Integrated Production of Xylitol, Ethanol, and Enzymes from Oil Palm Empty Fruit Bunch through Bioprocessing as an Application of the Biorefinery Concept
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation and Source
2.2. Pretreatment of Oil Palm Empty Fruit Bunches
2.3. Enzymatic Hydrolysis of Pretreated Oil Palm Empty Fruit Bunches Using Cellic Htech Enzyme
2.4. Xylitol Fermentation of Oil Palm Empty Fruit Bunches Hydrolysate by M. caribbica
2.5. Ethanol Production Using Semi-Simultaneous Saccharification and Fermentation (Semi-SSF) Method
2.6. Extracellular Enzymes Production by Aspergillus niger Unpad CC C42
2.7. Analysis Method
2.8. Enzyme Activity Test
2.9. Protein Content
2.10. Enzyme Molecular Weight
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Study for Oil Palm Empty Fruit Bunches Pretreatment
3.2. Enzymatic Hydrolysis of Oil Palm Empty Fruit Bunches
3.3. Xylitol Fermentation Using Meyerozyma caribbica
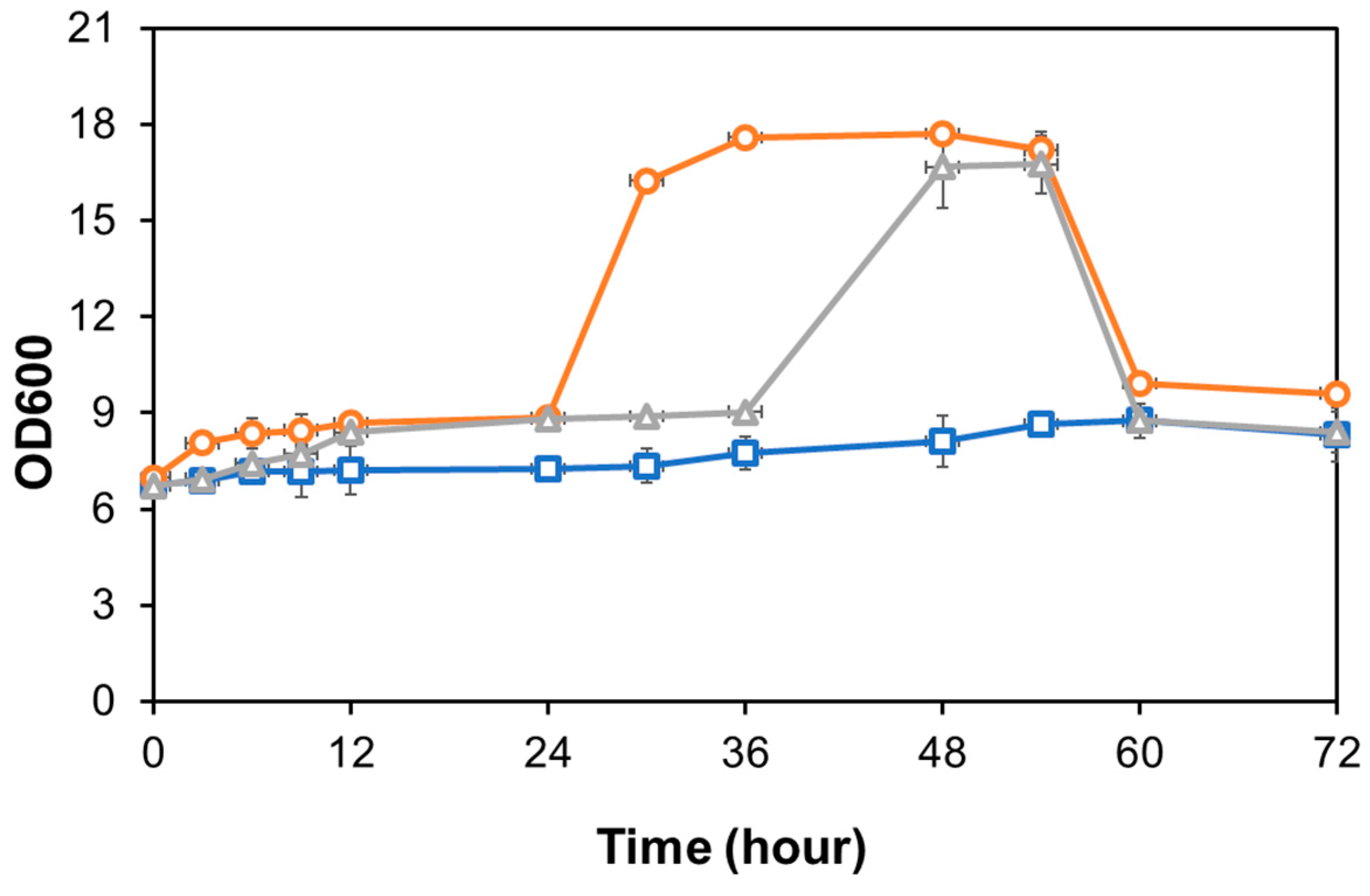
3.4. Ethanol Production Using Semi-SSF Method by Candida sp. Unpad CC Y26
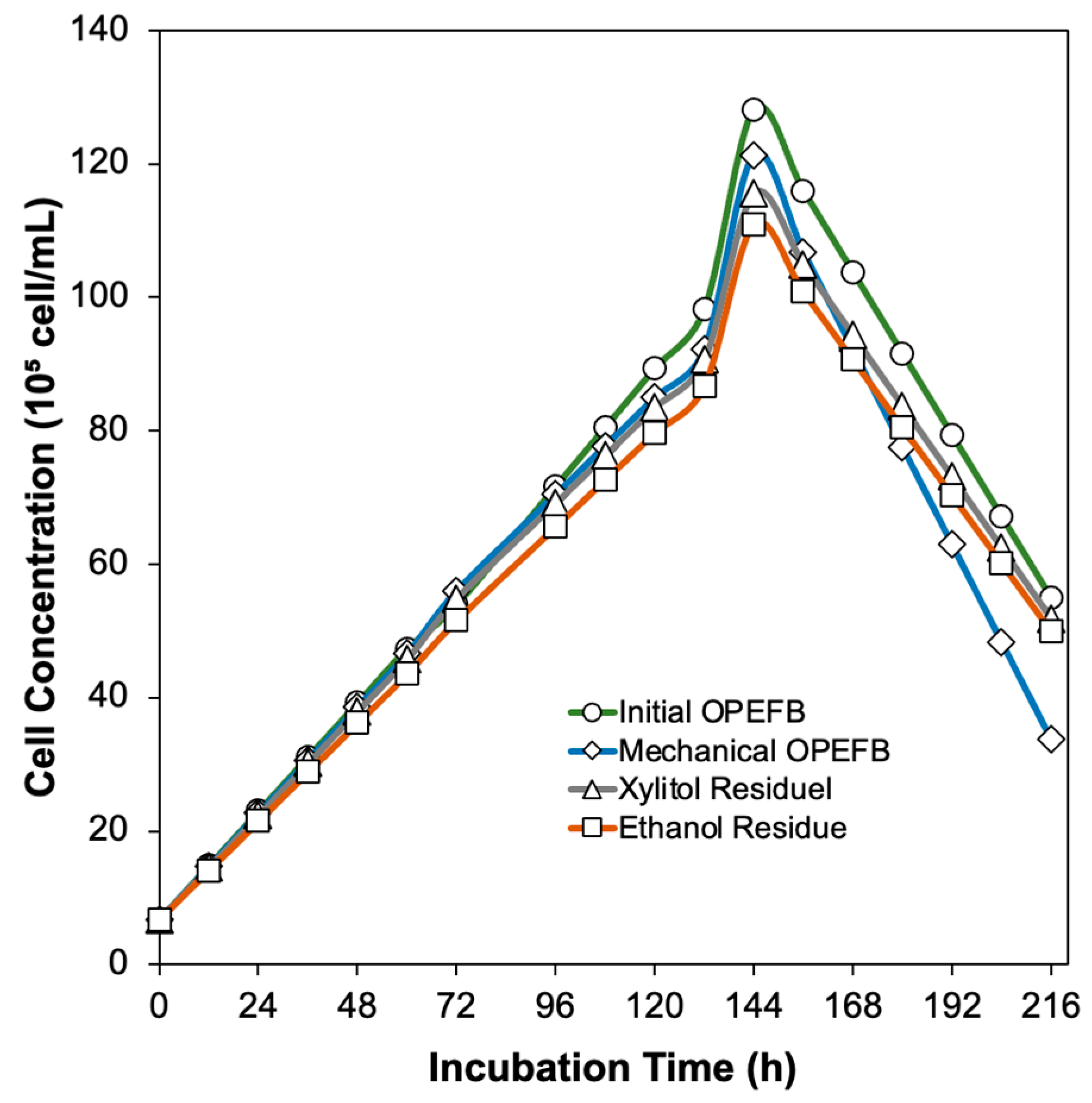
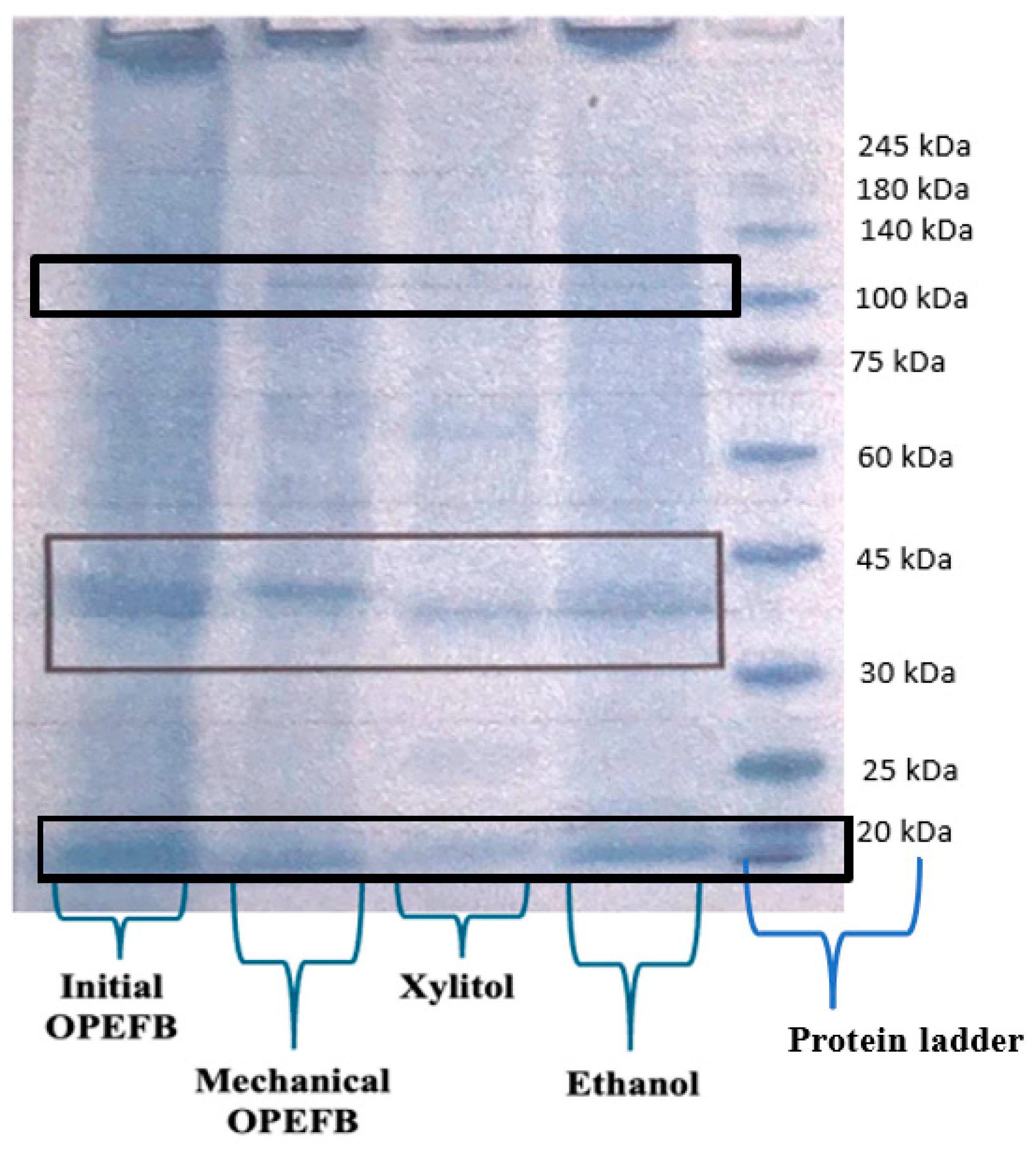
3.5. Extracellular Enzymes Production by Aspergillus niger Unpad CC C42 Using Various Oil Palm Empty Fruit Bunches Residue
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkusma, Y.M.; Hermawan, H.; Hadiyanto, H. Pengembangan Potensi Energi Alternatif Dengan Pemanfaatan Limbah Cair Kelapa Sawit Sebagai Sumber Energi Baru Terbarukan Di Kabupaten Kotawaringin Timur. J. Ilmu Lingkung. 2016, 14, 96–102. [Google Scholar] [CrossRef]
- Yanti, R.N.; Hutasuhut, I.L. Potensi Limbah Padat Perkebunan Kelapa Sawit Di Provinsi Riau. Wahana For. J. Kehutan. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Chang, S.H. An Overview of Empty Fruit Bunch from Oil Palm as Feedstock for Bio-Oil Production. Biomass Bioenergy 2014, 62, 174–181. [Google Scholar] [CrossRef]
- Mardawati, E.; Maharani, N.; Wira, D.W.; Harahap, B.M.; Yuliana, T.; Sukarminah, E. Xylitol Production from Oil Palm Empty Fruit Bunches (OPEFB) Via Simultaneous Enzymatic Hydrolysis and Fermentation Process. J. Ind. Inf. Technol. Agric. 2020, 2, 29–36. [Google Scholar] [CrossRef]
- Muryanto; Sudiyani, Y.; Abimanyu, H. Optimization of NaOH Alkali Pretreatment of Oil Palm Empty Fruit Bunch for Bioethanol. J. Kim. Terap. Indones 2016, 18, 27–35. [Google Scholar] [CrossRef]
- Utarti, E. Siswanto Limbah Berlignoselulosa Sebagai Media Produksi Xilanase Kapang Asal Jerami Padi Sawah Pantai. J. Ilmu Dasar 2018, 19, 117–124. [Google Scholar] [CrossRef]
- Rahman, S.H.A.; Choudhury, J.P.; Ahmad, A.L.; Kamaruddin, A.H. Optimization Studies on Acid Hydrolysis of Oil Palm Empty Fruit Bunch Fiber for Production of Xylose. Bioresour. Technol. 2007, 98, 554–559. [Google Scholar] [CrossRef]
- Sugiwati, S.; Suaidah, S.; Triwahyuni, E.; Muryanto, M.; Andriani, Y.; Abimanyu, H. Hydrolysis of Cellulose from Oil Palm Empty Fruit Bunch Using Aspergillus Niger. E3S Web Conf. 2021, 226, 00042. [Google Scholar] [CrossRef]
- Alencar, B.R.A.; de Freitas, R.A.A.; Guimarães, V.E.P.; Silva, R.K.; Elsztein, C.; da Silva, S.P.; Dutra, E.D.; de Morais Junior, M.A.; de Souza, R.B. Meyerozyma Caribbica Isolated from Vinasse-Irrigated Sugarcane Plantation Soil: A Promising Yeast for Ethanol and Xylitol Production in Biorefineries. J. Fungi 2023, 9, 789. [Google Scholar] [CrossRef]
- Miskah, S.; Istiqomah, U.; Malami, S. Pengaruh Konsentrasi Asam Pada Proses Hidrolisis Dan Waktu Fermentasi Pembuatan Bioetanol Dari Buah Sukun (Artocarpus Altilis). Jurnal Teknik Kimia 2016, 22, 45–57. [Google Scholar]
- Lu, M.; Chen, S.; Pan, S.; Huang, S.; Wen, S.; Li, F.; Liao, W.; Peng, L.; Huang, S. Production of Ethanol from Polysaccharides and Monosaccharides in in Laminaria Japonica with Pichia Angophorae. J. Biobased. Mater. Bioenergy 2014, 8, 415–421. [Google Scholar] [CrossRef]
- Zhai, L.; Manglekar, R.R.; Geng, A. Enzyme Production and Oil Palm Empty Fruit Bunch Bioconversion to Ethanol Using a Hybrid Yeast Strain. Biotechnol. Appl. Biochem. 2020, 67, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, J.; Du, G.; Chen, J.; Takahashi, S.; Liu, S. Developing Aspergillus Niger as a Cell Factory for Food Enzyme Production. Biotechnol. Adv. 2020, 44, 107630. [Google Scholar] [CrossRef] [PubMed]
- Kuka, E.; Cirule, D.; Andersone, I.; Andersons, B.; Fridrihsone, V. Conditions Influencing Mould Growth for Effective Prevention of Wood Deterioration Indoors. Appl. Sci. 2022, 12, 975. [Google Scholar] [CrossRef]
- Dermiyati, D.; Suharjo, R.; Telaumbanua, M.; Yosita, R.; Sari, A.W.; Andayani, A.P. Abundance and Characterization of Microorganisms Isolated from Oil Palm Empty Fruit Bunches Waste under Aerobic, Anaerobic, and Facultative Anaerobic Conditions. Biodiversitas 2020, 21, 4213–4220. [Google Scholar] [CrossRef]
- Akita, H.; Yusoff, M.Z.M.; Fujimoto, S. Preparation of Oil Palm Empty Fruit Bunch Hydrolysate. Fermentation 2021, 7, 81. [Google Scholar] [CrossRef]
- Sari, F.P.; Falah, F.; Anita, S.H.; Ramadhan, K.P.; Laksana, R.P.B.; Fatriasari, W.; Hermiati, E. Pretreatment of Oil Palm Empty Fruit Bunch (OPEFB) at Bench-Scale High Temperature-Pressure Steam Reactor for Enhancement of Enzymatic Saccharification. Int. J. Renew. Energy Dev. 2020, 10, 157–169. [Google Scholar] [CrossRef]
- Mardawati, E.; Mandra Harahap, B.; Ayu Febrianti, E.; Try Hartono, A.; Putri Siahaan, N.; Wulandari, A.; Yudiastuti, S.; Suhartini, S. Kasbawati Integrated and Partial Process of Xylitol and Bioethanol Production from Oil Palm Empty Fruit Bunches. Adv. Food Sci. Sustain. Agric. Agroind. Eng. 2022, 5, 49–67. [Google Scholar]
- Schütz, G.; Haltrich, D.; Atanasova, L. Influence of Spore Morphology on Spectrophotometric Quantification of Trichoderma Inocula. Biotechniques 2020, 68, 279–282. [Google Scholar] [CrossRef]
- Hindrichsen, I.K.; Kreuzer, M.; Madsen, J.; Knudsen, K.E.B. Fiber and Lignin Analysis in Concentrate, Forage, and Feces: Detergent Versus Enzymatic-Chemical Method. J. Dairy Sci. 2006, 89, 2168–2176. [Google Scholar] [CrossRef]
- Jampala, P.; Tadikamalla, S.; Preethi, M.; Ramanujam, S.; Uppuluri, K.B. Concurrent Production of Cellulase and Xylanase from Trichoderma Reesei NCIM 1186: Enhancement of Production by Desirability-Based Multi-Objective Method. 3 Biotech 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Adney, B.; Baker, J. Measurement of Cellulase Activities; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Yuliana, T.; Putri, R.; Hanidah, I.; Mardawati, E.; Tjaturina, H. Purification and Characterization Laccase from Trametes Versicolor (L.) Lloyd in Submerged Fermentation. Pak. J. Biol. Sci. 2022, 25, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Okafor, U.A.; Okochi, V.I.; Onyegeme-Okerenta, B.M.; Nwodo-Chinedu, S. Xylanase Production by Aspergillus Niger ANL 301 Using Agro-Wastes. Afr. J. Biotechnol. 2007, 6, 1710–1714. [Google Scholar]
- Fischer, T.; Elenko, E.; McCaffery, J.M.; DeVries, L.; Farquhar, M.G. Clathrin-coated vesicles bearing GAIP possess GTPase-activating protein activity in vitro. Proc. Nat. Acad. Sci. USA 1999, 96, 6722–6727. [Google Scholar] [CrossRef]
- Laser, M.; Schulman, D.; Allen, S.G.; Lichwa, J.; Antal, M.J.; Lynd, L.R. A Comparison of Liquid Hot Water and Steam Pretreatments of Sugar Cane Bagasse for Bioconversion to Ethanol. Bioresour. Technol. 2002, 81, 33–44. [Google Scholar] [CrossRef]
- Romaní, A.; Garrote, G.; López, F.; Parajó, J.C. Eucalyptus Globulus Wood Fractionation by Autohydrolysis and Organosolv Delignification. Bioresour. Technol. 2011, 102, 5896–5904. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Soontornchaiboon, W.; Chunhachart, O.; Pawongrat, R. Ethanol Production from Pineapple Waste by Co-Culture of Saccharomyces Cerevisiae TISTR 5339 and Candida Shehatae KCCM 11422. Asia-Pac. J. Sci. Technol. 2016, 21, 347–355. [Google Scholar] [CrossRef]
- Kresnowati, M.; Mardawati, E.; Setiadi, T. Production of Xylitol from Oil Palm Empty Friuts Bunch: A Case Study on Bioefinery Concept. Mod. Appl. Sci. 2015, 9, 206. [Google Scholar] [CrossRef]
- Harahap, B.M. Degradation Techniques of Hemicellulose Fraction from Biomass Feedstock for Optimum Xylose Production: A Review. J. Keteknikan Pertan. Trop. Dan Biosist. 2020, 8, 107–124. [Google Scholar] [CrossRef]
- Sugiharto, Y.E.C.; Harimawan, A.; Kresnowati, M.T.A.P.; Purwadi, R.; Mariyana, R.; Andry; Fitriana, H.N.; Hosen, H.F. Enzyme Feeding Strategies for Better Fed-Batch Enzymatic Hydrolysis of Empty Fruit Bunch. Bioresour. Technol. 2016, 207, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Dragosits, M.; Mattanovich, D. Adaptive Laboratory Evolution—Principles and Applications for Biotechnology. Microb. Cell Fact. 2013, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Mardawati, E.; Febrianti, E.A.; Fitriana, H.N.; Yuliana, T.; Putriana, N.A.; Suhartini, S. Kasbawati An Integrated Process for the Xylitol and Ethanol Production from Oil Palm Empty Fruit Bunch (OPEFB) Using Debaryomyces Hansenii and Saccharomyces Cerevisiae. Microorganisms 2022, 10, 2036. [Google Scholar] [CrossRef] [PubMed]
- Nobre, A.; Duarte, L.; Roseiro, J.; Girio, F. A Physiological and Enzymatic Study of Debaryomyces Hansenii Growth on Xylose- and Oxygen-Limited Chemostats. Appl. Microbiol. Biotechnol. 2002, 59, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Mardawati, E.; Wira, D.W.; Kresnowati, M.; Purwadi, R.; Setiadi, T. Microbial Production of Xylitol from Oil Palm Empty Fruit Bunches Hydrolysate: The Effect of Glucose Concentration. J. Jpn. Inst. Energy 2015, 94, 769–774. [Google Scholar] [CrossRef]
- Karunia, Y.A.I.; Silvina, F. Murniarti Pemberian Kombinasi Pupuk AB Mix Dan Pupuk Organik Cair Limbah Rumah Tangga Pada Tanaman Tomat (Lycopersicum Esculentum Mill.) Secara Hidroponik. J. Online Mhs. (JOM) Bid. Pertan. 2019, 6, 1–12. [Google Scholar]
- Mardawati, E.; Putri, S.H.; Fitriana, H.N.; Nurliasari, D.; Rahmah, D.M.; Rosanti; Maulana, I.; Dewantoro, A.I.; Hermiati, E.; Balia, R.L. Application of Biorefinery Concept to the Production of Bromelain, Ethanol, and Xylitol from Pineapple Plant Waste. Fermentation 2023, 9, 816. [Google Scholar] [CrossRef]
- Pant, S.; Ritika; Prakash, A.; Kuila, A. Integrated Production of Ethanol and Xylitol from Brassica Juncea Using Candida Sojae JCM 1644. Bioresour. Technol. 2022, 351, 126903. [Google Scholar] [CrossRef]
- Tesfaw, A.; Assefa, F. Current Trends in Bioethanol Production by Saccharomyces Cerevisiae: Substrate, Inhibitor Reduction, Growth Variables, Coculture, and Immobilization. Int. Sch. Res. Not. 2014, 2014, 532852. [Google Scholar] [CrossRef]
- Alminderej, F.M.; Hamden, Z.; El-Ghoul, Y.; Hammami, B.; Saleh, S.M.; Majdoub, H. Impact of Calcium and Nitrogen Addition on Bioethanol Production by S. Cerevisiae Fermentation from Date By-Products: Physicochemical Characterization and Technical Design. Fermentation 2022, 8, 583. [Google Scholar] [CrossRef]
- Rejeki, D.S.; Aminin, A.L.N.; Suzery, M. Preliminary Study of Hyptis Pectinata (L.) Poit Extract Biotransformation by Aspergillus Niger. IOP Conf. Ser. Mater. Sci. Eng. 2018, 349, 012004. [Google Scholar] [CrossRef]
- Legodi, L.M.; La Grange, D.C.; van Rensburg, E.L.J. Production of the Cellulase Enzyme System by Locally Isolated Trichoderma and Aspergillus Species Cultivated on Banana Pseudostem during Solid-State Fermentation. Fermentation 2023, 9, 412. [Google Scholar] [CrossRef]
- Kiribayeva, A.; Mukanov, B.; Silayev, D.; Akishev, Z.; Ramankulov, Y.; Khassenov, B. Cloning, Expression, and Characterization of a Recombinant Xylanase from Bacillus Sonorensis T6. PLoS ONE 2022, 17, e0265647. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Bhattacharya, S.; Roopa, K.S.; Yashoda, S.S. Microbial Utilization of Agronomic Wastes for Cellulase Production by Aspergillus Niger and Trichoderma Viride Using Solid-State Fermentation. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2011, 5, 18–22. [Google Scholar]
- Vantamuri, A.B.; Kaliwal, B.B. Purification and Characterization of Laccase from Marasmius Species BBKAV79 and Effective Decolorization of Selected Textile Dyes. 3 Biotech 2016, 6, 189. [Google Scholar] [CrossRef]


| % Dry Weight | |||||||
|---|---|---|---|---|---|---|---|
| Lignocellulose Component | Raw OPEFB | OPEFB after Pretreatment | |||||
| A15 | A30 | A60 | B15 | B30 | B60 | ||
| Neutral detergent fiber (NDF) | 79.7 | 77.8 | 75.6 | 72.8 | 76.0 | 75.0 | 71.2 |
| Acid detergent fiber (ADF) | 56.3 | 58.4 | 58.4 | 54.8 | 52.8 | 54.8 | 51.8 |
| Hemicellulose | 23.4 | 19.4 | 17.2 | 18.0 | 23.2 | 20.2 | 19.4 |
| Lignin | 19.1 | 16.6 | 14.6 | 13.6 | 9.5 | 8.6 | 7.6 |
| Cellulose | 36.0 | 41.0 | 43.0 | 40.6 | 42.9 | 45.4 | 43.0 |
| Ash | 1.2 | 0.8 | 0.8 | 0.6 | 0.4 | 0.8 | 1.2 |
| No | Composition | Dry Weight (%) | ||
|---|---|---|---|---|
| Raw Material | Pretreated OPEFB | Xylanase-Hydrolyzed OPEFB | ||
| 1 | NDF | 79.7 | 75.6 | 79.9 |
| 2 | ADF | 56.3 | 52.6 | 72.4 |
| 3 | Hemicellulose | 23.4 | 23.0 | 7.5 |
| 4 | Cellulose | 36.0 | 42.6 | 62 |
| 5 | Lignin | 19.1 | 9.6 | 8.9 |
| Sample | Type of Enzyme | Xylose | Glucose | ||
|---|---|---|---|---|---|
| Concentration (g/L) | Theoretical Xylose in the Feed Stock (%) | Concentration (g/L) | Theoretical Xylose in the Feed Stock (%) | ||
| Hydrolysate I | Cellic Htech | 12.27 | 60.62 | 31.73 | 83.9 |
| Hydrolysate II | Cellic Ctech | 0 | 0 | 35.86 | 66 |
| Parameter | Exponential End (t = 54) | Fermentation End (t = 72) | ||||
|---|---|---|---|---|---|---|
| Medium A | Medium B | Medium C | Medium A | Medium B | Medium C | |
| Initial Xylose Concentration (g/L) | 12.265 | 12.265 | 12.265 | 12.2650 | 12.2650 | 12.2650 |
| Final Xylose Concentration (g/L) | 5.839 | 5.919 | 3.233 | 3.6970 | 4.5250 | 2.9780 |
| Xylose Utilization (%) | 52.393 | 51.741 | 73.640 | 69.8573 | 63.1064 | 75.7195 |
| Initial Glucose Concentration (g/L) | 31.726 | 31.726 | 31.726 | 31.7260 | 31.7260 | 31.7260 |
| Final Glucose Concentration (g/L) | 11.888 | 1.9060 | 19.436 | 7.0770 | 0.259 | 17.9160 |
| Glucose Utilization (%) | 62.529 | 93.992 | 38.738 | 77.6934 | 99.184 | 43.5290 |
| Xylitol Concentration (g/L) | 0.041 | 0.000 | 0.043 | 0.0400 | 0.0000 | 0.0360 |
| Xylitol Yield from Xylose Consumption (Yp/s) (g/g) | 0.005 | 0.000 | 0.005 | 0.0047 | 0.0000 | 0.0039 |
| Xylitol Yield from Cell Count (Yp/x) (g/g) | 0.022 | 0 | 0.0042 | 0.0253 | 0.0000 | 0.0215 |
| Ethanol Concentration (g/L) | 6.326 | 5.397 | 0.504 | 7.206 | 5.1370 | 0.000 |
| Ethanol Yield from Glucose Consumption (Yp/s) (g/g) | 0.3189 | 0.199 | 0.0330 | 0.2923 | 0.163 | 0.0000 |
| Ethanol Yield from Biomass Formation (Yp/x) (g/g) | 3.3383 | 0.5809 | 0.0400 | 4.5608 | 3.2513 | 0.0000 |
| Biomass Concentration (g/L) | 8.645 | 17.20 | 16.75 | 8.325 | 9.61 | 8.395 |
| Biomass Yield from Xylose Consumption (Yx/s xil) (g/g) | 0.295 | 1.6105 | 1.1216 | 0.1844 | 0.3398 | 0.1804 |
| Biomass Yield from Glucose Consumption (Y x/s glu) (g/g) | 0.0955 | 0.3248 | 0.8242 | 0.0641 | 0.0530 | 0.1144 |
| Specific Growth Rate (µ) (1/h) | 0.0049 | 0.0208 | 0.0144 | 0.0049 | 0.0208 | 0.0144 |
| Parameter | t = 48 | t = 96 | ||||
|---|---|---|---|---|---|---|
| Medium 1 | Medium 2 | Medium 3 | Medium 1 | Medium 2 | Medium 3 | |
| Initial Glucose Concentration (g/L) | 36.86 | 36.86 | 36.86 | 36.86 | 36.86 | 36.86 |
| Final Glucose Concentration (g/L) | 2.62 | 2.80 | 3.04 | 0.50 | 1.66 | 1.50 |
| Glucose Utilization (%) | 92.88 | 92.40 | 91.73 | 98.64 | 95.48 | 95.92 |
| Ethanol Concentration (g/L) | 0.03 | 21.35 | 0.04 | 0.02 | 1.32 | 0.04 |
| Ethanol Yield from Glucose Consumption (Yp/s) (g/g) | 0.0008 | 0.6260 | 0.0011 | 0.0006 | 0.0374 | 0.0012 |
| Ethanol Yield from biomass (Yp/x) (g/g) | 0.003 | 2.476 | 0.004 | 1.00 | 1.375 | 0.042 |
| Biomass Yield from Glucose Consumption (Yx/s glu) (g/g) | 0.253 | 0.253 | 0.255 | 0.001 | 0.028 | 0.027 |
| Initial Biomass Concentration (g/L) | 0.11 | 0.14 | 0.13 | 0.11 | 0.14 | 0.13 |
| Final Biomass Concentration (g/L) | 8.76 | 8.76 | 8.76 | 0.13 | 1.11 | 1.08 |
| Specific Growth Rate (µ/hour) | 0.07918 | 0.14828 | 0.07958 | 0.07918 | 0.14828 | 0.07958 |
| No | Composition | % Dry Weight | |||
|---|---|---|---|---|---|
| Raw Material | Mechanically Treated OPEFB | Xylanase-Hydrolyzed OPEFB | Ethanol Fermentation Solid Residue | ||
| 1 | NDF | 79.7 | 73.5 | 79.9 | 66.9 |
| 2 | ADF | 56.3 | 56.9 | 72.4 | 59.7 |
| 3 | Hemicellulose | 23.4 | 16.6 | 7.5 | 7.2 |
| 4 | Cellulose | 36.0 | 37.3 | 62 | 38.7 |
| 5 | Lignin | 19.1 | 7.6 | 8.9 | 19.8 |
| Substrate Type | Incubation Time (Days) | Cellulase Activity (U/mL) | Xylanase Activity (U/mL) | Laccase Activity (U/mL) |
|---|---|---|---|---|
| Raw OPEFB | 3 | 30.66 ± 6.68 | 24.46 ± 7.00 | 0.0000148 ± 0.0000035 |
| 6 | 34.98 ± 1.14 | 38.19 ± 0.26 | 0.0000234 ± 0.0000070 | |
| 9 | 39.49 ± 6.86 | 39.96 ± 0.04 | 0.0000139 ± 0.0000044 | |
| Mechanically treated OPEFB | 3 | 30.39 ± 2.88 | 22.92 ± 7.52 | 0.0000127 ± 0.0000019 |
| 6 | 35.78 ± 16.67 | 34.30 ± 6.56 | 0.0000102 ± 0.0000013 | |
| 9 | 39.51 ± 45.89 | 35.61 ± 6.20 | 0.0000108 ± 0.0000031 | |
| Xylanase-hydrolyzed OPEFB | 3 | 20.98 ± 4.04 | 21.69 ± 9.10 | 0.0000099 ± 0.0000086 |
| 6 | 25.11 ± 3.14 | 29.47 ± 7.65 | 0.0000143 ± 0.0000035 | |
| 9 | 37.60 ± 5.97 | 38.41 ± 1.47 | 0.0000150 ± 0.0000045 | |
| Ethanol fermentation solid residue OPEFB | 3 | 30.93 ± 0.89 | 32.53 ± 0.60 | 0.0000147 ± 0.0000174 |
| 6 | 42.14 ± 5.02 | 36.42 ± 0.83 | 0.0000143 ± 0.0000032 | |
| 9 | 55.16 ± 20.24 | 36.60 ± 0.89 | 0.0000052 ± 0.0000036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardawati, E.; Nawawi, M.I.S.; Caroline, V.; Imanisa, T.W.; Amanda, P.; Mahardika, M.; Masruchin, N.; Fitriana, H.N.; Rachmadona, N.; Lani, M.N. Integrated Production of Xylitol, Ethanol, and Enzymes from Oil Palm Empty Fruit Bunch through Bioprocessing as an Application of the Biorefinery Concept. Fermentation 2023, 9, 882. https://doi.org/10.3390/fermentation9100882
Mardawati E, Nawawi MIS, Caroline V, Imanisa TW, Amanda P, Mahardika M, Masruchin N, Fitriana HN, Rachmadona N, Lani MN. Integrated Production of Xylitol, Ethanol, and Enzymes from Oil Palm Empty Fruit Bunch through Bioprocessing as an Application of the Biorefinery Concept. Fermentation. 2023; 9(10):882. https://doi.org/10.3390/fermentation9100882
Chicago/Turabian StyleMardawati, Efri, Maisyarah Isnaini S. Nawawi, Viola Caroline, Tania Widani Imanisa, Putri Amanda, Melbi Mahardika, Nanang Masruchin, Hana Nur Fitriana, Nova Rachmadona, and Mohd Nizam Lani. 2023. "Integrated Production of Xylitol, Ethanol, and Enzymes from Oil Palm Empty Fruit Bunch through Bioprocessing as an Application of the Biorefinery Concept" Fermentation 9, no. 10: 882. https://doi.org/10.3390/fermentation9100882
APA StyleMardawati, E., Nawawi, M. I. S., Caroline, V., Imanisa, T. W., Amanda, P., Mahardika, M., Masruchin, N., Fitriana, H. N., Rachmadona, N., & Lani, M. N. (2023). Integrated Production of Xylitol, Ethanol, and Enzymes from Oil Palm Empty Fruit Bunch through Bioprocessing as an Application of the Biorefinery Concept. Fermentation, 9(10), 882. https://doi.org/10.3390/fermentation9100882






