Synergistic Effect of Surfactant on Disperser Energy and Liquefaction Potential of Macroalgae (Ulva intestinalis) for Biofuel Production
Abstract
1. Introduction
2. Materials and Methods
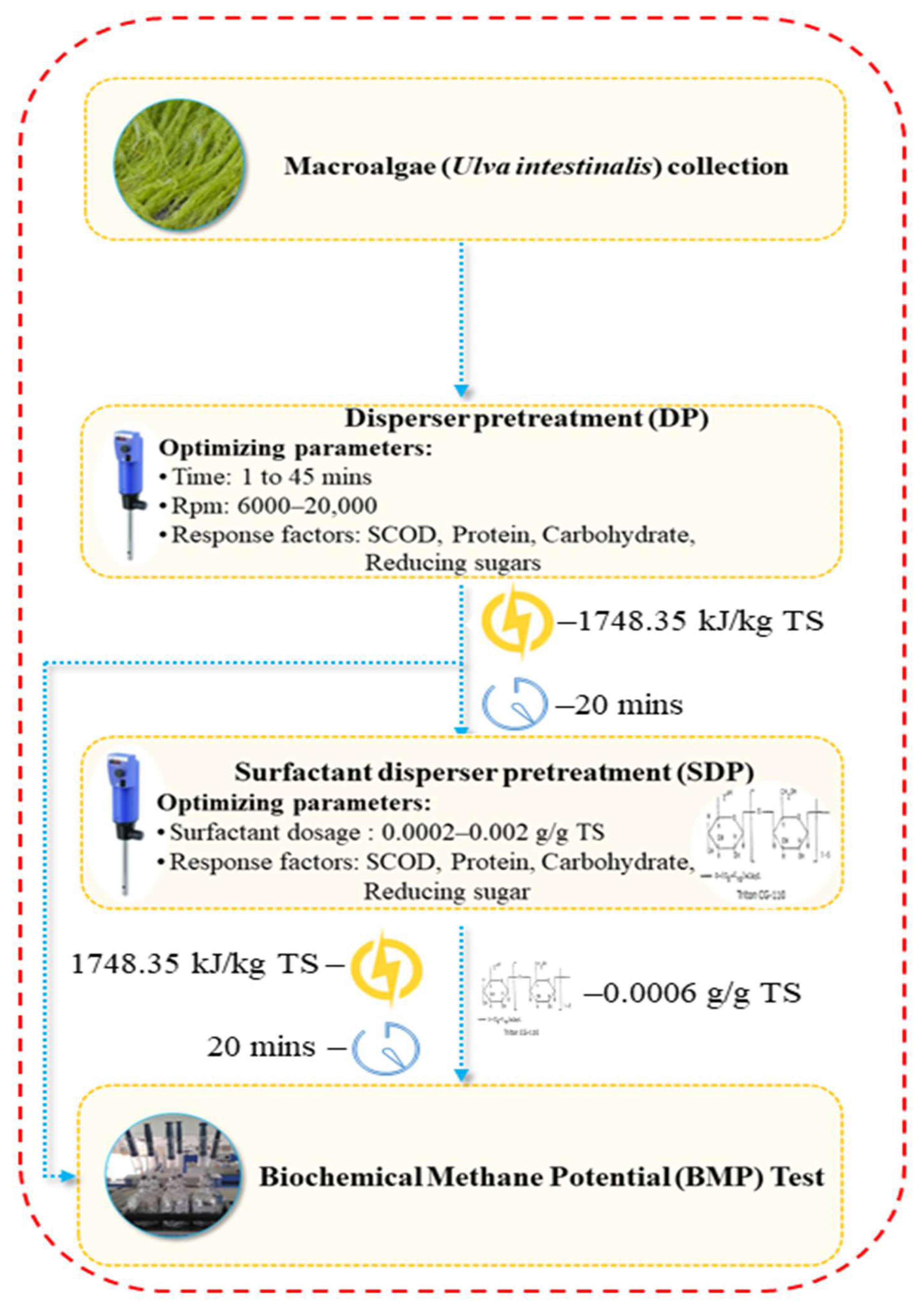
2.1. Collection of Macroalgae
2.2. Disperser Pretreatment (DP)
2.3. Surfactant Disperser Pretreatment (SDP)
2.4. Biochemical Methane Potential (BMP) Test
2.5. Analytical Methods
2.6. Energy and Cost Analysis
2.6.1. Specific Energy Calculation
2.6.2. Output Energy (OE)
2.6.3. Net Energy (EN)
2.6.4. Energy Ratio (ER)
2.6.5. Energy Used for Pumping
2.6.6. Energy Used for AD Process
3. Result and Discussion
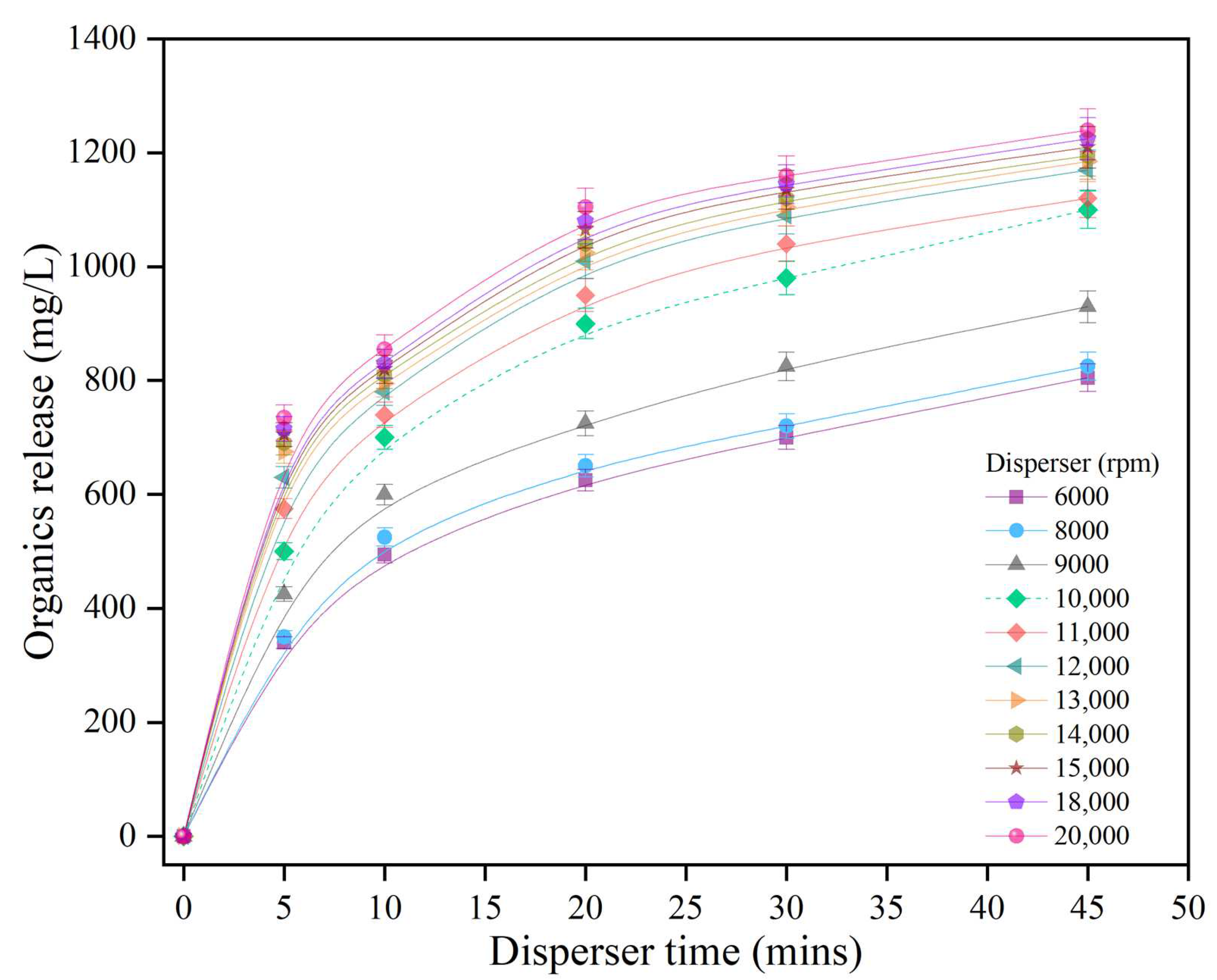
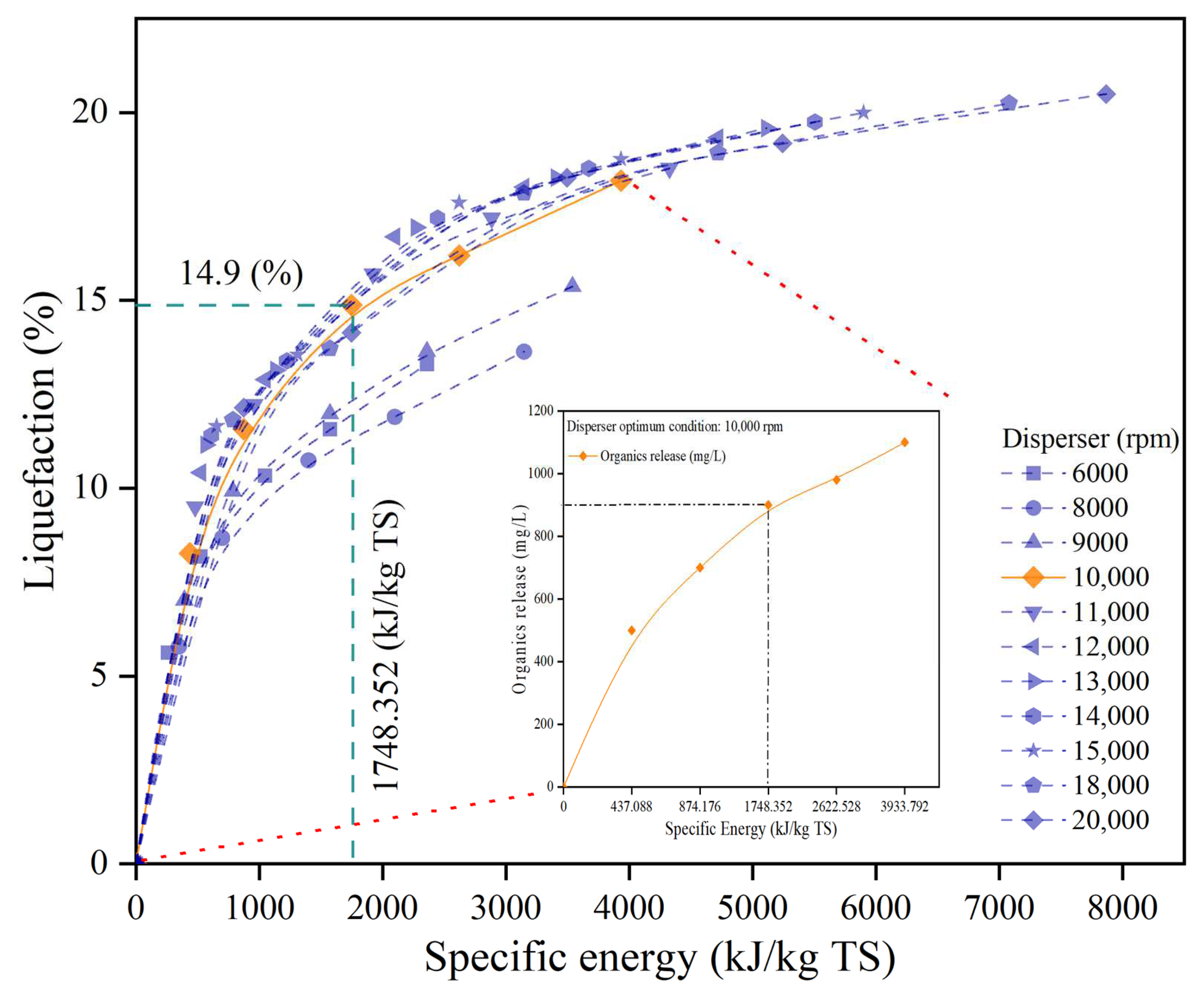
3.1. Disperser Pretreatment (DP)
3.1.1. Effect of Disperser Pretreatment on Organic Release
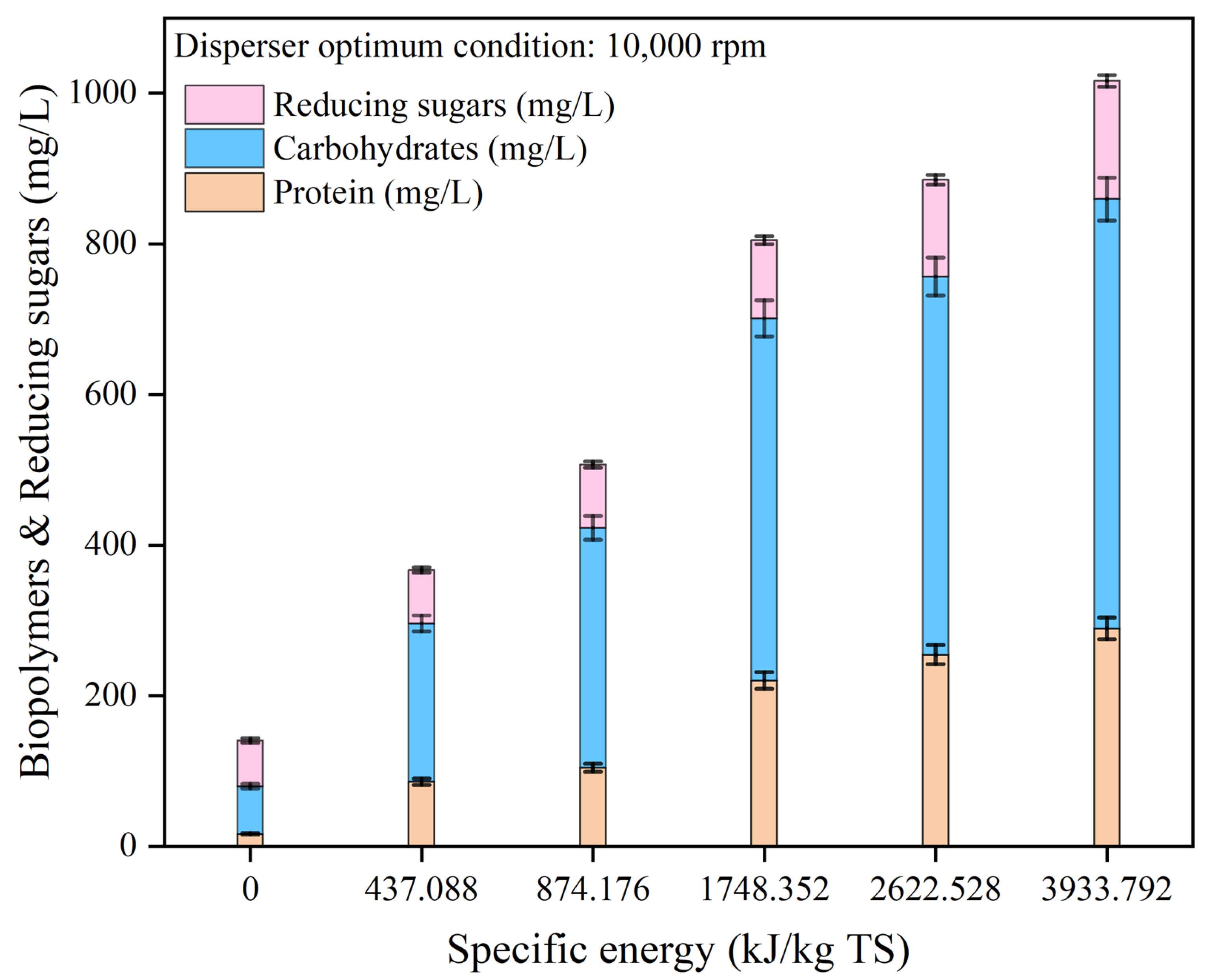
3.1.2. Effect of disperser pretreatment on biopolymer release
3.2. Surfactant-Induced Disperser Pretreatment (SDP)
3.2.1. Effect of SDP on Organic Release
3.2.2. Effect of SDP on Biopolymer Release
3.3. Biomethane Production
3.4. Energy and Economic Assessment of DP and SDP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, G.; Burgess, J.G.; Wu, M.; Wang, S.; Gao, K. Using Macroalgae as Biofuel: Current Opportunities and Challenges. Bot. Mar. 2020, 63, 355–370. [Google Scholar] [CrossRef]
- Godvin Sharmila, V.; Dinesh Kumar, M.; Pugazhendi, A.; Bajhaiya, A.K.; Gugulothu, P.; Rajesh Banu, J. Biofuel Production from Macroalgae: Present Scenario and Future Scope. Bioengineered 2021, 12, 9216–9238. [Google Scholar] [CrossRef]
- Jermsittiparsert, K. Linkage between Energy Consumption, Natural Environment Pollution, and Public Health Dynamics in Asean. Int. J. Econ. Financ. Stud. 2021, 13, 1–21. [Google Scholar]
- Hassaan, M.A.; El Nemr, A.; Elkatory, M.R.; Eleryan, A.; Ragab, S.; El Sikaily, A.; Pantaleo, A. Enhancement of Biogas Production from Macroalgae Ulva Latuca via Ozonation Pretreatment. Energies 2021, 14, 1703. [Google Scholar] [CrossRef]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva Intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef] [PubMed]
- Suutari, M.; Leskinen, E.; Fagerstedt, K.; Kuparinen, J.; Kuuppo, P.; Blomster, J. Macroalgae in Biofuel Production. Phycol. Res. 2015, 63, 1–18. [Google Scholar] [CrossRef]
- Jones, E.S.; Raikova, S.; Ebrahim, S.; Parsons, S.; Allen, M.J.; Chuck, C.J. Saltwater Based Fractionation and Valorisation of Macroalgae. J. Chem. Technol. Biotechnol. 2020, 95, 2098–2109. [Google Scholar] [CrossRef]
- Feng, R.Z.; Zaidi, A.A.; Zhang, K.; Shi, Y. Optimisation of Microwave Pretreatment for Biogas Enhancement through Anaerobic Digestion of Microalgal Biomass. Period. Polytech. Chem. Eng. 2019, 63, 65–72. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A Critical Review on Anaerobic Digestion of Microalgae and Macroalgae and Co-Digestion of Biomass for Enhanced Methane Generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Kumar, M.; Godvin Sharmila, V.; Kumar, G.; Park, J.H.; Al-Qaradawi, S.Y.; Rajesh Banu, J. Surfactant Induced Microwave Disintegration for Enhanced Biohydrogen Production from Macroalgae Biomass: Thermodynamics and Energetics. Bioresour. Technol. 2022, 350, 126904. [Google Scholar] [CrossRef] [PubMed]
- Edward, M.; Edwards, S.; Egwu, U.; Sallis, P. Bio-Methane Potential Test (BMP) Using Inert Gas Sampling Bags with Macroalgae Feedstock. Biomass Bioenergy 2015, 83, 516–524. [Google Scholar] [CrossRef]
- Ushani, U.; Rajesh Banu, J.; Tamilarasan, K.; Kavitha, S.; Tae Yeom, I. Surfactant Coupled Sonic Pretreatment of Waste Activated Sludge for Energetically Positive Biogas Generation. Bioresour. Technol. 2017, 241, 710–719. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2012; p. 1496. [Google Scholar]
- Kavitha, S.; Jayashree, C.; Adish Kumar, S.; Kaliappan, S.; Rajesh Banu, J. Enhancing the Functional and Economical Efficiency of a Novel Combined Thermo Chemical Disperser Disintegration of Waste Activated Sludge for Biogas Production. Bioresour. Technol. 2014, 173, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Tamilarasan, K.; Kavitha, S.; Selvam, A.; Rajesh Banu, J.; Yeom, I.T.; Nguyen, D.D.; Saratale, G.D. Cost-Effective, Low Thermo-Chemo Disperser Pretreatment for Biogas Production Potential of Marine Macroalgae Chaetomorpha Antennina. Energy 2018, 163, 533–545. [Google Scholar] [CrossRef]
- Kumar, M.D.; Tamilarasan, K.; Kaliappan, S.; Banu, J.R.; Rajkumar, M.; Kim, S.H. Surfactant Assisted Disperser Pretreatment on the Liquefaction of Ulva Reticulata and Evaluation of Biodegradability for Energy Efficient Biofuel Production through Nonlinear Regression Modelling. Bioresour. Technol. 2018, 255, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Banu, J.R.; Priya, A.A.; Uan, D.K.; Yeom, I.T. Liquefaction of Food Waste and Its Impacts on Anaerobic Biodegradability, Energy Ratio and Economic Feasibility. Appl. Energy 2017, 208, 228–238. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Jie Ying, A.N.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and Strategies for the Synthesis of Diverse Nanoparticles and Their Applications: A Comprehensive Overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Mitra, A. Macroalgal Proximate Information. Pdf. January 2009. Available online: https://www.researchgate.net/publication/343063967_Macroalgal_proximate_informationh (accessed on 28 November 2022).
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puthiya Veettil, R.; Rabia; Mathew, D.K.; Gondi, R.; Sankarapandian, K.; Kannan, M.; Kumar, G.; Al-Qaradawi, S.Y.; Jeyakumar, R.B. Synergistic Effect of Surfactant on Disperser Energy and Liquefaction Potential of Macroalgae (Ulva intestinalis) for Biofuel Production. Fermentation 2023, 9, 55. https://doi.org/10.3390/fermentation9010055
Puthiya Veettil R, Rabia, Mathew DK, Gondi R, Sankarapandian K, Kannan M, Kumar G, Al-Qaradawi SY, Jeyakumar RB. Synergistic Effect of Surfactant on Disperser Energy and Liquefaction Potential of Macroalgae (Ulva intestinalis) for Biofuel Production. Fermentation. 2023; 9(1):55. https://doi.org/10.3390/fermentation9010055
Chicago/Turabian StylePuthiya Veettil, Rinsha, Rabia, Dinesh Kumar Mathew, Rashmi Gondi, Kavitha Sankarapandian, Meganathan Kannan, Gopalakrishnan Kumar, Siham Y. Al-Qaradawi, and Rajesh Banu Jeyakumar. 2023. "Synergistic Effect of Surfactant on Disperser Energy and Liquefaction Potential of Macroalgae (Ulva intestinalis) for Biofuel Production" Fermentation 9, no. 1: 55. https://doi.org/10.3390/fermentation9010055
APA StylePuthiya Veettil, R., Rabia, Mathew, D. K., Gondi, R., Sankarapandian, K., Kannan, M., Kumar, G., Al-Qaradawi, S. Y., & Jeyakumar, R. B. (2023). Synergistic Effect of Surfactant on Disperser Energy and Liquefaction Potential of Macroalgae (Ulva intestinalis) for Biofuel Production. Fermentation, 9(1), 55. https://doi.org/10.3390/fermentation9010055








