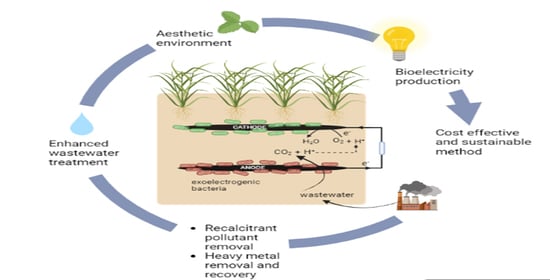
Constructed Wetland Coupled Microbial Fuel Cell: A Clean Technology for Sustainable Treatment of Wastewater and Bioelectricity Generation
Abstract
1. Introduction
2. Critical Pollutants in Industrial Wastewater and Their Ecological Effects
2.1. Heavy Metals
2.2. Radionuclides
2.3. Pharmaceuticals
2.4. Dyes
2.5. Pesticides and Endocrine Disruptors
2.6. Hydrocarbon Compounds
2.7. Microplastics and Other Micropollutants
2.8. Persistent Organic Pollutants (POPs)
3. Conventional Wastewater Technologies
3.1. Primary Treatment
3.2. Secondary Treatment
3.3. Tertiary Treatment
3.4. Limitations of Conventional Waste Water Treatment Method
4. Constructed Wetland
4.1. Components of a Constructed Wetland System
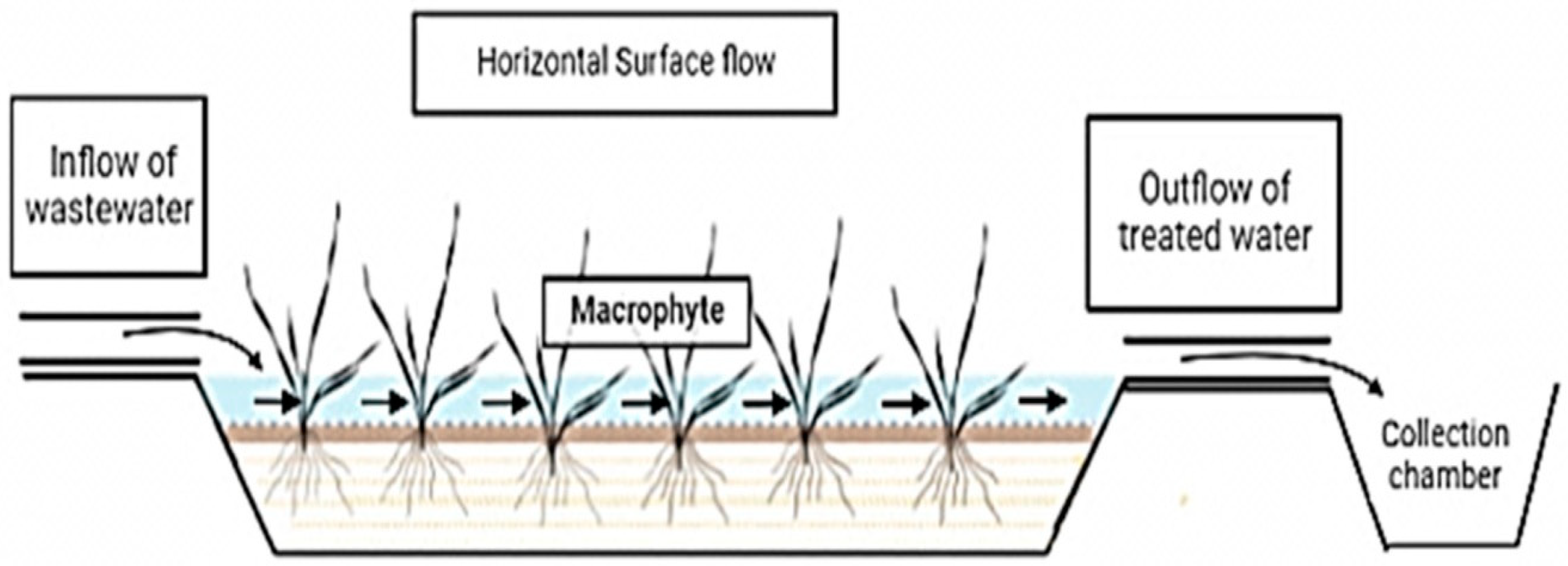
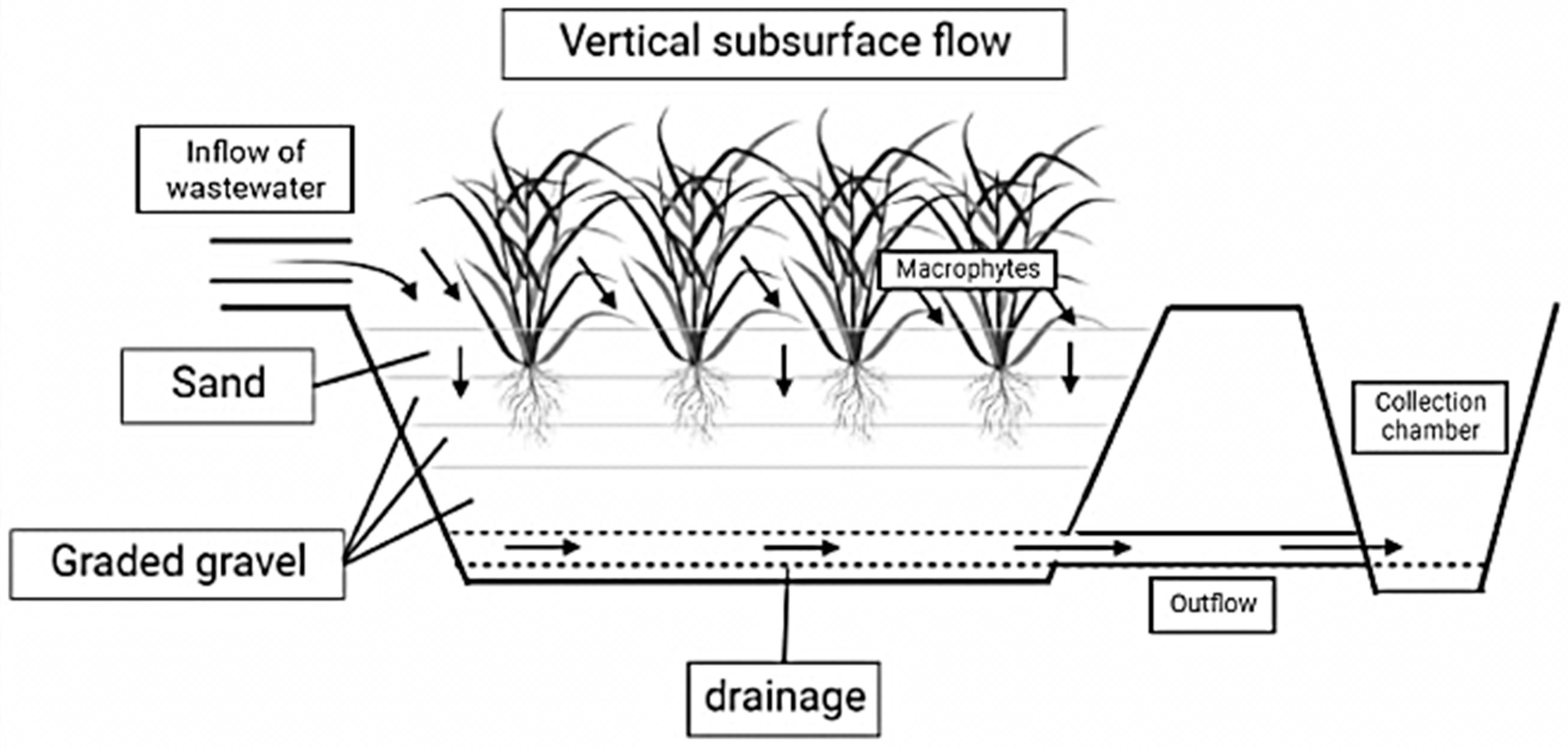
4.2. Constructed Wetland can Be of Three Types According to Flow Regimes
5. Microbial Fuel Cell (Microbial Fuel Cell)
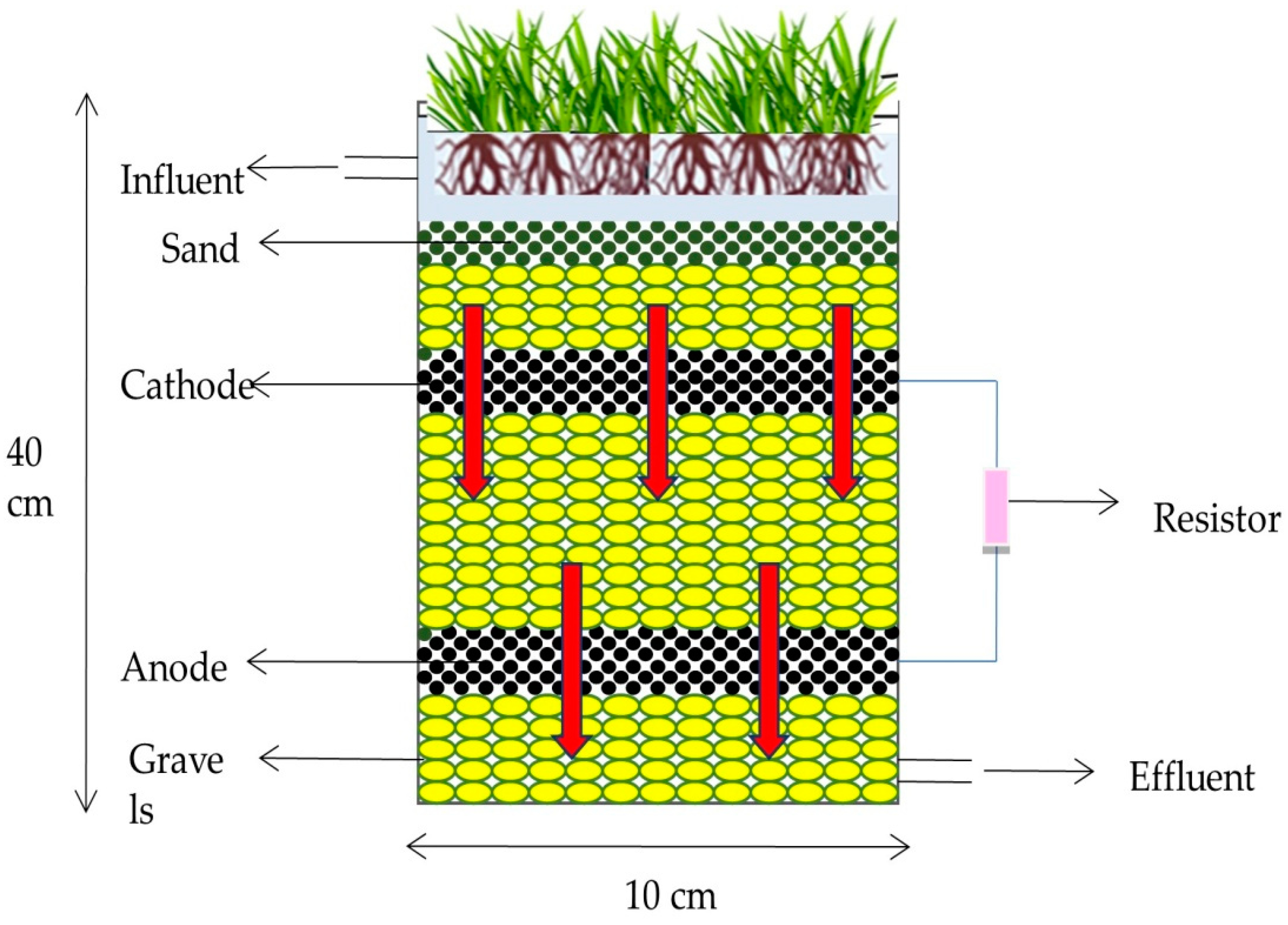
6. Constructed Wetland Integrated Microbial Fuel Cell
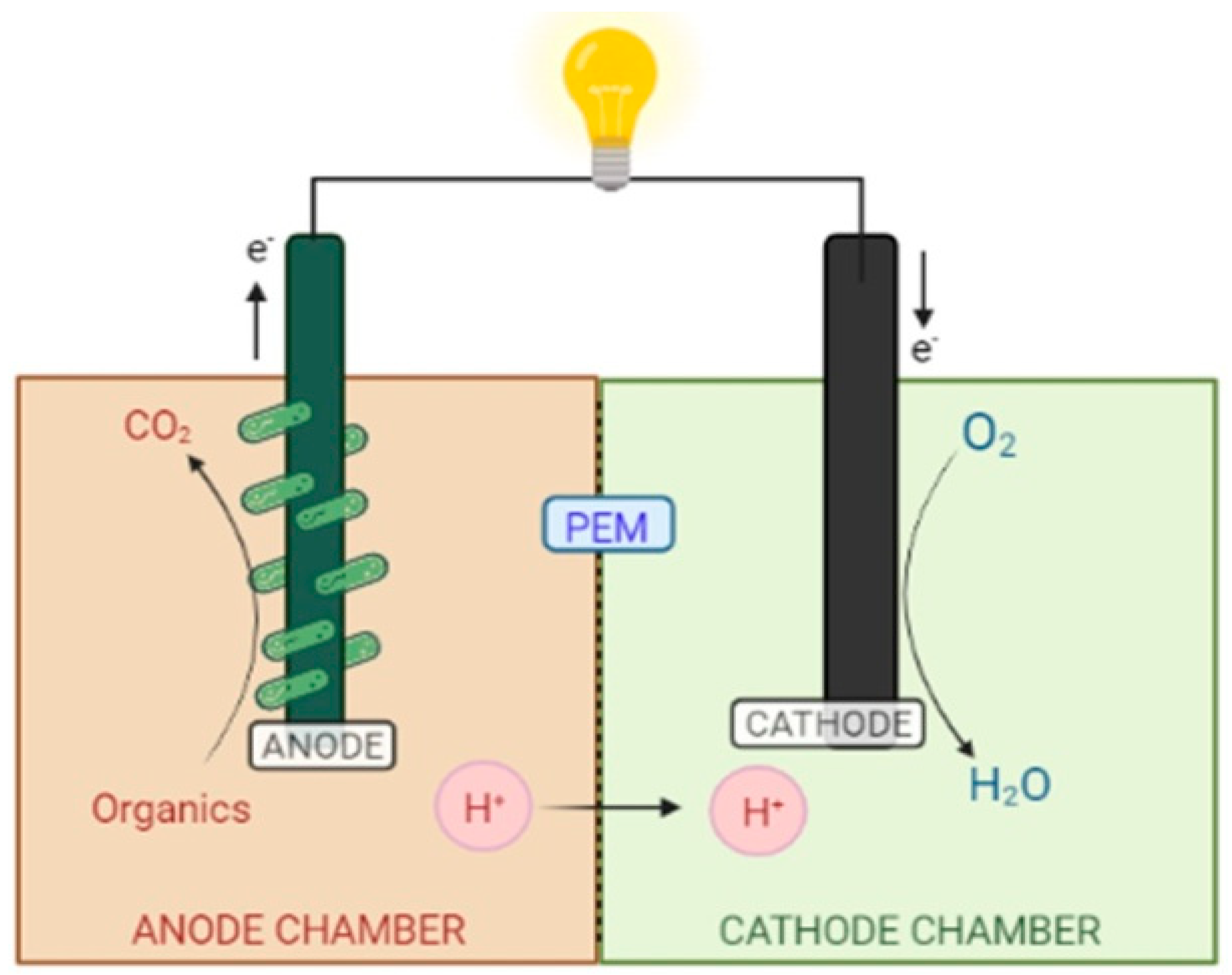
6.1. Configuration of a CW-MFC
6.2. Principle Reactions Occurring in CW-MFC during the Operation
6.3. Factors Affecting CW-MFC Performance
6.3.1. Wetland Macrophyte
6.3.2. Exoelectrogenic Microorganisms
6.3.3. Microorganisms in Cathode
6.3.4. Carbon Mass Balance in CW-MFC System
6.3.5. Type of Wastewater/Substrate
6.3.6. Support Matrix/Media
6.3.7. Electrodes
6.3.8. Hydraulic Retention/Resistance Time (HRT)
7. CW-MFC for Enhanced Wastewater Treatment and Electricity Generation
| Dimension (h × d) (cm) | Vol (L) | Waste Water | Plants | COD Removal Efficiency (%) | TN Removal (%) | TP Removal (%) | Max. OCV (mv) | HRT (h) | Max. Power Density (mW/m2) | Max. Current Density (mAm−2) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 × 14.5 | 3.7 | swine wastwater | Phragmites australis | 76.5% | 49.7 | 65.9 | 495 | - | 9.35 | - | [63] |
| 115 × 47 | 150 | synthetic wastewater | Phragmites australis | 90–95% | - | - | 748 | 76.8 | 0.15 mW/m2 | 1.1 mA/m2 | [27] |
| 30 × 52.5 | 12.4 | synthetic wastwater with azo dye | Ipomoea aquatica | 86 | - | - | - | 72 | 0.302 W/m3 | - | [58] |
| 10.5 × 62 | 5.4 | synthetic wastewater with methylene dye | Canna indica | 74.9 | - | - | - | 96 | 15.7 mW/m2 | 69.75 | [22] |
| 30 × 50 | 35.3 | synthetic wastewater | Ipomoea aquatica | 94.8 | 90.8 | - | 530 | 48 | 12.42 | - | [31] |
| 25 × 45 | 6 | synthetic | Cyperus | 72 | 47 | 86 | 440 | 9 | 30 | 70 | [64] |
| 30 × 9 | 1.9 | synthetic | Carex nigra (Common Sedge) | 99.5 | 90 | - | 80 | 15 | - | 80 | [65] |
| 0.7 × 0.17 | 8.1 | sswine wastewater | Phragmites australis | 93 | 85 | 98 | 280 | 24 | 383 | 856 | [66] |
| 18 × 75 | _ | synthetic | Typha latifolia | 100 | - | - | 421.7 | 24 | 6.12 | - | [67] |
| 20 × 55 | municipal wastewater | Phragmites australis | 82.32 | 82.46 | 95.06 | 265 | 72 | 3714 | - | [55,56] | |
| 30 × 50 | 35.3 | synthetic wastewater | Phragmites australis | 94.9 | - | - | 741 | 48 | 0.2 | - | [68] |
8. Economic Considerations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doherty, L.; Zhao, Y.; Zhao, X.; Hu, Y.; Hao, X.; Xu, L.; Liu, R.A. Review of A Recently Emerged Technology: ConstructedWetland–Microbial Fuel Cells. Water Res. 2015, 85, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Baker, B. Water: Availability and Use MississippiS, tate University Extension Service. [online] Extension. msstate.edu. 2016. Available online: http://extension.msstate.edu/publications/water-availability-and-use (accessed on 21 September 2022).
- Dhawan, V. Water and Agriculture in India. Oav. De. 2022. Available online: https://www.oav.de/fileadmin/user_upload/5_Publikationen/5_Studien/170118_Study_Water_Agriculture_India.pdf (accessed on 10 October 2022).
- Niti Aayog, Govt. of India.Water Index. Social. Niti. Gov. In. 2022. Available online: http://social.niti.gov.in/water-index (accessed on 21 October 2022).
- Sahoo, A.K.; Singh, S.; Nath, A.; Sunani, S.K. Natural Resource Management and Environmental Security, 1st ed.; Integrated Publication: Delhi, India, 2021. [Google Scholar]
- Wang, Y.; Zhao, Y.; Xu, L.; Wang, W.; Doherty, L.; Tang, C.; Ren, B.; Zhao, J. Constructed Wetland Integrated Microbial Fuel Cell System: Looking Back, Moving Forward. Water Sci. Technol. 2017, 76, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kong, F. Electricity Production and The Analysis of The Anode Microbial Community in A Constructed Wetland-Microbial Fuel Cell. RSC Adv. 2019, 9, 21460–21472. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.E.; Bin Halmi, M.I.E.; Bin Abd Samad, M.Y.; Uddin, M.K.; Mahmud, K.; Abd Shukor, M.Y.; Shamsuzzaman, S.M. Design, Operation and Optimization of Constructed Wetland for Removal of Pollutant. Int. J. Environ. Res. Public Health 2020, 17, 8339. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A Review on Removing Pharmaceutical Contaminants from Wastewater by ConstructedWetlands: Design, Performance and Mechanism. Sci. Total Environ. 2014, 468–469, 908–932. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, A.K.; Kushawaha, J.; Chauhan, J.S.; Sharma, S.; Singh, P. Water Pollutants: Sources and Impact on The Environment and Human Health. In Sensors in Water Pollutants Monitoring: Role of Material; Springer Nature: Singapore, 2019; pp. 43–62. [Google Scholar] [CrossRef]
- Busby, C. Aspects of DNA Damage from Internal Radionuclides. In New Research Directionsin DNA Repair; Chen, C., Ed.; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef][Green Version]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds—Natural Sources, Importance and Applications; Chapter 17; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Pereira, P.; Enguita, F.J.; Ferreira, J.; Leitão, A.L. DNA Damage Induced by Hydroquinone Can Be Prevented by Fungal Detoxification. Toxicol. Rep. 2014, 1, 1096–1105. [Google Scholar] [CrossRef]
- Ateia, M.; Zheng, T.; Calace, S.; Tharayil, N.; Pilla, S.; Karanfil, T. Sorption Behavior of Real Microplastics (Mps): Insights for Organic Micro pollutants Adsorption on ALarge Set of Well-Characterized Mps. Sci. Total Environ. 2020, 720. [Google Scholar] [CrossRef]
- Atkinson, S.; Crocker, D.E.; Ortiz, R.M. Endo crine Systems. In Encyclopedia of Marine Mammals; Academic Press: Cambridge, MA, USA, 2018; pp. 318–328. [Google Scholar] [CrossRef]
- Von Sperling, M. Wastewater Characteristics, Treatmentand Disposal. In Water Intelligence Online. 6:9781780402086; IWA Publishing: London, UK, 2015. [Google Scholar] [CrossRef]
- Narayan, M.; Solanki, P.; Srivastava, R.K. Treatment of Sewage (Domestic Wastewater or Municipal Wastewater) and Electricity Production by Integrating Constructed Wetland with Microbial Fuel Cell. In Sewage; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, S.; Sharma, C. Constructed wetlands: An approach for wastewater treatment. Elixir Pollut. 2011, 37, 3666–3672. [Google Scholar]
- Ucar, D.; Zhang, Y.; Angelidaki, I. An Overview of Electron Acceptors in Microbial Fuel Cells. Front. Microbiol. 2017, 8, 643. [Google Scholar] [CrossRef]
- Corbella, C.; Jaume, P.J. Microbial Fuel Cells Implemented in Constructed Wetlands: Fundamentals, Current Research and Future Perspectives. Hdl. Handle. Net. 2015. Available online: http://hdl.handle.net/2117/87020 (accessed on 27 September 2022).
- Yadav, A.K.; Dash, P.; Mohanty, A.; Abbassi, R.; Mishra, B.K. Performance Assessment of InnovativeC onstructed Wetland-Microbial Fuel Cell for Electricity Production and Dye Removal. Ecol. Eng. 2012, 47, 126–131. [Google Scholar] [CrossRef]
- Zhou, F.; Cheng, S.; Wang, H.; Cao, X.; Li, X. Feasibility Study of Simultaneous Azo Dye Decolorization and Bioelectricity Generation by Microbial Fuel Cell-Coupled Constructed Wetland: Substrate Effects. RSC Adv. 2017, 7, 16542–16552. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, Y.; Zhao, X.; Wang, W. Nutrient and Organics Removal from Swine Slurry with Simultaneous Electricity Generation in An Alum Sludge-Based Constructed Wetland Incorporating Microbial Fuel Cell Technology. Chem. Eng. J. 2015, 266, 74–81. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, C.; Wu, Z.-X.; Liang, S.-N.; Liu, M.-H. Activated Carbon Clogging Analysis in An Integration of ConstructedWetland with Microbial Fuel Cell. E3S Web Conf. 2018, 53, 01025. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Liu, R.; Morgan, D. Global Development of Various Emerged Substrates Utilized in Constructed Wetlands. Bioresour. Technol. 2018, 261, 441–452. [Google Scholar] [CrossRef]
- Villaseñor, J.; Capilla, P.; Rodrigo, M.A.; Cañizares, P.; Fernández, F.J. Operation of A Horizontal Subsurface Flow Constructed Wetland—Microbial Fuel Cell Treating Wastewater under Different Organic Loading Rates. Water Res. 2013, 47, 6731–6738. [Google Scholar] [CrossRef]
- Huang, J.; Miwornunyuie, N.; Ewusi-Mensah, D.; Koomson, D.A. Assessing the Factors Influencing the Performance of Constructed Wetland–Microbial Fuel Cell Integration. Water Sci. Technol. 2020, 81, 631–643. [Google Scholar] [CrossRef]
- Sierra, A.A. Integrating Microbial Electro Chemical Systems in Constructed Wetlands, A New Paradigm for Treating Wastewater in Small Communities. Memoria Present Adaparaop Taraltítulode Doctor Porla Universidadde Alcalá. Por. Ph.D. Thesis, Universidad de Alcalá, Alcalá de Henares, Spain, 2017; pp. 100–165. [Google Scholar]
- Wang, J.; Song, X.; Wang, Y.; Bai, J.; Bai, H.; Yan, D.; Cao, Y.; Li, Y.; Yu, Z.; Dong, G. Bio electricity Generation, Contaminat Removal and Bacterial Community Distribution as Affected by Substrate Material Size and Aquatic Macrophyte in Constructed Wetland-Microbial Fuel Cell. Bioresour. Technol. 2017, 245, 372–378. [Google Scholar] [CrossRef]
- Liu, S.; Song, H.; Li, X.; Yang, F. Power Generation Enhancement by Utilizing Plant Photosynthate in Microbial Fuel CellCoupled Constructed Wetland System. Int. J. Photoenergy 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Liu, F.; Sun, L.; Wan, J.; Shen, L.; Yu, Y.; Hu, L.; Zhou, Y. Performance of Different Macrophytes in the Decontamination of and Electricity Generation from Swine Wastewater Via an Integrated Constructed Wetland-Microbial Fuel Cell Process. J. Environ. Sci. 2020, 89, 252–263. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Yan, B.; Xu, Y.; Shutes, B. Treatment of Typical Antibiotics in Constructed Wetlands Integrated with Microbial Fuel Cells: Roles of Plant and Circuit Operation Mode. Chemosphere 2020, 250, 126252. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Pérez, O.; Bahena-Rabadan, K.Y.; Dehesa-Carrasco, U.; Pérez, V.H.G.; Estrada-Arriaga, E.B. Bioelectricity Production Using Shade Macrophytes in Constructed Wetlands-Microbial Fuel Cells. Environ. Technol. 2020, 43, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Yadav, A.K.; Garaniya, V.; Abbassi, R. Constructed Wetland Coupled Microbial Fuel Cell Technology. In Microbial Electrochemical Technology; Venkata Mohan, S., Varjani, S., Panday, A., Eds.; Sustainable Platform for Fuels, Chemicals and Remediation, Biomass, Biofuels and Biochemicals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1021–1036. [Google Scholar] [CrossRef]
- Greenway, M. The Role of Macrophytesin Nutrient Removal Using Constructed Wetlands. Environ. Bioremediation Technol. 2022, 331–351. [Google Scholar] [CrossRef]
- Oodally, A.; Gulam hussein, M.; Randall, D.G. Investigating the Performance of Constructed Wetland Microbial Fuel Cells Using Three Indigenous South African Wetland Plants. J. Water Process Eng. 2019, 32, 100930. [Google Scholar] [CrossRef]
- Guang, L.; Koomson, D.A.; Jingyu, H.; Ewusi-Mensah, D.; Miwornunyuie, N. Performance of Exoelectro genic Bacteria Used in Microbial Desalination Cell Technology. Int. J. Environ. Res. Public Health 2020, 17, 1121. [Google Scholar] [CrossRef]
- Richter, H.; McCarthy, K.; Nevin, K.P.; Johnson, J.P.; Rotello, V.M.; Lovley, D.R. Electricity Generation by Geobacter Sulfurreducens Attached to Gold Electrodes. Langmuir 2008, 24, 4376–4379. [Google Scholar] [CrossRef]
- Min, B.; Cheng, S.; Logan, B.E. Electricity Generation Using Membrane and Salt Bridge Microbial Fuel Cells. Water Res. 2005, 39, 1675–1686. [Google Scholar] [CrossRef]
- Sathiyanarayanan, G.; Chabert, N.; Tulumello, J.; Achouak, W. Building Efficient Bio cathodes with Acidithiobacillus Ferrooxidans for the High Current Generation. J. Power Sources 2021, 514, 230586. [Google Scholar] [CrossRef]
- Gregory, K.B.; Bond, D.R.; Lovley, D.R. Graphite Electrodes as Electron Donors for Anaerobic Respiration. Environ. Microbiol. 2004, 6, 596–604. [Google Scholar] [CrossRef]
- Xafenias, N.; Zhang, Y.; Banks, C.J. Enhanced Performance of Hexavalent Chromium Reducing Cathodes in the Presence Ofshewanella Oneidensismr-1 and Lactate. Environ. Sci. Technol. 2013, 47, 4512–4520. [Google Scholar] [CrossRef]
- Freguia, S.; Tsujimura, S.; Kano, K. Electron Transfer Pathways in Microbial Oxygen Biocathodes. Electrochim. Acta 2010, 55, 813–818. [Google Scholar] [CrossRef]
- Lovley, D.R. Happy together: Microbial communities that hookup to swap electrons. ISMEJ 2017, 11, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Chakraborty, I.; Ghangrekar, M.M. Methanogenesis inhibitors used in bio-electro chemical systems: A review revealing reality to decide future direction and applications. Bioresour. Technol. 2021, 319, 124141. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.-J.; Choi, M.-J.; Lee, J.-W.; Kim, K.-Y.; Kim, I.S. Effect of Different Substrates on the Performance, Bacterial Diversity, and Bacterial Viability in Microbial Fuel Cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, X.; Ning, X.; Yang, Q. Research Progress of Microbial Fuel Cell and Constructed Wetland Coupling System. IOP Conf. Ser. Earth Environ. Sci. 2018, 199, 052014. [Google Scholar] [CrossRef]
- Kalathil, S.; Patil, S.A.; Pant, D. Microbial Fuel Cells: Electrode Materials. Encycl. Interfacial Chem. 2018, 309–318. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, X.; Zhao, Y.; Wang, W. The Effects of Electrode Spacing and Flow Direction on46 the Performance of Microbial Fuel Cell-Constructed Wetland. Ecol. Eng. 2015, 79, 8–14. [Google Scholar] [CrossRef]
- Abdelgadir, A.; Chen, X.; Liu, J.; Xie, X.; Zhang, J.; Zhang, K.; Wang, H.; Liu, N. Characteristics, Process Parameters, and Inner Components of Anaerobic Bioreactors. Biomed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Yang, G.W. Biological Power Generation Constructed Wetland System for Treatment of Domestic Sewage. J. Zhejiang Univ. 2015, 49, 1186–1192. [Google Scholar]
- Fang, Z.; Song, H.-L.; Cang, N.; Li, X.-N. Electricity Production from AzoDye Wastewater Using a Microbial Fuel Cell Coupled Constructed Wetland Operating under Different Operating Conditions. Biosens. Bioelectron. 2015, 68, 135–141. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D.; Zhang, Q.; Liu, T.; Tao, Z. Simultaneous Removal of Heavy Metals and Bioelectricity Generation in Microbial Fuel Cell Coupled with Constructed Wetland: An Optimization Study on Substrate and Plant Types. Environ. Sci. Pollut. Res. 2021, 29, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Cao, F.-Q.; Kong, Q.; Zhou, L.-L.; Yuan, Q.; Zhu, Y.-J.; Wang, Q.; Du, Y.-D.; Wang, Z.-D. Electricity Production and Evolution of Microbial Community in the Constructed Wetland-Microbial Fuel Cell. Chem. Eng. J. 2018, 339, 479–486. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Tang, C.; Doherty, L. Influence of Glass Wool as Separator on Bioelectricity Generation in A ConstructedWetland-Microbial Fuel Cell. J. Environ. Manag. 2018, 207, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Yadav, A.K.; Mishra, B.K. The Effects of Microbial Fuel Cell Integration into Constructed Wetland on the Performance of Constructed Wetland. Bioresour. Technol. 2015, 195, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Song, H.-L.; Cang, N.; Li, X.-N. Performance of Microbial Fuel Cell Coupled Constructed Wetland System for Decolorization of AzoDye and Bioelectricity Generation. Bioresour. Technol. 2013, 144, 165–171. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Yu, L.; Li, W.; Huang, X.; Qin, Q.; Zhu, S. Performance Optimization and Microbial Community Evaluation for Domestic Wastewater Treatment in A Constructed Wetland-Microbial Fuel Cell. Environ. Res. 2022, 212, 113249. [Google Scholar] [CrossRef]
- Mu, C.; Wang, L.; Wang, L. Performance of Lab-Scale Microbial Fuel Cell Coupled with Unplanted Constructed Wetland forHexavalent Chromium Removal and Electricity Production. Environ. Sci. Pollut. Res. 2020, 27, 25140–25148. [Google Scholar] [CrossRef]
- Saz, Ç.; Türe, C.; Türker, O.C.; Yakar, A. Effect of Vegetation Type on Treatment Performance and Bioelectric Production of Constructed Wetland Modules Combined with Microbial Fuel Cell (CW-MFC) Treating Synthetic Wastewater. Environ. Sci. Pollut. Res. 2018, 25, 8777–8792. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Lehl, H.K.; Thung, W.-E.; Nordin, N. Role of Macrophyte and Effect of Supplementary Aeration in Up-Flow Constructed Wetland-Microbial Fuel Cell for Simultaneous Wastewater Treatment and Energy Recovery. Bioresour. Technol. 2017, 224, 265–275. [Google Scholar] [CrossRef]
- Zhao, Y.; Collum, S.; Phelan, M.; Good body, T.; Doherty, L.; Hu, Y. Preliminary Investigation of Constructed Wetland Incorporating Microbial Fuel Cell: Batch and Continuous Flow Trials. Chem. Eng. J. 2013, 229, 364–370. [Google Scholar] [CrossRef]
- Ren, B.; Wang, T.; Zhao, Y. Two-Stage Hybrid Constructed Wetland-Microbial Fuel Cells for Swine Wastewater Treatment and Bio energy Generation. Chemosphere 2021, 268, 128803. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Yadav, A.K.; Garaniya, V.; Lewis, T.; Abbassi, R.; Khan, S.J. Electrode Dependent Anaerobic Ammonium Oxidation in Microbial Fuel Cell Integrated Hybrid Constructed Wetlands: A New Process. Sci. Total Environ. 2020, 698, 134248. [Google Scholar] [CrossRef] [PubMed]
- Doherty, L.; Zhao, Y. Operating A Two-Stage Microbial Fuel Cell–Constructed Wetland for Fuller Waste water Treatment and Moreb Efficient Electricity Generation. Water Sci. Technol. 2015, 72, 421–428. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Lehl, H.K.; Thung, W.-E. Hybrid System Up-Flow Constructed Wetland Integrated with Microbial Fuel Cell for Simultaneous Waste water Treatment and Electricity Generation. Bioresour. Technol. 2015, 186, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, S.; Long, X.; Yang, X.; Li, H.; Xiang, W. Optimization of Bio electricity Generation in Constructed Wetland-Coupled Microbial Fuel Cell Systems. Water 2017, 9, 185. [Google Scholar] [CrossRef]
- Freeman, A.I.; Widdowson, S.; Murphy, C.; Cooper, D.J. Economic assessment of aerated constructed treatment wetland susing whole life costing. Water Sci.Technol. 2019, 80, 75–85. [Google Scholar] [CrossRef]
- Teng, C.J.; Leu, S.Y.; Ko, C.H.; Fan, C.; Sheu, Y.S.; Hu, H.Y. Economic and environmental analysis of using constructed riparian wetlands to support urbanized municipal waste water treatment. Ecol. Eng. 2012, 44, 249–258. [Google Scholar] [CrossRef]
- Alfranca, O.; Garcia, J.; Varela, H. Economic Valuation of a Created Wetland Fed with Treated Wastewater Located in a Peri-Urban Parkin Catalonia, Spain. Water Sci. Technol. 2011, 63, 891–898. [Google Scholar] [CrossRef][Green Version]
- Zhu, C.Y.; Wang, J.F.; Li, Q.S.; Wang, L.L.; Tang, G.H.; Chui, B.S.; Bai, J.H. Integration of CW-MFC and anaerobic granularsludge to explore the intensified ammonification-nitrification-denitrification processes for nitrogen removal. Chemosphere 2021, 278, 130428. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, P.; Patil, S.; Yadav, A.K. A comprehensive review on emerging constructed wetland coupled microbialfuel cell technology: Potential application sand challenges. Bioresour. Technol. 2021, 320, 124376. [Google Scholar] [CrossRef]
- Di Muro, J.L.; Guertin, F.M.; Helling, R.K.; Romar, S. A Financial and Environmental Analysis of Constructed Wetlands for Industrial Wastewater Treatment. J. Ind. Ecol. 2014, 18, 631–640. [Google Scholar] [CrossRef]



| Pollutant | Origin | Impact |
|---|---|---|
| Heavy metals (mercury, chromium, arsenic, lead etc.) | Electronic and electroplating plant Food and beverage processing industry Rubber processing industry | Highly toxic, can accumulate through the food chain. Mercury is highly toxic in the nervous system. Lead affects mental capabilities in children |
| Radionuclides | Naturally from Earth’s crust Nuclear power plants Nuclear weapon testing and manufacturing | Damages DNA Cancer |
| Xenobiotics | Pharmaceutical industries | Emerging antibiotic resistance in pathogens |
| Dyes (azo dye, Sulfur dyes) | Paper, printing, textile, and cosmetic industries | Increases BOD, COD in water Impairs the photosynthetic process Accumulates in the food chain and are recalcitrant Mutagenic and carcinogenic |
| Pesticides and herbicides | Agricultural run-off Mill waste | Allergenicity Affect neuro-endocrine system |
| Microplastics | 3D printing powders Industrial abrasives Tyre manufacturing | Can adsorb micropollutants Cannot be removed by conventional wastewater treatment |
| Persistent organic pollutants (POPs) | Pesticide industries By-products of industrial processes and combustion | Global circulation, accumulate in food web, noxious to living creatures, may act as endocrine disruptors |
| Group | Example |
|---|---|
| Denitrifying bacteria | Pseudomonas, Ochrobactrum |
| Dissimilatory metal-reducing bacteria | Geobacter, Shewanella, Geopsychrobacter, Geothrix |
| Sulfate-reducing bacteria | Desulfuromonas, Desulfolobus |
| Fermentative | Clostridium, Escherichia coli |
| Purple non-sulfur, photosynthetic | Rhodoferax ferrireducens |
| Purple non-sulfur, non-photosynthetic | Rhodopseudomonas palustris DX-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesarwani, S.; Panwar, D.; Mal, J.; Pradhan, N.; Rani, R. Constructed Wetland Coupled Microbial Fuel Cell: A Clean Technology for Sustainable Treatment of Wastewater and Bioelectricity Generation. Fermentation 2023, 9, 6. https://doi.org/10.3390/fermentation9010006
Kesarwani S, Panwar D, Mal J, Pradhan N, Rani R. Constructed Wetland Coupled Microbial Fuel Cell: A Clean Technology for Sustainable Treatment of Wastewater and Bioelectricity Generation. Fermentation. 2023; 9(1):6. https://doi.org/10.3390/fermentation9010006
Chicago/Turabian StyleKesarwani, Shiwangi, Diksha Panwar, Joyabrata Mal, Nirakar Pradhan, and Radha Rani. 2023. "Constructed Wetland Coupled Microbial Fuel Cell: A Clean Technology for Sustainable Treatment of Wastewater and Bioelectricity Generation" Fermentation 9, no. 1: 6. https://doi.org/10.3390/fermentation9010006
APA StyleKesarwani, S., Panwar, D., Mal, J., Pradhan, N., & Rani, R. (2023). Constructed Wetland Coupled Microbial Fuel Cell: A Clean Technology for Sustainable Treatment of Wastewater and Bioelectricity Generation. Fermentation, 9(1), 6. https://doi.org/10.3390/fermentation9010006