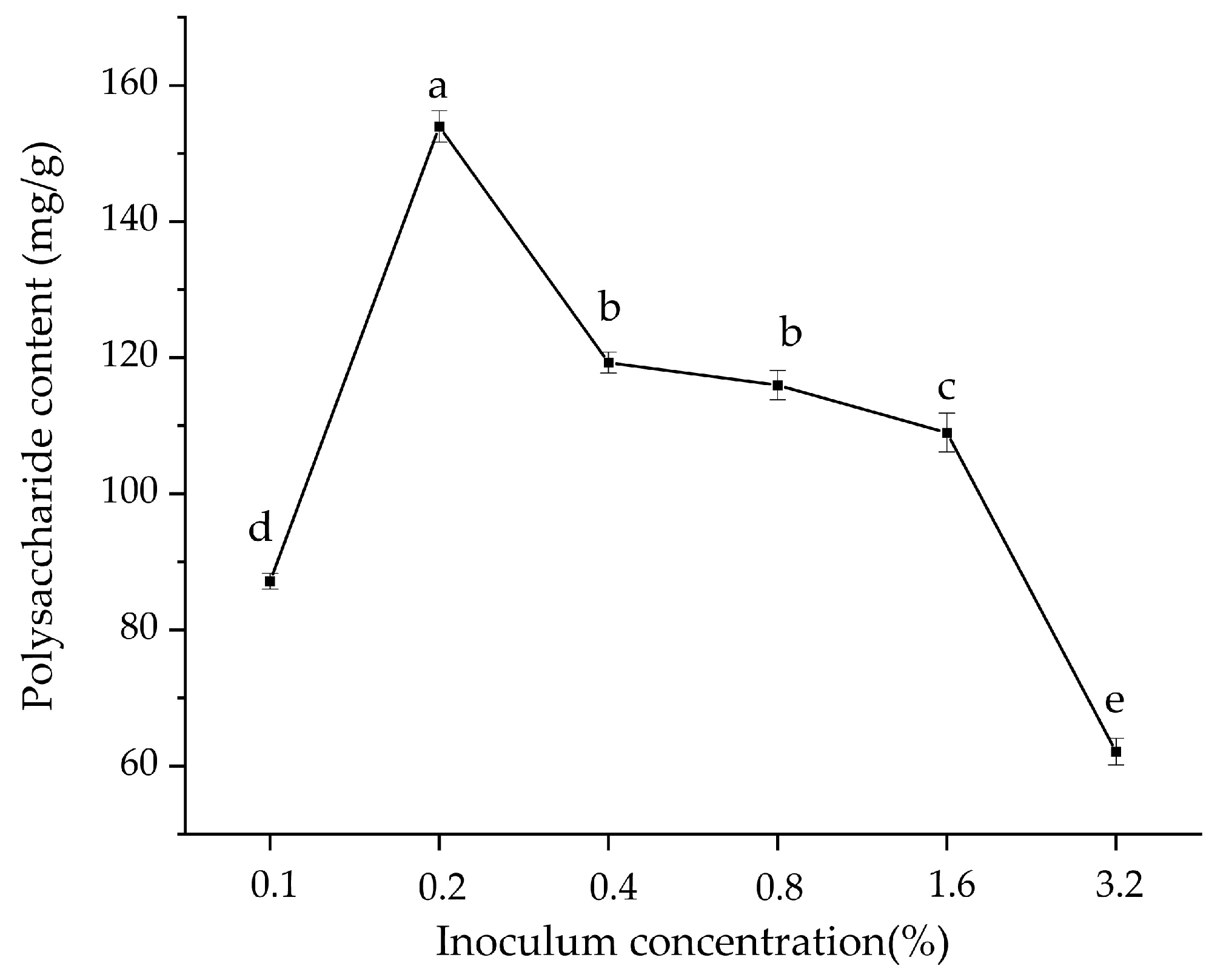
3.1. Polysaccharide Content of Fermented Glycyrrhiza Stems and Leaves with Different Bacterial Additions
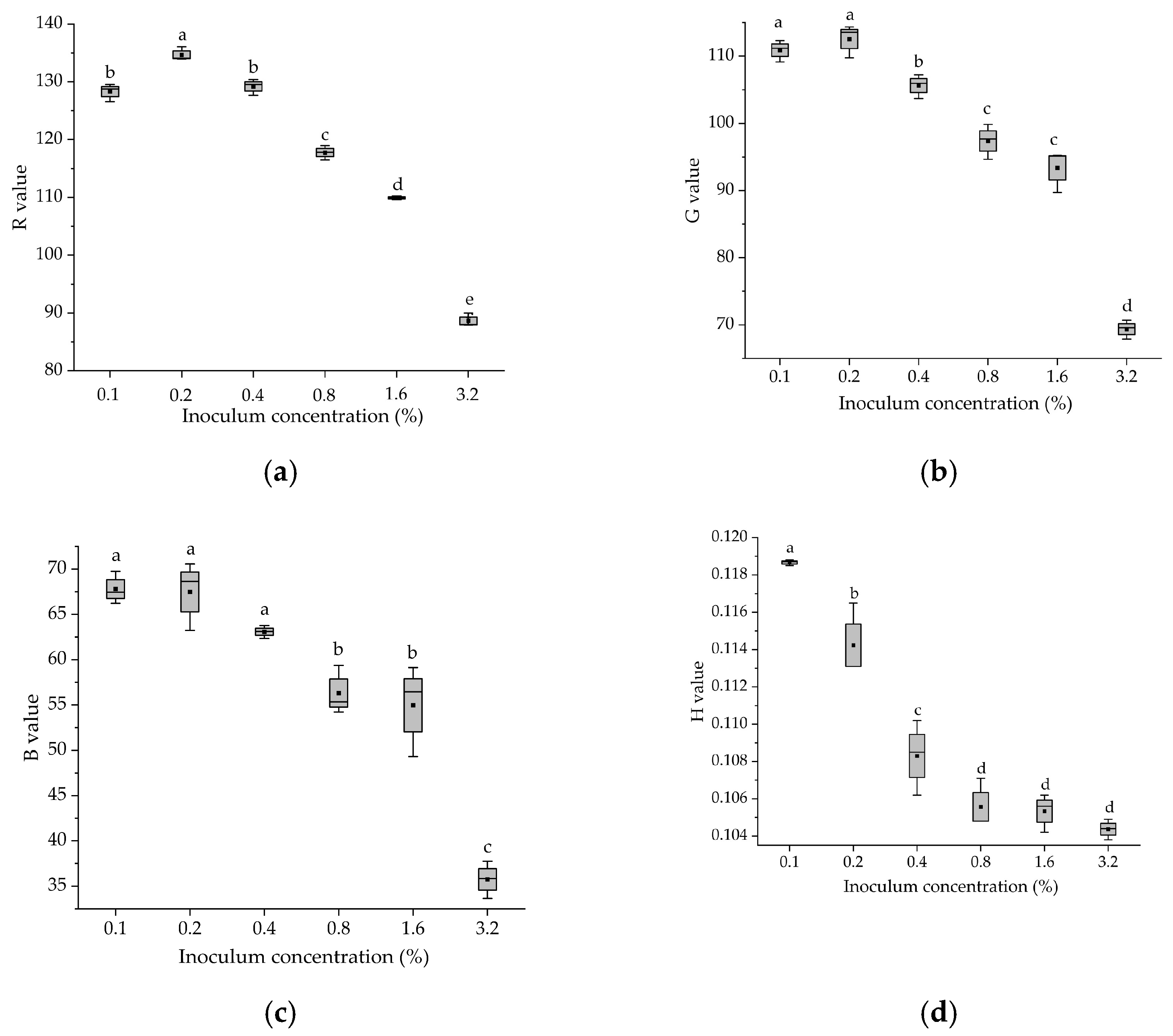
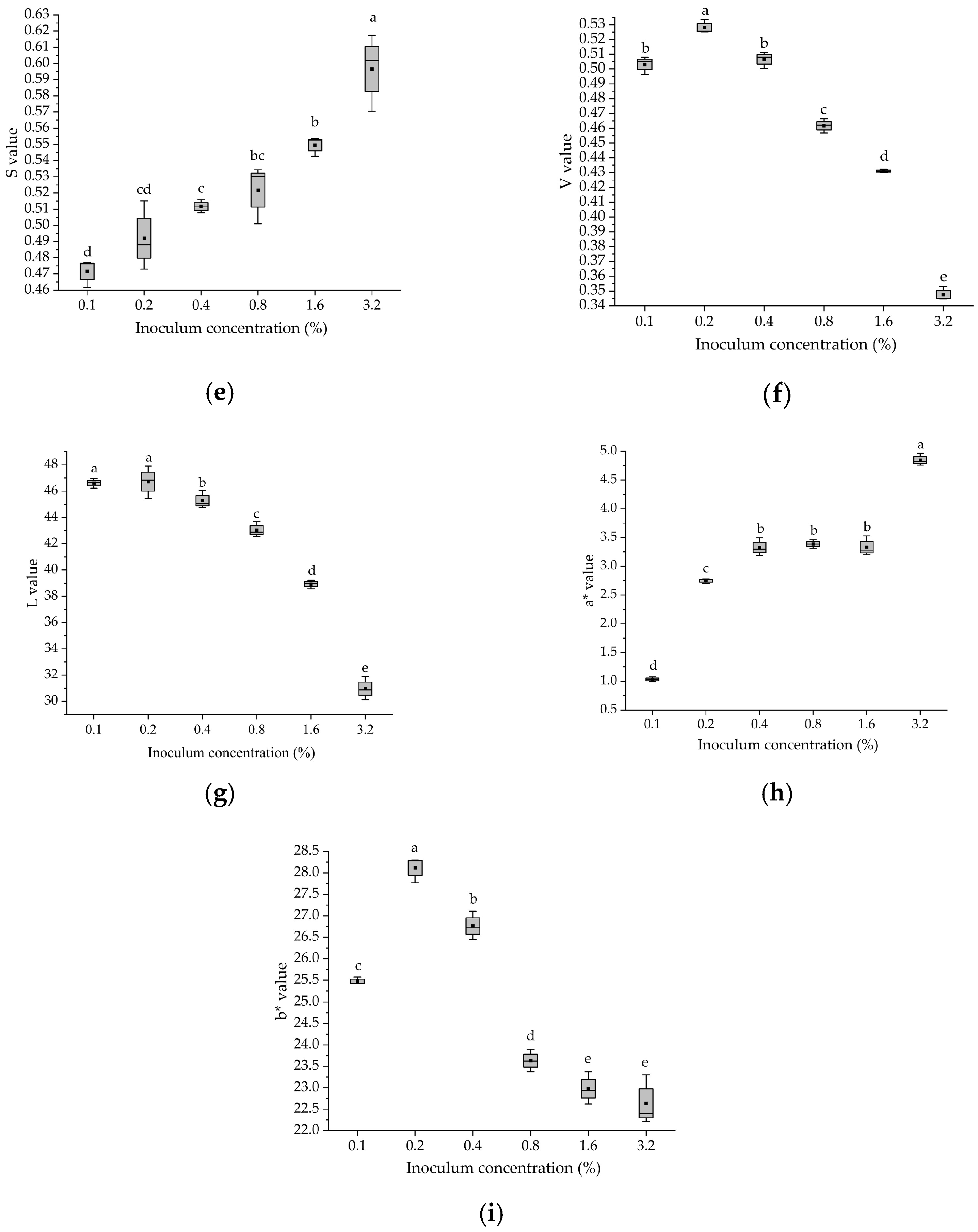
3.2. Color Parameters of Fermented Glycyrrhiza Stems and Leaves with Different Bacterial Additions
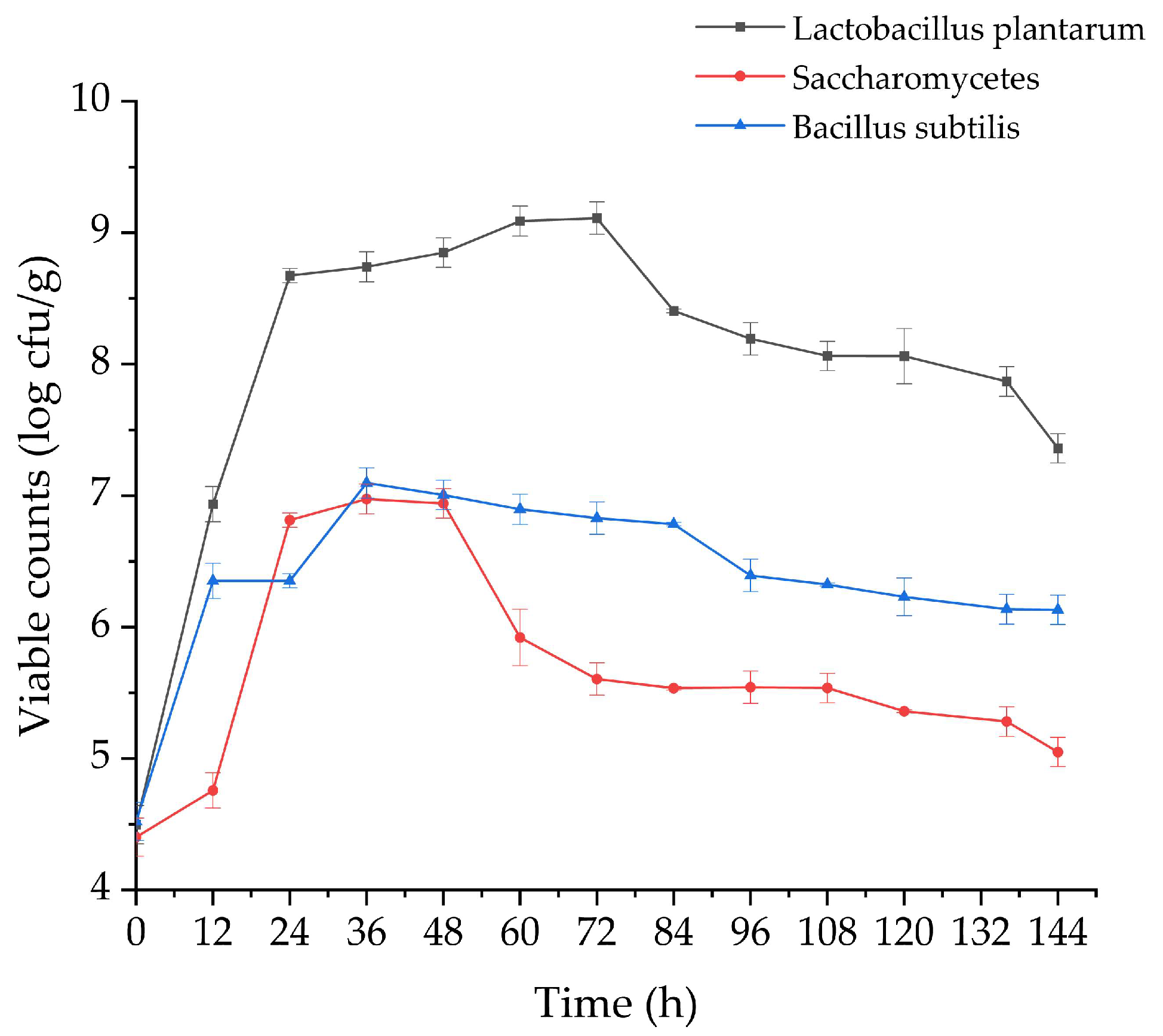
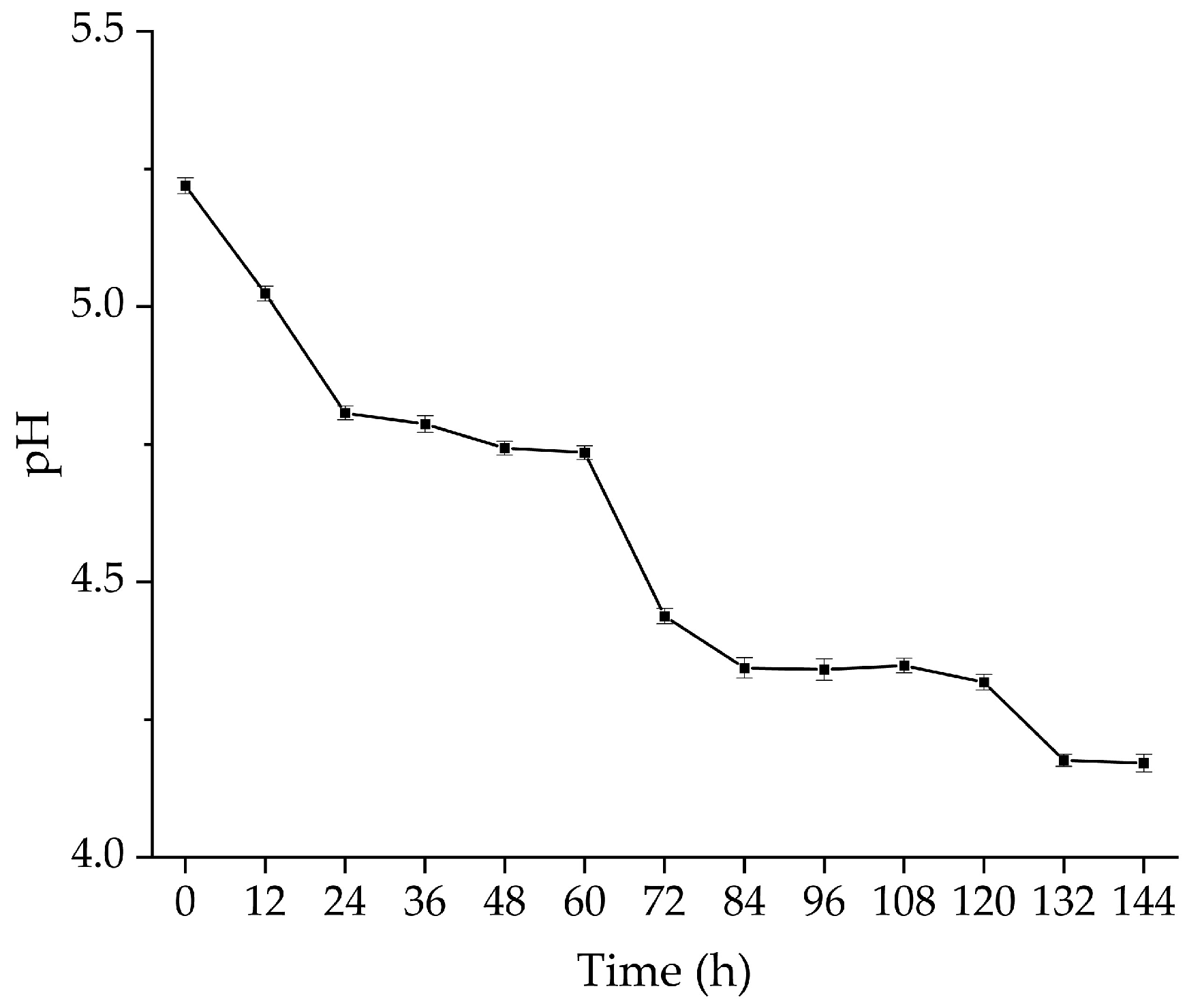
3.3. Dynamic Changes in the Viable Counts of the Probiotic Strains and pH Values of Glycyrrhiza Stems and Leaves during Fermentation
3.4. Dynamic Changes in Polysaccharide Content of Glycyrrhiza Stems and Leaves during Fermentation
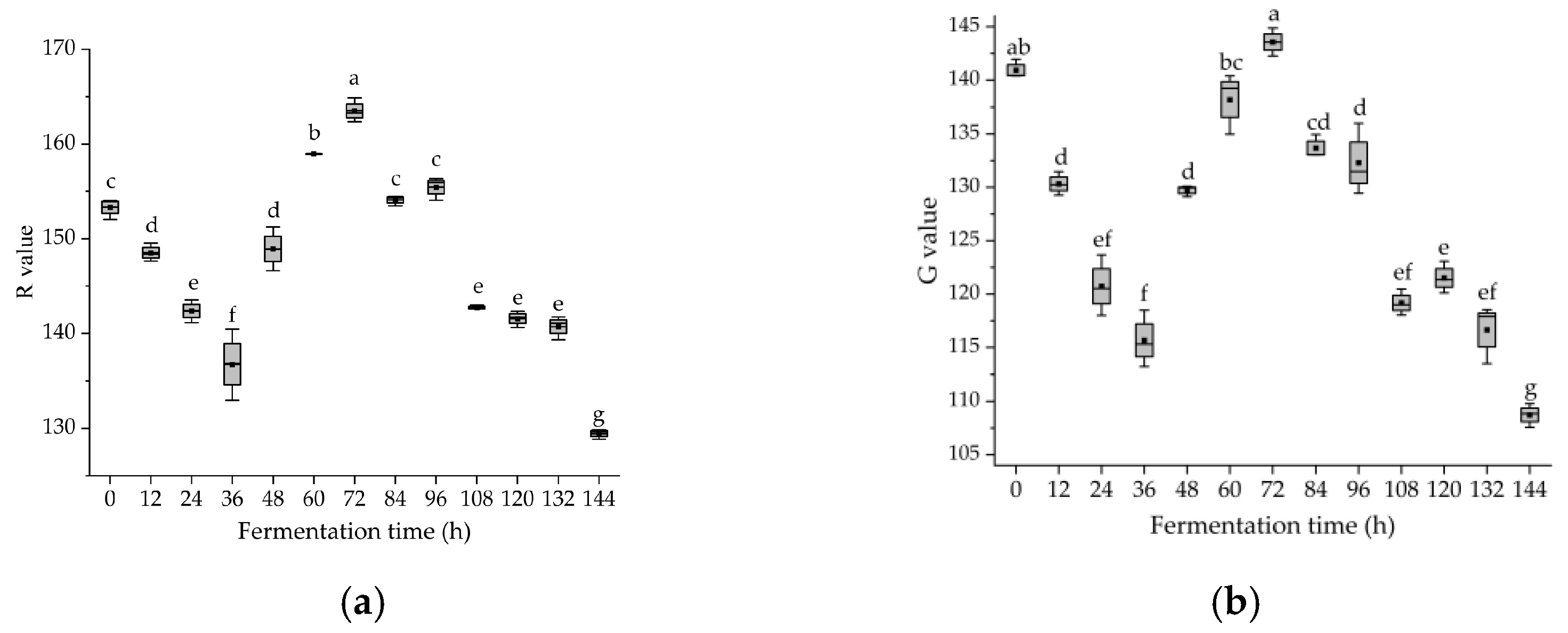
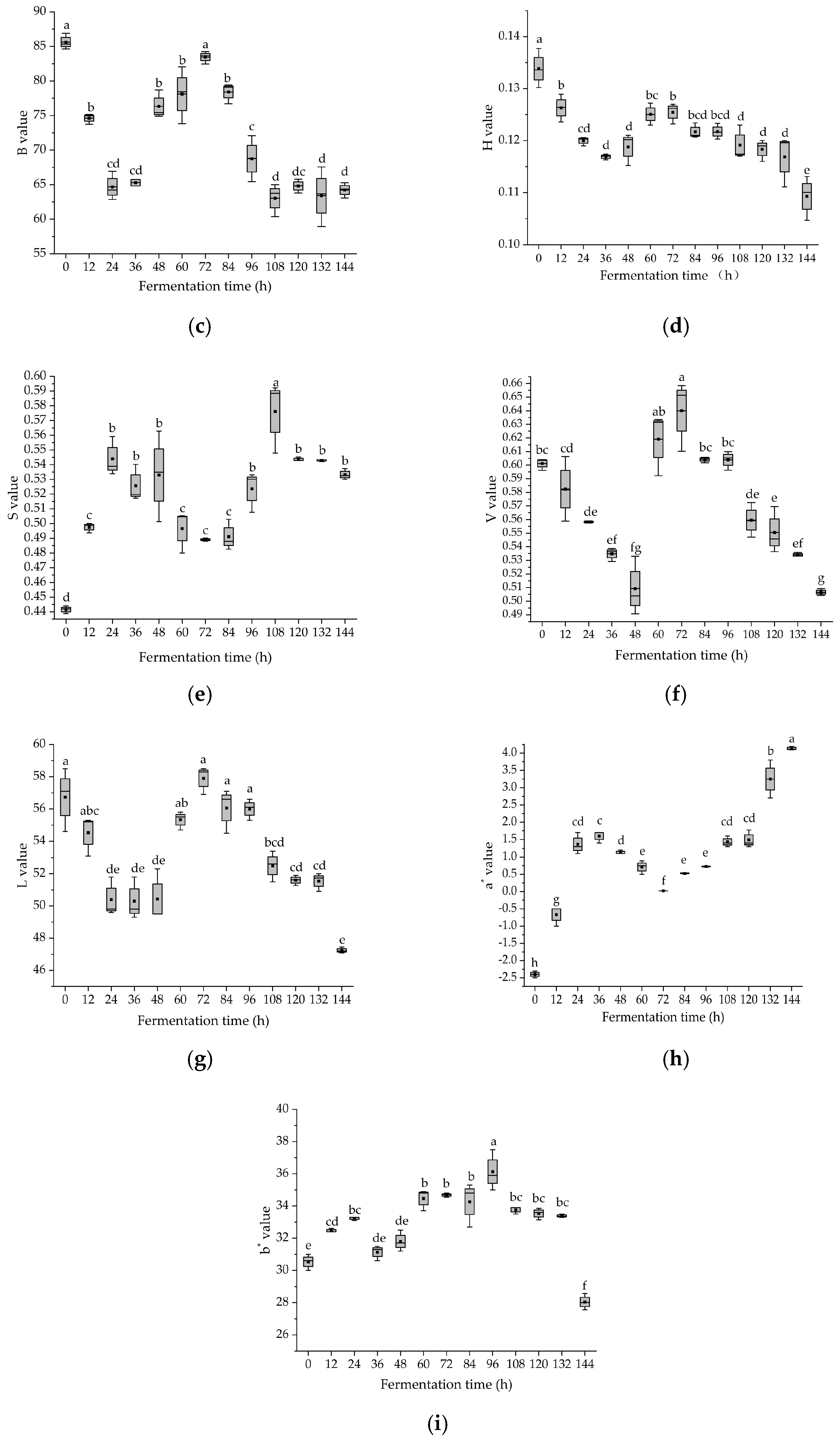
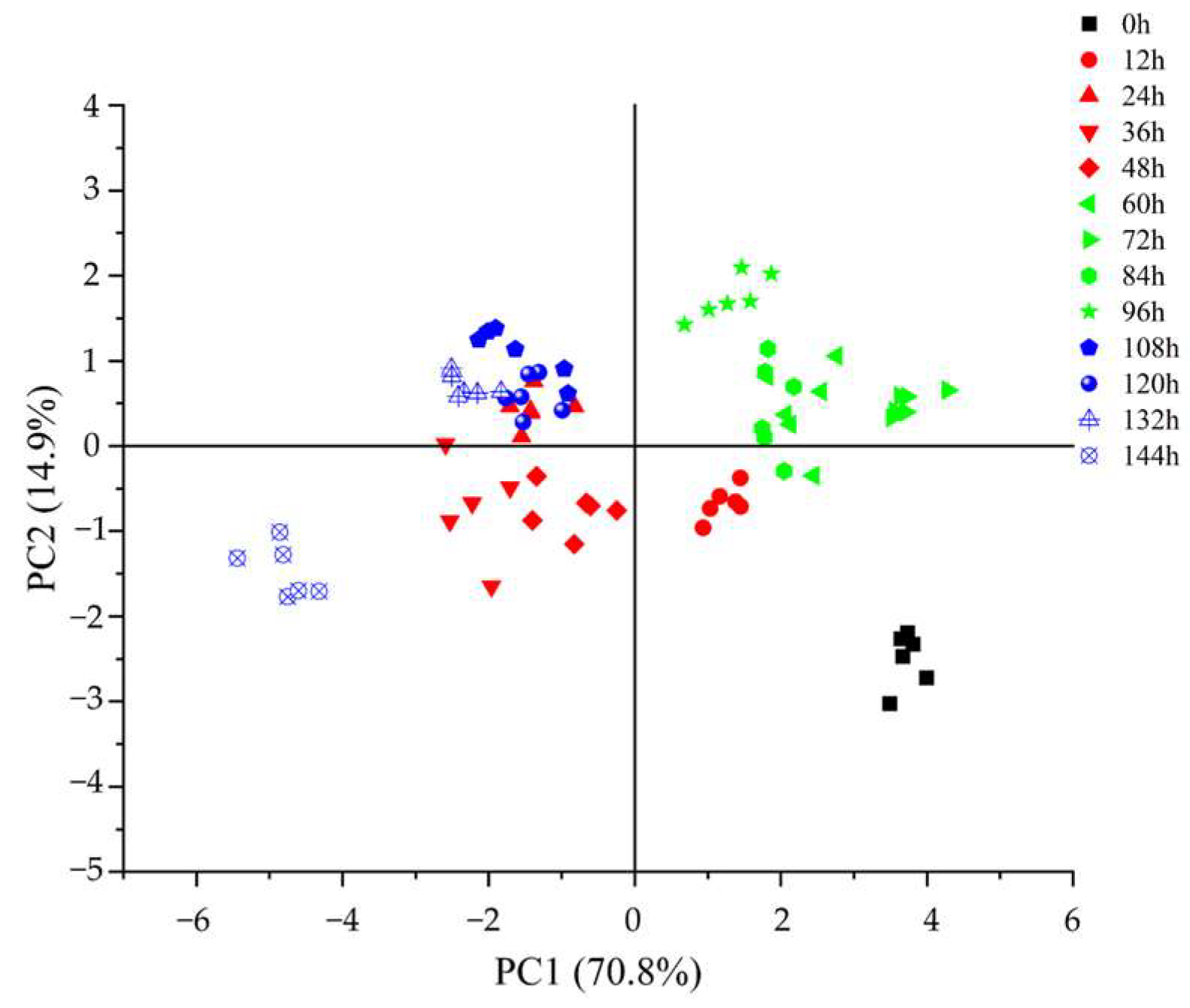
3.5. Dynamic Changes in Color Parameters of Glycyrrhiza Stems and Leaves during Fermentation
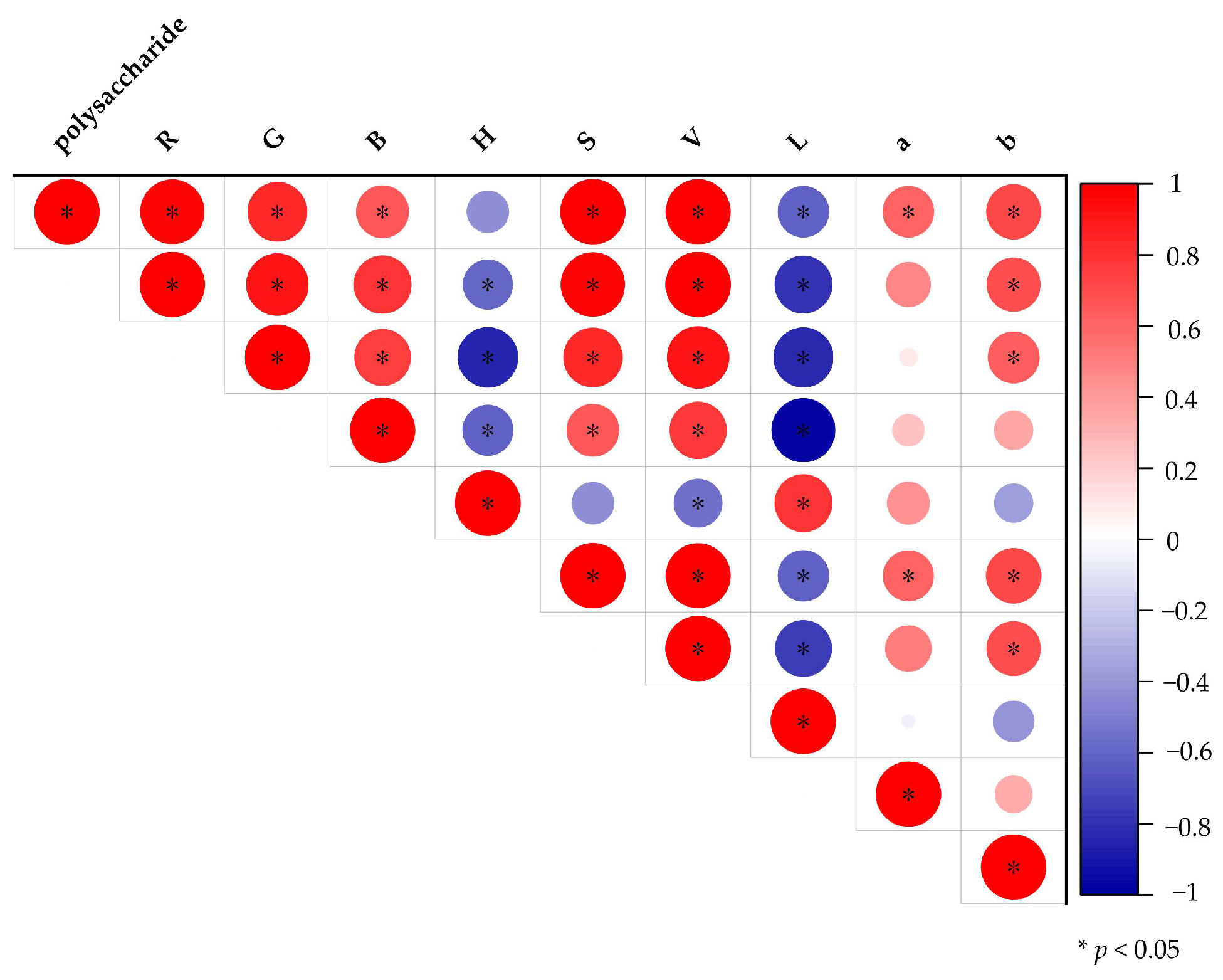
3.6. Correlations between Imaging Variables and Quality Indicators
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch Toxicol. 2020, 94, 651–715. [Google Scholar]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological effects of Glycyrrhiza spp. and its bioactive constituents: Update and review. Phytother. Res. 2015, 29, 1868–1886. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used chinese herb. Pharm. Biol. 2017, 55, 7586–7597. [Google Scholar] [CrossRef]
- Simayi, Z.; Rozi, P.; Yang, X.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.; Ma, S.; Askar, G.; Yadikar, N. Isolation, structural characterization, biological activity, and application of glycyrrhiza polysaccharides: Systematic review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef]
- Hu, X.; Chen, J.; Jiang, L.; Shikov, A. Metabolomic and pharmacologic insights of aerial and underground parts of glycyrrhiza uralensis fisch for maximum utilization of medicinal resources. Front. Pharmacol. 2021, 12, 658–670. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Qiao, S. Advances in research on solid-state fermented feed and its utilization: The pioneer of private customization for intestinal microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef]
- Cui, Y.; Peng, S.; Deng, D. Solid-state fermentation improves the quality of chrysanthemum waste as an alternative feed ingredient. J. Environ. Manag. 2023, 330, 117060. [Google Scholar] [CrossRef]
- Wu, T.X.; Wang, N.; Zhang, Y.; Xu, X.B. Advances in the study on microbial fermentation and transformation of traditional Chinese medicine. Afr. J. Microbiol. Res. 2013, 7, 1644–1650. [Google Scholar] [CrossRef]
- Liu, N.; Song, M.; Wang, N.F.; Wang, Y.; Wang, R.F.; An, X.P.; Qi, J.W. The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, X.; Yue, Y.; Yue, T.; Yuan, Y. Improved flavonoid content in mulberry leaves by solid-state fermentation: Metabolic profile, activity, and mechanism. Innov. Food Sci. Emerg. 2023, 84, 103308. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Luis, F.V.; Nuria, L.R.; Antonio, M.O.; Erenas, M.M.; Palma, A.J. Recent developments in computer vision-based analytical chemistry: A tutorial review. Anal. Chim. Acta 2015, 899, 23–56. [Google Scholar] [CrossRef]
- Meenu, M.; Kurade, C.; Neelapu, B.C.; Kalra, S.; Ramaswamy, H.S.; Yu, Y. A concise review on food quality assessment using digital image processing. Trends Food Sci. Tech. 2021, 118, 106–124. [Google Scholar] [CrossRef]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G. Digital image colorimetry on smartphone for chemical analysis: A review. Measurement 2021, 171, 108829. [Google Scholar] [CrossRef]
- Chun, W.D.; Gao, Z.L.; Bin, H.; Hai, B.Y.; Yong, W.J.; Hong, K.Z. Prediction of congou black tea fermentation quality indices from color features using non-linear regression methods. Sci. Rep. 2018, 8, 10535. [Google Scholar] [CrossRef]
- Alberto, L.; Anelis, Q.; Meily Sánchez, A.; Elias, N.; Rodríguez, B.; José Cremata, C.; Julio, C.; Sánchez, A. Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biologicals 2008, 36, 134–141. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; An, X.P.; Qi, J.W. Study on the Enhancement of Antioxidant Properties of Rice Bran Using Mixed-Bacteria Solid-State Fermentation. Fermentation 2022, 8, 212. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, S.; Wu, T. Inoculum size of co-fermentative culture affects the sensory quality and volatile metabolome of fermented milk over storage. J. Dairy Sci. 2022, 105, 5654–5668. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Naziri, E.; Tsimidou, M.Z. Squalene versus ergosterol formation using Saccharomyces cerevisiae: Combined effect of oxygen supply, inoculum size, and fermentation time on yield and selectivity of the bioprocess. J. Agric. Food Chem. 2009, 57, 6189–6198. [Google Scholar] [CrossRef]
- Zhao, C.; Su, W.; Mu, Y.; Jiang, L.; Mu, Y. Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res. Int. 2020, 138, 109800. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, X.J.; Li, C.Q.; He, L.P.; Wang, X.; Zeng, X.F. Mixed fermentation with Lactobacillus plantarum, Bifidobacteriµm animalis subsp. lactis and Candida utilis improves the fermentation quality of Hong Suan Tang. Food Chem. 2023, 402, 134488. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Chen, C.Y.; Lin, C.P.; Huang, C.L.; Lin, C.H.; Cheng, C.Y.; Chung, Y.C. Tyrosinase inhibitory and antioxidant activities of three Bifidobacterium bifidum-fermented herb extracts. Ind. Crops Prod. 2016, 89, 376–382. [Google Scholar] [CrossRef]
- Gao, Q.; Song, Y.; Liang, Y.; Li, Y.; Chang, Y.; Ma, R.; Cao, X.; Wang, S. Dynamics of physicochemical properties, functional compounds and antioxidant capacity during spontaneous fermentation of Lycium ruthenicum Murr (Qinghai–Tibet Plateau) Natural Vinegar. Foods 2022, 11, 1344. [Google Scholar] [CrossRef] [PubMed]
- Verón, H.E.; Cano, P.G.; Fabersani, E.; Sanz, Y.; Torres, S. Cactus pear (opuntia ficus-indica) juice fermented with autochthonous lactobacillus plantarum s-811. Food Funct. 2019, 10, 1085–1097. [Google Scholar] [CrossRef]
- Adetuyi, F.O.; Ibrahim, T.A. Effect of fermentation time on the phenolic, flavonoid and vitamin C contents and antioxidant activities of okra (Abelmoschus esculentus) seeds. Niger. Food J. 2014, 32, 128–137. [Google Scholar] [CrossRef]
- Shakoor, A.; Zhang, C.P.; Xie, J.C.; Yang, X.L. Maillard reaction chemistry in formation of critical intermediates and flavour compounds and their antioxidant properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef]
- Hu, S.; He, C.; Li, Y.; Yu, Z.; Ni, D. Changes of fungal community and non-volatile metabolites during pile-fermentation of dark green tea. Food Res. Int. 2021, 147, 110472. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Song, Y.; Li, X.; Liu, N.; An, X.; Qi, J. Dynamic Changes and Correlation Analysis of Polysaccharide Content and Color Parameters in Glycyrrhiza Stems and Leaves during Fermentation. Fermentation 2023, 9, 900. https://doi.org/10.3390/fermentation9100900
Du J, Song Y, Li X, Liu N, An X, Qi J. Dynamic Changes and Correlation Analysis of Polysaccharide Content and Color Parameters in Glycyrrhiza Stems and Leaves during Fermentation. Fermentation. 2023; 9(10):900. https://doi.org/10.3390/fermentation9100900
Chicago/Turabian StyleDu, Juan, Yifeng Song, Xia Li, Na Liu, Xiaoping An, and Jingwei Qi. 2023. "Dynamic Changes and Correlation Analysis of Polysaccharide Content and Color Parameters in Glycyrrhiza Stems and Leaves during Fermentation" Fermentation 9, no. 10: 900. https://doi.org/10.3390/fermentation9100900
APA StyleDu, J., Song, Y., Li, X., Liu, N., An, X., & Qi, J. (2023). Dynamic Changes and Correlation Analysis of Polysaccharide Content and Color Parameters in Glycyrrhiza Stems and Leaves during Fermentation. Fermentation, 9(10), 900. https://doi.org/10.3390/fermentation9100900






