Effect of Lactiplantibacillus plantarum X22-2 on Biogenic Amine Formation and Quality of Fermented Lamb Sausage during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sausage Sample Preparation
2.3. Microbiological Index Determination
2.4. Determination of Physical and Chemical Indicators
2.4.1. pH Value
2.4.2. Water Activity (Aw)
2.4.3. Quality Characteristics
2.4.4. Color
2.4.5. Thiobarbituric Acid Values (TBA)
2.4.6. Total Volatile Basic Nitrogen (TVB-N)
2.4.7. Peroxide Value (POV)
2.4.8. Biogenic Amines (BAs) in Fermented Sausages
2.5. Data Analysis
3. Results and Discussion
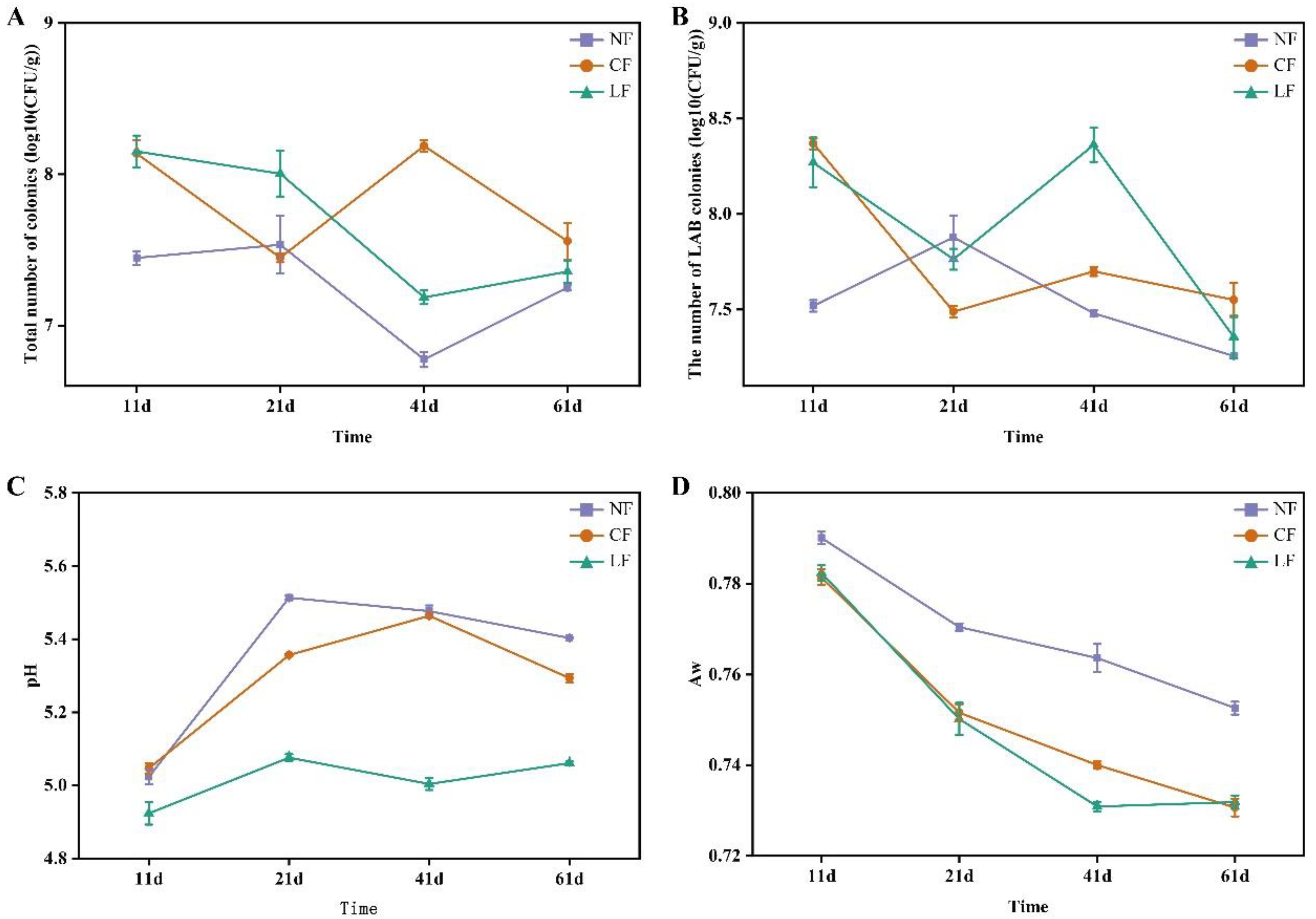
3.1. Analysis of the Total Viable Count, LAB Count, pH, and Water Activity
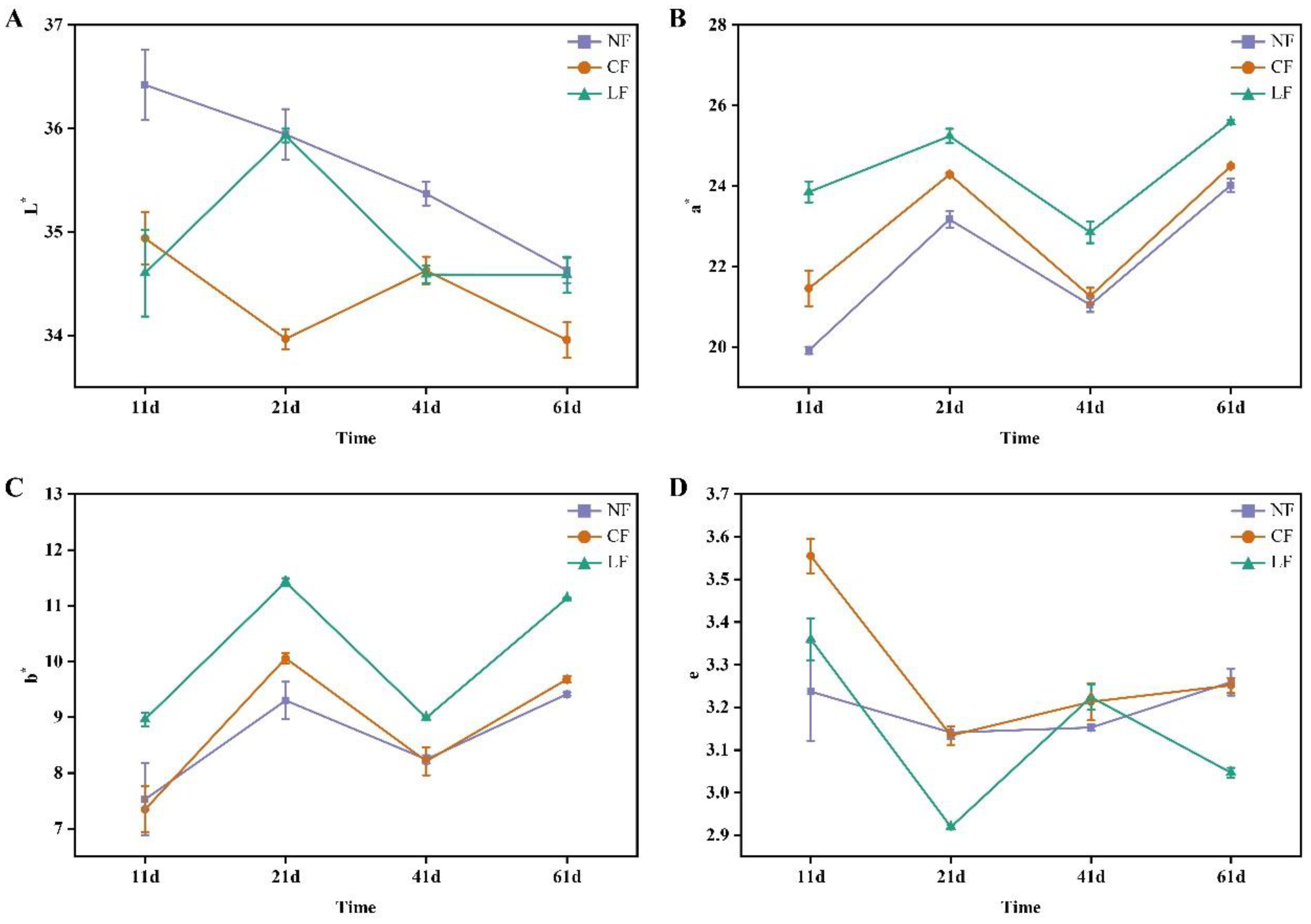
3.2. Analysis of Color
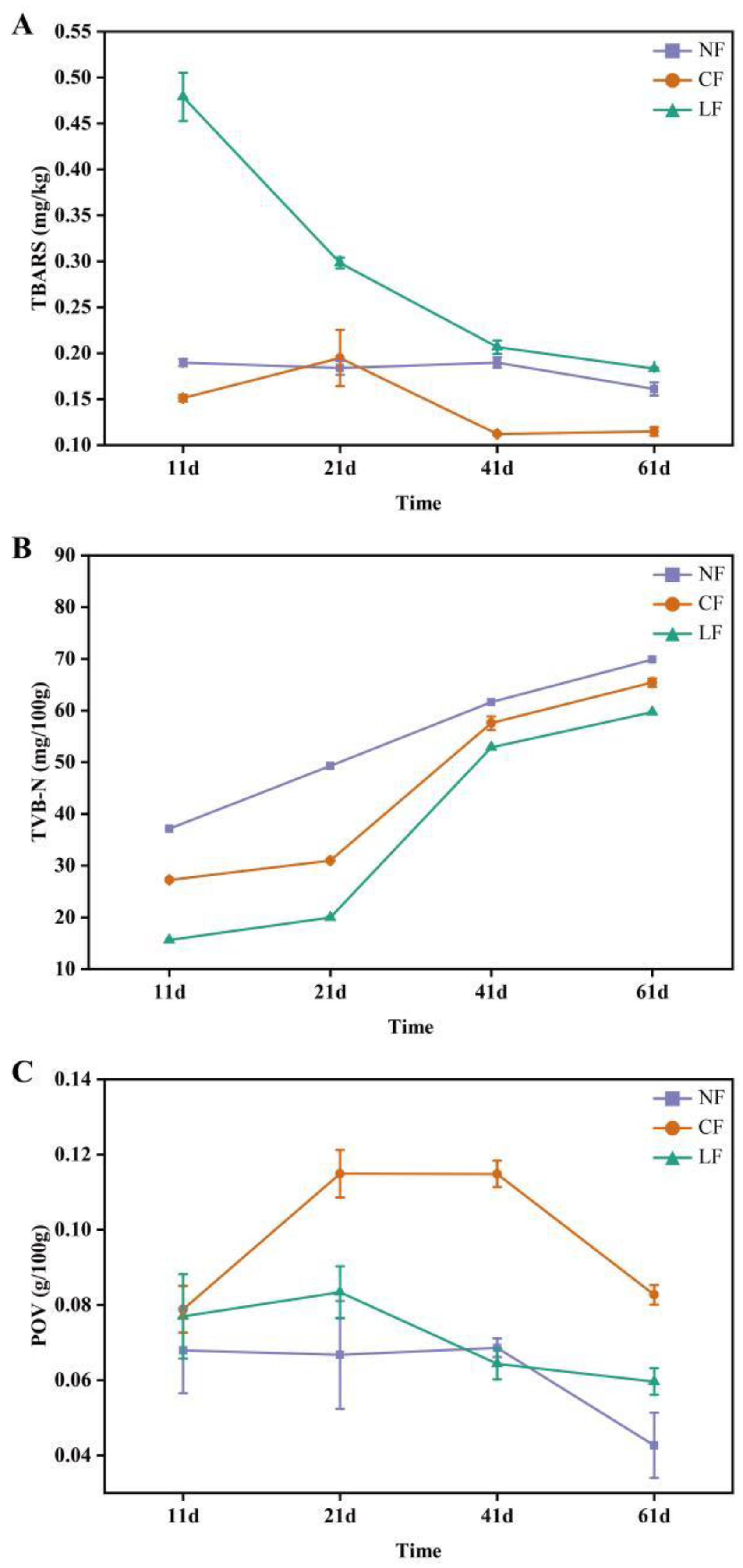
3.3. Analysis of TBARS, TVB-N, and POV
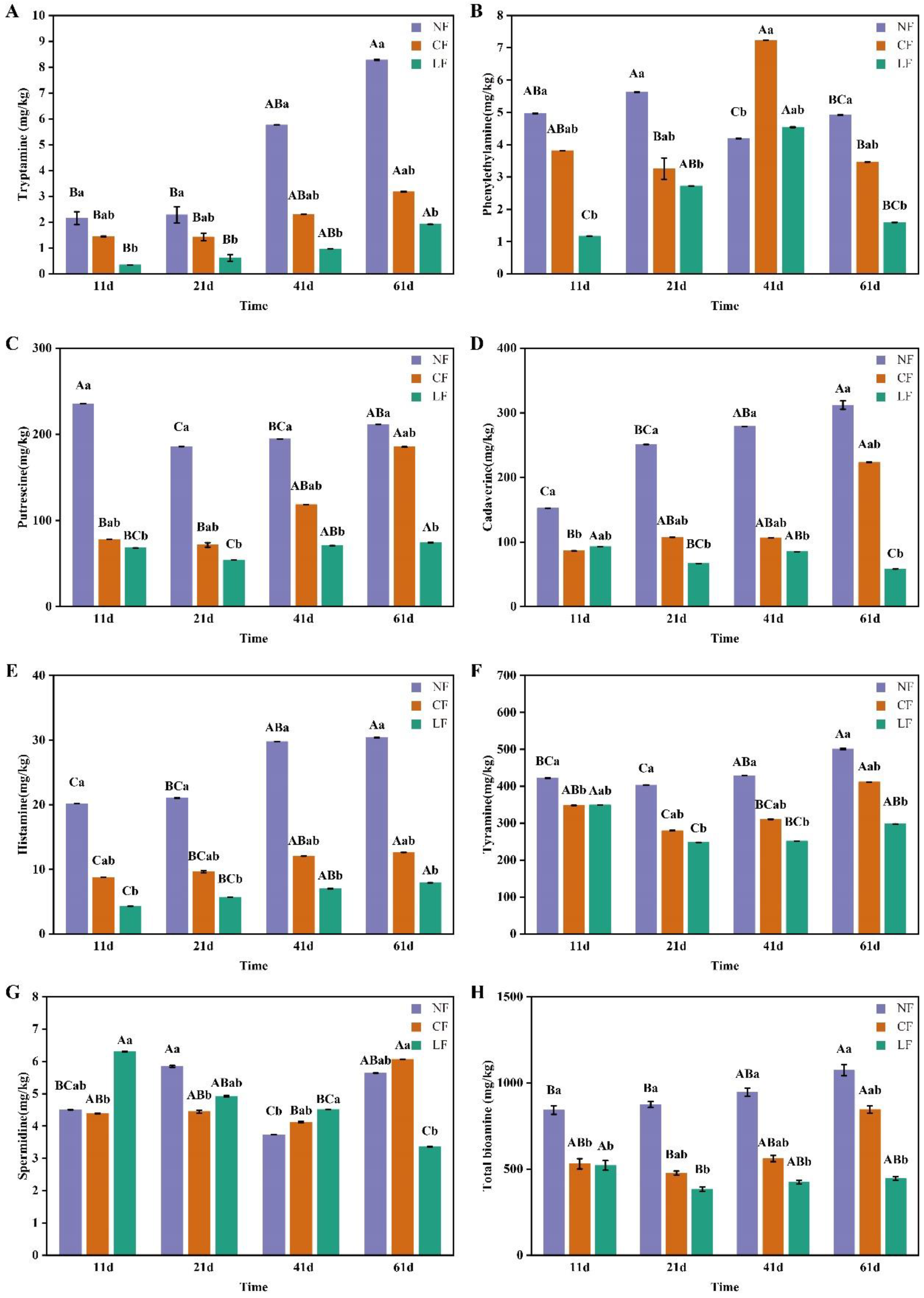
3.4. Analysis of Biogenic Amines (BAs)
3.4.1. Biogenic Amine Standard Curve
3.4.2. Biogenic Amines
3.5. Principle Component Analysis (PCA)
3.6. Texture Profile Analysis (TPA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, P.; Chatli, M.K.; Verma, A.K.; Mehta, N.; Malav, O.P.; Kumar, D.; Sharma, N. Quality, functionality, and shelf life of fermented meat and meat products: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2844–2856. [Google Scholar] [CrossRef]
- Jastrzębska, A.; Kowalska, S.; Szłyk, E. Studies of levels of biogenic amines in meat samples in relation to the content of additives. Food Addit. Contam. A 2016, 33, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Geng, Y.; Shi, D.; Li, R.; Tang, J.; Lu, S. Impact of Coreopsis tinctoria Nutt. Essential oil microcapsules on the formation of biogenic amines and quality of smoked horsemeat sausage during ripening. Meat Sci. 2023, 195, 109020. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Hur, S.J. Effect of in vitro human digestion on biogenic amine (tyramine) formation in various fermented sausages. J. Food Prot. 2018, 81, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Biogenic amines in meat and meat products. Crit. Rev. Food Scince Nutr. 2005, 44, 489–599. [Google Scholar] [CrossRef]
- Pegg, A.E. Toxicity of Polyamines and Their Metabolic Products. Chem. Res. Toxicol. 2013, 26, 1782–1800. [Google Scholar]
- Park, Y.K.; Lee, J.H.; Mah, J.H. Occurrence and reduction of biogenic amines in traditional Asian fermented soybean foods: A review. Food Chem. 2019, 278, 1–9. [Google Scholar]
- Papamanoli, E.; Tzanetakis, N.; Litopoulou-Tzanetaki, E.; Kotzekidou, P. Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci. 2003, 65, 859–867. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Khalifa, I.; Mesak, M.A.; Lorenzo, J.M.; Farag, M.A. A comprehensive review of the role of microorganisms on texture change, flavor and biogenic amines formation in fermented meat with their action mechanisms and safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 3538–3555. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, L.; Yang, Q. Partial replacement of nitrite with a novel probiotic Lactobacillus plantarum on nitrate, color, biogenic amines and gel properties of Chinese fermented sausages. Food Res. Int. 2020, 137, 109351. [Google Scholar] [CrossRef]
- Li, S.; Du, X.; Feng, L.; Mu, G.; Tuo, Y. The microbial community, biogenic amines content of soybean paste, and the degradation of biogenic amines by Lactobacillus plantarum HM24. Food Sci. Nutr. 2021, 9, 6458–6470. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, S. The Importance of Amine-degrading Enzymes on the Biogenic Amine Degradation in Fermented Foods: A review. Process Biochem. 2020, 99, 331–339. [Google Scholar]
- Manca, G.; Ru, A.; Siddi, G.; Murittu, G.; Luigi De Santis, E.P. The effect of seasonality on the biogenic amines, free amino acids, and physico-chemical composition of raw milk Fiore Sardo cheese produced in Sardinia (Italy). Food Control. 2023, 145, 109486. [Google Scholar]
- Önal, A.; Tekkeli, S.E.K.; Önal, C. A review of the liquid chromatographic methods for the determination of biogenic amines in foods. Food Chem. 2013, 138, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Ekici, K.; Omer, A.K. The determination of some biogenic amines in Turkish fermented sausages consumed in Van. Toxicol. Rep. 2018, 5, 639–643. [Google Scholar]
- Sun, L.; Guo, W.; Zhai, Y.; Zhao, L.; Liu, T.; Yang, L.; Jin, Y.; Duan, Y. Screening and the ability of biogenic amine-degrading strains from traditional meat products in Inner Mongolia. LWT 2023, 176, 114533. [Google Scholar]
- Fang, M. The Study on the Quality Evaluation Technology of Mutton Processing Suitability to Mutton Sausage. Master’s Thesis, Beijing Forestry University, Beijing, 16 December 2008. [Google Scholar]
- Mazzucco, E.; Gosetti, F.; Bobba, M.; Marengo, E.; Robotti, E.; Gennaro, M.C. High-Performance Liquid Chromatography−Ultraviolet Detection Method for the Simultaneous Determination of Typical Biogenic Amines and Precursor Amino Acids. Appl. Food Chemistry. J. Agric. Food Chem. 2010, 58, 127–134. [Google Scholar] [CrossRef]
- Tasić, T.; Ikonić, P.; Mandić, A.; Jokanović, M.; Tomović, V.; Savatić, S.; Petrović, L. Biogenic amines content in traditional dry fermented sausage Petrovská klobása as possible indicator of good manufacturing practice. Food Control. 2012, 23, 107–112. [Google Scholar] [CrossRef]
- Eerola, S.; Sagués, A.; Lilleberg, L.; Aalto, H. Biogenic amines in dry sausages during shelf-life storage. Z. Leb. Unters Forsch. 1997, 205, 351–355. [Google Scholar] [CrossRef]
- Hüseyin, B.; Osman, E. Effects of some commercial additives on the quality of sucuk (Turkish dry-fermented sausage). Food Chem. 2007, 4, 1465–1473. [Google Scholar]
- Sundararajan, M.; Neese, F. Distal Histidine Modulates the Unusual O-Binding of Nitrite to Myoglobin: Evidence from the Quantum Chemical Analysis of EPR Parameters. Inorg. Chem. 2015, 54, 7209–7217. [Google Scholar] [CrossRef]
- Santos, C.; Roseiro, L.C.; Gonçalves, H.; Melo, R.S. Incidence of different pork quality categories in a Portuguese slaughterhouse: A survey. Meat Sci. 1994, 38, 279–287. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, S.; Li, Q.; Xue, D.; Jiang, S.; Han, Y.; Li, C. Effect of Thermal Processing on the Conformational and Digestive Properties of Myosin. Foods 2023, 12, 1249. [Google Scholar] [PubMed]
- Zhang, Y.; Li, S.; Zhao, L. Effects of thermal processing and temperature on the quality, protein oxidation, and structural characteristics of yak meat. J. Texture Stud. 2023, 54, 12780. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hua, Y.; Qiu, A. Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res. Int. 2006, 39, 240–249. [Google Scholar] [CrossRef]
- Fernández, J.; Pérez-Álvarez, J.A.; Fernández-López, J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997, 59, 345–353. [Google Scholar]
- Holman, B.W.B.; Bekhit, A.E.-D.A.; Waller, M.; Bailes, K.L.; Kerr, M.J.; Hopkins, D.L. The association between total volatile basic nitrogen (TVB-N) concentration and other biomarkers of quality and spoilage for vacuum packaged beef. Meat Sci. 2021, 179, 108551. [Google Scholar]
- Sohn, J.-H.; Ohshima, T. Control of lipid oxidation and meat color deterioration in skipjack tuna muscle during ice storage. Fish. Sci. 2010, 76, 703–710. [Google Scholar] [CrossRef]
- Latorre-Moratalla, M.L.; Bover-Cid, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Control of Biogenic Amines in Fermented Sausages: Role of Starter Cultures. Front. Microbiol. 2012, 3, 169. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar]
- Shakila, R.J.; Vasundhara, T.S.; Vijaya Rao, D. Inhibitory effect of spices on in vitro histamine production and histidine decarboxylase activity ofMorganella morganii and on the biogenic amine formation in mackerel stored at 30 °C. Z. Für Lebensm. -Unters. Forsch. 1996, 203, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Ladero, V.; Fernandez, M.; Lopez, P.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 3, S95–S100. [Google Scholar]
- Zhang, Q.; Lin, S.; Nie, X. Reduction of biogenic amine accumulation in silver carp sausage by an amine-negative Lactobacillus plantarum. Food Control. 2013, 32, 496–500. [Google Scholar]
- Kim, D.H.; Kim, K.B.W.R.; Cho, J.Y.; Ahn, D.H. Inhibitory Effects of Brown Algae Extracts on Histamine Production in Mackerel Muscle via Inhibition of Growth and Histidine Decarboxylase Activity of Morganella morganii. J. Microbiol. Biotechnol. 2014, 24, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Tosukhowong, A.; Visessanguan, W.; Pumpuang, L.; Tepkasikul, P.; Panya, A.; Valyasevi, R. Biogenic amine formation in Nham, a Thai fermented sausage, and the reduction by commercial starter culture, Lactobacillus plantarum BCC 9546. Food Chem. 2011, 129, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Corrado, M.L.; Knaus, T.; Schwaneberg, U.; Mutti, F.G. High-Yield Synthesis of Enantiopure 1,2-Amino Alcohols from L -Phenylalanine via Linear and Divergent Enzymatic Cascades. Org. Process Res. Dev. 2022, 26, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhang, R.; Shi, L.; Zhang, F.; Chang, J.; Chu, X. Effects of spices on the formation of biogenic amines during the fermentation of dry fermented mutton sausage. Food Chem. 2020, 321, 126723. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Wang, Y.; Huang, Y.; Li, P.; Li, P. Lactobacillus plantarum LPL-1, a bacteriocin producing strain, changed the bacterial community composition and improved the safety of low-salt fermented sausages. LWT 2020, 128, 109385. [Google Scholar] [CrossRef]
- Anlı, R.E.; Vural, N.; Yılmaz, S.; Vural, Ỳ. The determination of biogenic amines in Turkish red wines. J. Food Compos. Anal. 2004, 17, 53–62. [Google Scholar] [CrossRef]
- Gençcelep, H.; Kaban, G.; Kaya, M. Effects of starter cultures and nitrite levels on formation of biogenic amines in sucuk. Meat Sci. 2007, 77, 424–430. [Google Scholar]
- Ercan, S.Ş.; Soysal, Ç.; Bozkurt, H. Biogenic amine contents of fresh and mature kashar cheeses during refrigerated storage. Food Health 2019, 5, 19–29. [Google Scholar] [CrossRef]
- Anderegg, J.; Fischer, M.; Dürig, J.; Die, A.; Lacroix, C.; Meile, L. Detection of biogenic amines and tyramine-producing bacteria in fermented sausages from Switzerland. J. Food Prot. 2020, 9, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Kalhotka, L.; Cwiková, O.; Čírtková, V.; Matoušová, Z.; Přichystalová, J. Changes in counts of microorganisms and biogenic amines production during the manufacture of fermented sausages Poličan. J. Microbiology. Biotechnol. Food Sci. 2012, 2, 2. [Google Scholar]
- Komprda, T. Effect of starter culture, spice mix and storage time and temperature on biogenic amine content of dry fermented sausages. Meat Sci. 2004, 67, 607–616. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Konstantinoudis, G. Protein isoelectric point distribution in the interactomes across the domains of life. Biophys. Chem. 2020, 256, 106269. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Ennouri, K.; Chakchouk-Mtibaa, A.; Karray-Rebai, I.; Hmidi, M.; Bouchaala, K.; Mellouli, L. Relationships Between Textural Modifications, Lipid and Protein Oxidation and Sensory Attributes of Refrigerated Turkey Meat Sausage Treated with Bacteriocin BacTN635. Food Bioprocess Technol. 2017, 10, 1655–1667. [Google Scholar] [CrossRef]

| Items | Groups | 11d | 21d | 41d | 61d |
|---|---|---|---|---|---|
| Hardness/g | NF | 1721.05 ± 302.3917 Cb | 2481.2 ± 96.6158 ABCb | 3088.54 ± 69.9037 ABb | 3432.13 ± 630.0595 Ab |
| CF | 2848.75 ± 423.9597 Ba | 2789.89 ± 372.1003 Bab | 4226.39 ± 203.0002 ABa | 4537.09 ± 65.8715 Aab | |
| LF | 2846.38 ± 221.572 Ba | 4098.07 ± 398.1491 ABa | 4348.78 ± 385.0237 Aba | 7049.03 ± 652.2639 Aa | |
| Elasticity | NF | 0.62 ± 0.0859 ABa | 0.62 ± 0.0694 Aa | 0.51 ± 0.0004 Bb | 0.52 ± 0.029 ABa |
| CF | 0.53 ± 0.0094 ABa | 0.55 ± 0.0098 Aab | 0.55 ± 0.0246 ABa | 0.51 ± 0.0128 Ba | |
| LF | 0.62 ± 0.4058 Aa | 0.52 ± 0.0203 Ab | 0.53 ± 0.0108 Aab | 0.52 ± 0.0331 Aa | |
| Cohesiveness | NF | 0.49 ± 0.0797 Aab | 0.48 ± 0.0091 Aa | 0.47 ± 0.0075 Aab | 0.48 ± 0.0311 Aab |
| CF | 0.52 ± 0.0389 Aa | 0.48 ± 0.010 Aa | 0.49 ± 0.0115 Aa | 0.49 ± 0.0175 Aa | |
| LF | 0.45 ± 0.0275 Bb | 0.49 ± 0.0099 Aa | 0.45 ± 0.0053 Bb | 0.45 ± 0.0303 ABb | |
| Chewiness | NF | 501.84 ± 102.6476 Bb | 732.96 ± 164.9974 ABb | 722.81 ± 62.2123 Ab | 581.97 ± 150.4956 ABb |
| CF | 762.72 ± 14.5363 ABa | 731.49 ± 110.7989 Bb | 1153.67 ± 69.9084 Aa | 1159.01 ± 64.3266 Aab | |
| LF | 782.35 ± 76.8353 Ba | 992.5 ± 71.7348 ABa | 980.8 ± 59.6045 ABab | 2071.3 ± 105.899 Aa | |
| Adhesion | NF | 834.2 ± 222.8949 Bb | 1187.16 ± 57.7083 Ba | 1382.72 ± 176.5026 ABb | 1827.81 ± 78.7324 Ab |
| CF | 1445.32 ± 184.6078 Ba | 1408.52 ± 330.1148 Ba | 1998.9 ± 189.3273 ABa | 2189.24 ± 104.8466 Aab | |
| LF | 1261 ± 207.7469 Bb | 1902.54 ± 524.1228 ABa | 2084.45 ± 219.8095 ABa | 3153.98 ± 202.0457 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Zhang, T.; Zhai, Y.; Sun, L.; Zhai, M.; Kang, L.; Zhao, X.; Wang, B.; Duan, Y.; Jin, Y. Effect of Lactiplantibacillus plantarum X22-2 on Biogenic Amine Formation and Quality of Fermented Lamb Sausage during Storage. Fermentation 2023, 9, 883. https://doi.org/10.3390/fermentation9100883
Liu T, Zhang T, Zhai Y, Sun L, Zhai M, Kang L, Zhao X, Wang B, Duan Y, Jin Y. Effect of Lactiplantibacillus plantarum X22-2 on Biogenic Amine Formation and Quality of Fermented Lamb Sausage during Storage. Fermentation. 2023; 9(10):883. https://doi.org/10.3390/fermentation9100883
Chicago/Turabian StyleLiu, Ting, Taiwu Zhang, Yujia Zhai, Lina Sun, Maoqin Zhai, Letian Kang, Xin Zhao, Bohui Wang, Yan Duan, and Ye Jin. 2023. "Effect of Lactiplantibacillus plantarum X22-2 on Biogenic Amine Formation and Quality of Fermented Lamb Sausage during Storage" Fermentation 9, no. 10: 883. https://doi.org/10.3390/fermentation9100883
APA StyleLiu, T., Zhang, T., Zhai, Y., Sun, L., Zhai, M., Kang, L., Zhao, X., Wang, B., Duan, Y., & Jin, Y. (2023). Effect of Lactiplantibacillus plantarum X22-2 on Biogenic Amine Formation and Quality of Fermented Lamb Sausage during Storage. Fermentation, 9(10), 883. https://doi.org/10.3390/fermentation9100883







