Abstract
Probiotics have extensive use in daily life, due to the function of the changing intestinal metabolism and material conversion processes, wherein they remodel the intestinal microbiota, regulate the intestinal function and affect the organism’s health. Limosilactobacillus reuteri (L. reuteri), originally discovered in breast milk and currently reported to be present within the gut of almost all vertebrates and mammals, is an intestinal probiotic with prebiotic efficacy. Most L. reuteri have good intestinal colonization and bacteriocin secretion abilities, which can increase the expression of the mucin (mucoprotein) genes 2 MUC2 and MUC13, which in turn promote the development and maturation of intestinal organoids, and augment mucin secretion. In enteritis patients, L. reuteri downregulates α Tumor necrosis factor-α, (TNF-α), Interleukin-6 (IL-6), IL-8, and IL-12 expression to attenuate inflammation. It also induces the host’s production of immunoglobulin A (IGA), which manipulates the intestinal microbial community, inhibiting the growth of pathogens. L. reuteri has been widely used in daily life. with in-depth studies having been conducted on the prebiotic effects of L. reuteri. However, the complexity of its application in a clinical setting is still unclear because the pathogenesis of various diseases still requires a large amount of data and theoretical support.
1. Source and Diversity of L. Reuteri
L. reuteri is a Gram-positive bacterium of heterotypic lactic-acid fermentation and is a facultative anaerobic organism. The morphology shows a slightly irregular campylobacter with a rounded end. It was first isolated in breast milk in 1962. Its suitable culture environment is 35~40 °C, with a pH of between 6.7 and 7.0. Its metabolites include lactic acid, carbon dioxide, formic acid, ethanol, acetic acid, higher alcohols, etc. [1]. It has good biocompatibility with lactic acid bacteria in the intestines of most vertebrates and mammals and is non-toxic to human beings, without causing disease. It has been proven to be one of the resident flora of the human gastrointestinal tract [1]; thus, it has a high theoretical research and production application value.
Currently, L. reuteri is commonly used for screening and amplification cultures of lactic acid bacteria, such as MRS and M17, and some researchers have chosen LRIM medium with cottonseed sugar as the carbon source to screen and culture it from feces [2].
L. reuteri was first found in breast milk. It is often distributed in the intestines and feces of animals, and it is found in some fermented products. L. reuteri from different sources has diverse physiological characteristics, with strong adhesive capacity. Most L. reuteri can colonize intestinal mucosa to form site competition, effectively resist harmful bacteria colonization, regulate intestinal flora, and avoid intestinal diseases. Its characteristics are summarized in Table 1.

Table 1.
Distribution, sources and characteristics of L. reuteri.
2. Physiological Role of L. Reuteri
Probiotics can regulate intestinal microbial communities and inhibit the growth of harmful microorganisms by inducing the host to produce bacteriocins and immunoglobulins [30]. Probiotics can also fortify the intestinal barrier through maintenance of the tight junctions and induction of mucin production, and they can mediate immune regulation, which may occur through the secretion of cytokines mediated by signaling pathways, such as nuclear factor kappa-B (NF-κB), which affects the proliferation and differentiation of immune cells or epithelial cells. Many studies have proven that L. reuteri has an important regulatory effect on animal growth, intestinal microflora composition and metabolism, and intestinal immune function [1,31].
2.1. Improvement of Intestinal Physiological Function
2.1.1. Modulation of Gut Flora
Probiotics are able to influence the diversity, composition, and metabolic function of the gut microbiota. Proper supplementation of L. reuteri can promote the gut microbiota to enhance inosine production, which can reduce Th1/Th2 cells and their related cytokines under the action of adenosine A2A receptor, and the L. reuteri intestinal microbiota inosine adenosine A2A receptor axis can be used as a potential treatment for Treg cell-deficient diseases. L. reuteri C10-2-1 has been shown to regulate the diversity of intestinal microbiota in rat ileum [32], and the addition of L. reuteri DMS17938 to the diet of infants improves their intestinal health by reducing pathogen colonization, the expression levels of enterohemorrhagic E. coli virulence genes and deleterious bacteriotoxic effects [33].
2.1.2. Improvement of Intestinal Structure
L. reuteri can promote the development of intestinal villi, increase the expression of intestinal defense peptides and tight junction proteins, and increase the integrity of the intestinal barrier. Other studies haves compared and analyzed the effect of L. reuteri on the miRNA expression profile of piglet jejunum, and they found that it significantly up-regulated jejunal miR-423-5p. L. reuteri can affect the intestinal immune function of piglets by inhibiting the synthesis of the immunoglobulin λ light chain C region [34], and L. reuteri I5007 can increase the height of the ileum villi and reduce the depth of duodenal crypts in piglets, significantly improving the intestinal development of newborn piglets, promoting the expression of intestinal epithelial cell proteins, and enhancing the mechanical barrier function of a piglet’s intestinal mucosa [35,36].
2.2. Inhibition of the Growth and Colonization of Intestinal Pathogenic Bacteria
2.2.1. Production of Antimicrobial Metabolites
L. reuteri is a heterotype lactic-acid fermentation that can produce lactic acid, acetic acid, ethanol, short-chain fatty acid, and other metabolites. At the same time, some L. reuteri also produce bacteriocin Reuterin or Reutericyclin, Reutericin 6, and Ap48-MapA to effectively inhibit the growth of harmful microorganisms.
Metabolites
L. reuteri metabolites such as lactic acid can inhibit the growth of harmful microorganisms by changing the permeability of the pathogenic bacteria cell membrane, chelating metal ions and reducing the pH of the growth environment [37].
Secretion of reuterin
Reuterin’s main components are monomers, hydrates, and the cyclodimers of 3-Hydroxypropionaldehyde (3-HPA), which are associated with glycerol metabolism. The antibacterial activity of reuterin, with its stronger stability, is not destroyed by nuclease, protease, and lipase [38,39]. It also has broad-spectrum antimicrobial properties and can inhibit the growth of some Gram-positive bacteria, Gram-negative bacteria, Saccharomyces, Aspergillus, and Fusarium fungi. However, its mechanism of action is tentatively unclear, and the current explanation is that reuterin can inhibit harmful microbial RNase activity [40,41]. It interacts with thiol genes in cells [42] to inhibit the growth and reproduction of harmful bacteria.
Secretion of Reutericyclin
Reutericyclin is a tetrachloric acid derivative, and its main component, 1,3-diacylpyrrolidine-2,4-dione compounds, can be produced by some L. reuteri strains. It is a class of proton carrier with broad-spectrum antimicrobial properties, but it is ineffective against Gram-negative bacteria, acting through the protons and into the cytoplasm and acidic cytoplasmic-effect transmembrane pH gradient dissipation and the inhibition of harmful microbial growth [42,43]. At present, it is mostly used in the preservation of food, as it can inhibit protein hydrolysis in dough and the thermal inactivation caused by baking, and it inhibits the growth of streptomyces [6].
Secretion of Reutericin 6
Reutericin 6 is a Class II bacteriocin, which is derived from L. reuteri LA6 and screened from infant feces. Reutericin 6 has strong hydrophobicity, good thermal stability, and has bactericidal and dissolving effects on sensitive bacteria [44,45]. It can destroy the integrity of the cell membranes of harmful microorganisms through depolarizing the membranes and exudating cell fluid, leading to cell death and achieving antibacterial effects [46,47].
Production of antibacterial peptide Ap48 MapA
Peptide Ap48 MapA is composed of 48 amino acids. It is an antimicrobial peptide with a PI of 9.63, and it has a good antibacterial effect [48].
2.2.2. Site Competition with Pathogenic Bacteria through Colonization
Probiotics are a type of living bacteria that can promote the ecological balance of intestinal flora. By adhering to the host’s intestinal tract, they form a biological barrier with normal flora, releasing active antibacterial substances or blocking the combination of pathogenic bacteria with cell receptors through space, which affects the colonization, occupation, and reproduction of pathogenic bacteria. Probiotics produce biological enzymes, active peptides, and metabolites in their host, which can prevent the infection and colonization of pathogenic bacteria in the body, playing the role of a chemical barrier and achieving the purpose of preventing gastrointestinal mucositis (see Figure 1)
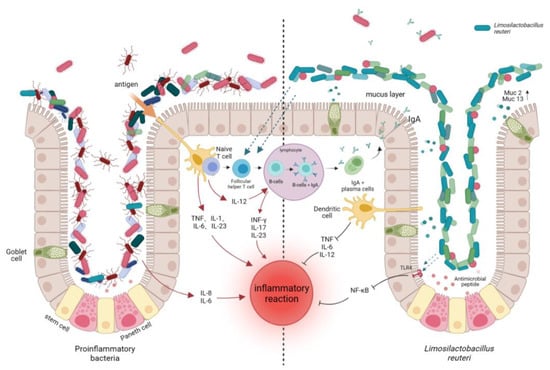
Figure 1.
Immune regulation of L. reuteri in intestinal tract. IL-1: Interleukin 1; IL-6: Interleukin 6; IL-23: Interleukin 23; IL-12: Interleukin 12; INF-γ: γInterferon; IL-17: Interleukin 17; IL-8: Interleukin 8; NF-kB: κ-light chain enhancement in nuclear factor-activated B cells; mucus layer; MUC2: Mucin gene 2; MUC13: Mucin gene 13.
L. reuteri, as the primary bacterium in human and most animal intestines, has good tolerance to the low pH of gastric acid and bile salt [49], and it has the ability to reject and replace pathogenic bacteria in intestinal cell adhesion. L. reuteri ATCC 55730 has been proven to adhere to Caco-2 human intestinal epithelial cells and combine with intestinal epithelial cells to form site competition, reduce the colonization of harmful bacteria, and inhibit the gene expression of pathogenic bacteria [50].
The ability of probiotics to inhibit the adhesion of pathogenic bacteria mainly depends on the specific adhesins of lactobacillus and pathogenic bacteria and the carriers on the cell surface. The adhesins mainly include secreted proteins, glycoproteins, and adhesive polysaccharides. L. reuteri contains gene-encoding proteins and collagen-binding proteins related to the adhesion of type collagen [51], which is conducive to its colonization in the intestinal tract. Some L. reuteri fermentation supernatants and their bacteria have the ability to inhibit the adhesion of pathogenic bacteria [52]. Through experiments, researchers have proven that L. reuteri can adhere to intestinal epithelial cells, form a biological barrier, and prevent the adhesion and colonization of pathogenic bacteria in intestinal epidermis. These findings further support the assumptions of the adhesion mechanism of L. reuteri.
2.2.3. Promotion of Mucus Secretion and Reduction in the Colonization of Harmful Microorganisms
The surfaces of intestinal epithelial cells are covered with a layer of mucus, which is mainly composed of mucin secreted by goblet cells. The mucus layer separates intestinal epithelial cells from the intestinal cavity environment, providing a habitat for obligate or facultative anaerobic microorganisms, attachment sites, and growth substrates for intestinal symbiotic flora [53]. It can also promote the colonization of probiotics in the intestinal tract and inhibit the growth and reproduction of harmful microorganisms (see Figure 1)
The genes related to mucin secretion that are stimulated by L. reuteri are muc2, muc3A, muc13, muc17, clca1, fcgbp, zg16, tff3, etc. Ye Seul Son et al. [54]. found through immunofluorescence staining that L. reuteri can promote intestinal organoid maturation and mucin secretion by increasing the expression of mucin barrier formation and the secretion of the gel-associated mucin MUC2 and mucin MUC13 that protect the mature gut from inflammation. L. reuteri I5007 can restore the number of goblet cells and repair damaged tissues by stimulating the gene expression of the mucin MUC2, and it can also promote the expression of proteins related to cell structure and vitality, as well as lipid metabolism in jejunal mucosal proteins [55]. In addition, the metabolites of L. reuteri, such as short-chain fatty acids, modulate the integrity of the epithelial barrier and tight junctions, which enhances mucus release and promotes the differentiation of intestinal epithelial cells.
2.3. Immunomodulatory Effects
Gut immune regulation is realized by the interaction of trillions of microorganisms and many model receptor gene products, and the gut microbiota drive immune system function and development, thus maintaining the homeostasis of the gut.
2.3.1. Influence on the Production of Immunoglobulin A
The presence of probiotics can affect the production of immunoglobulin A (IgA), whose metabolites, such as short-chain fatty acids, aryl hydrocarbon receptor ligands, and polyamines, communicate with their host and secrete outer membrane vesicles containing microbial-associated patterns vesicles. They can pass through the epithelial barrier or directly recognize pattern recognition receptors, promoting polymeric immunoglobulin receptors and transmembrane mucins to mediate the transformation of B cells into plasma cells and produce IgA, thus reducing the interaction between pathogens and the surface of intestinal epithelial cells, thereby reducing the corresponding exposure, colonization, and inflammatory damage [56]. It has been proved that various strains of L. reuteri can induce intestinal IgA production and modulate gut microbiota diversity in a strain-dependent manner [32]. However, whether they affect IgA levels through the direct regulation of B cells requires further study.
2.3.2. Regulation of TREG Cells
Regulatory T cells (Treg) are T lymphocytes with high immune tolerance that have a negative feedback regulation function. The decrease in the activity of Treg cells usually leads to abnormal immune activation. L. reuteri has been shown to modulate anti-inflammatory Treg cells, which can help them exert probiotic effects in a variety of disease and non-disease conditions [1]. L. reuteri CCFM1072 and CCFM1040 can regulate Treg cell effects. The modulation of gut microbiota in allergic asthmatic mice reduces airway inflammation [57]. L. reuteri DSM 17938 can promote the development or migration of Treg cells, reduce the generation of effective memory T cells (Tem), and suppress immunity in the necrotizing enterocolitis (NEC) model of the small intestine’s activated and reduced inflammatory response [33]. Studies have shown that L. reuteri can also inhibit an excessive inflammatory responses by inhibiting Toll-like receptors (TLR) 4 and NF-κB in small intestinal epithelial cells, intervening in the balance between Treg cells and Tem cells to make the immune system remain immune and to interrupt and avoid excessive immunity and exert anti-inflammatory effects [33,58,59]. It should be noted that L. reuteri is not helpful for all patients with inflammation. In lupus erythematosus patients, L. reuteri can intervene in the TLR7 pathway to affect immune regulation, making lupus-susceptible hosts more susceptible to disease and aggravating the patient’s condition [60].
2.3.3. Inhibition of the Production of Pro-inflammatory Factors
Some L. reuteri have been found to reduce the production of pro-inflammatory factors, as it is a common genus of Lactobacillus with therapeutic properties [61]. Anukam et al. [62] found that L. reuteri RC-14 downregulated TNF-α, IL-6, IL-8, IL-12, and other levels, and it relieved some symptoms of cystitis. L. reuteri GMNL-263 can reduce the levels of MCP-1, TNF, and IL-6 in mice fed a high-fat diet [63]. In addition, some L. reuteri also inhibit pro-inflammatory motor-protein cells in rodent models [64].
2.3.4. More Studies
Some L. reuteri can affect the production of inflammatory factors through their special structures and metabolites.
Exopoly saccharides (EPSs): EPSs are a type of carbohydrate compound secreted outside the cell wall of lactic acid bacteria during the growth and metabolism processes. They have the effect of inhibiting the pro-inflammatory immune response of macrophages. High levels of capsular polysaccharides can protect against intestinal innate immune factors [65], such as antimicrobial peptides. Enterotoxins released by enterotoxigenic Escherichia coli can induce the upregulation of CAMKII and cAMP in intestinal cells and destroy the electrolyte homeostasis of intestinal cells, leading to intestinal cell dehydration and diarrhea [66]. L. reuteri L26 can secrete an EPS (MW = 8.2 × 105Da) composed of α-D-glucose (1→3) and (1→6) glycosidic bonds [67], which can interfere with the overexpression of calmodulin-dependent protein kinase II and the cAMP-dependent kinase protein-related gene PRKAR1A, which is induced by enterotoxigenic Escherichia coli. Additionally, it reduces the effect of enterotoxigenic Escherichia coli on the tight junction of intestinal epithelial cells, preventing the development of diarrhea in piglets infected with enterotoxigenic Escherichia coli [68]. EPSs are important factors in the induction of T cells by L. reuteri. L. reuteri 100-23 produces EPSs by decomposing sucrose. This EPS is a levan (β-2, 6-linked fructan), and it can induce the proliferation of regulatory T cells in the spleen, affecting the immune response [69,70,71].
Surface layer proteins (SLPs): SLPs are part of the complex structure of the cell wall of L. reuteri. With the exception of its adhesive function, understanding the other functions of SLPs is still in the hypothetical stage. It has been experimentally demonstrated that SLPs can be recognized by dendritic cell-specific intercellular adhesion molecules combined with non-integrin molecules and induce a pro-inflammatory cytokine profile in the human dendritic cell DSC92 [72].
Histamine: Histamine in the metabolites of L. reuteri can stimulate the host H2 histamine receptor, derive histamine to inhibit THP-1 cells, produce tumor necrosis factor (TNF), and inhibit TNF production by transcriptional regulation [73].
Tryptophan catabolite can activate aromatic hydrocarbon receptors, promote innate lymphocytes, and produce local IL-22 [74].
In conclusion, L. reuteri has a good immunomodulatory effect, which can exert an immune effect by affecting the expression of mucin genes, producing antibodies and inflammation-related factors (as shown in Figure 1). However, some studies have shown that L. reuteri has little effect on immunity, and single use of L. reuteri does not improve immunity and has a negative effect on some diseases. Therefore, the adjustment and improvement of L. reuteri on the body’s immunity is still controversial.
Pro-inflammatory microorganisms (shown on the left side of the figure) can directly or indirectly interact with immune cells and intestinal epithelial cells to produce pro-inflammatory factors such as IL-6, IL-8, etc. In addition to B lymphocytes producing IgA, various stimulated proinflammatory cytokines produced by epithelial cells, monocytes, and lymphocytes also elicit an immune stimulatory response. L. reuteri can directly or indirectly alter gut microbiota through its metabolites, adhesion mechanisms, and gene regulation, reducing pro-inflammatory bacterial colonization and attenuating inflammatory responses (shown on the right side of the figure).
2.4. Amelioration of Obesity
Current studies suggest that low-grade systemic inflammation is the main mechanism leading to insulin resistance in obese individuals, and the correlation between gut microbiota and obesity has been well documented. L. reuteri is significantly associated with the development of obesity. In a high-fat diet (HFD)-induced obesity mouse model, through treatment with L. reuteri GMNL-263, the weight, fat, tissue, and liver, as a percentage of body weight, decreased significantly [74]. Li-Han Chen [28] and others have found that L. reuteri 263 supplementation triggered the remodeling of energy metabolism in white adipose tissue and enhanced browning-related genes including pparγ, prdm16, bmp7, fgf21, etc., promoting the conversion of white adipose tissue to faster energy-consuming beige adipose tissue and noticeably ameliorating obesity in HFD-induced obese mice.
2.5. Other Functions
As with many other Lactobacillus species, some L. reuteri strains produce different types of vitamins, such as vitamins B12 and B9. A recent study by Ker-Ping et al. [75] found that heat-inactivated L. reuteri GMNL-263 can activate the IGF–IR signaling pathway to reduce hyperglycemia-induced Fas-dependent, and mitochondria-dependent apoptosis pathways, and at the same time, it can alleviate hyperglycemia-caused heart damage. In addition, L. reuteri can lower serum cholesterol levels and is an antidiarrheal [76].
3. Application of L. Reuteri
L. reuteri, with its strong intestinal adhesion, widely exists in the intestines of humans and animals, and it can inhibit the reproduction of gastrointestinal pathogens, regulate the immune function of animals, and effectively reduce animal stress responses and feed-to-weight ratios, and thus, it is widely used and applied in daily life.
3.1. Application in the Feed Industry
L. reuteri can effectively inhibit the growth of harmful bacteria and reduce or replace the use of antibiotics in animals in the breeding process, and so it is used to promote the growth of livestock and poultry and weaken the resistance of pathogens caused by antibiotics. In animal husbandry, L. reuteri BSA131 causes significant weight gain when introduced into pigs, turkeys, and rats, and it can be isolated at higher concentrations from feces after feeding with probiotics [77]. L. reuteri and its mixed preparations can improve the apparent nitrogen digestibility, total energy digestibility, and average daily gain (ADG) of weaned piglets. It can also increase piglet body weight, reduce the proportion of feed materials, and improve animal survival rates [78]. Lei Qingzhi et al. [27] extracted L. reuteri LP4 from worker bees, which can inhibit the growth of the enteric pathogens Salmonella Typhimurium, Escherichia coli and Shigella flexneri. By feeding LP4, the survival rates of oriental honeybees to adulthood worker bees can be increased.
3.2. Application in Functional Food
L. reuteri, as a dominant, heterofermentative lactic acid bacteria, is a commonly used probiotic in food production.
The bacteriocin reuterin secreted by L. reuteri has strong broad-spectrum antibacterial activity, and it is often used as a biological preservative in food [76].However, its fermentation effect is not strong and its curdling ability is poor. Therefore, L. reuteri is often used as an auxiliary starter in cheese and yogurt to extend the shelf life of food and enhance product performance. At present, the probiotics used in commercial yogurt fermentation have poor acid resistance, bile salt resistance and adhesion, and colonization, which limit their beneficial effects. They do not have an antagonistic effect on Lactobacillus bulgaricus and Streptococcus thermophilus, and they can effectively inhibit the growth of Penicillium and prolong the shelf life of yogurt. L. reuteri LT018 has strong stability and excellent self-polymerization [79]. It can enhance the survival of live bacteria and intestinal colonization in dairy products. The growth state of fermented milk obviously increases the content of riboflavin in fermented milk, and this improves this commodity value.
Vitamins are essential trace elements for the human body; however, the body cannot synthesize vitamins by itself. L. reuteri is an active vitamin B12-producing bacteria in which cobalamin is involved in the metabolic pathway of methionine, contributing to the production of glutathione and to its anti-oxidation activity [58] and enhancing vitamin B12-related gene pathways in fermented foods mixed with fermented glucose, fructose, and glycerol to increase vitamin B12 production [80]. L. reuteri has also been used in the brewing of vinegar and wine, as it can adjust the total acid content of vinegar and improve the flavor [81,82] while also reducing the urea content in liquor-fermented grains and the formation of ethyl carbamate [83]. However, there are relatively few studies on urease in liquor, and the application of L. reuteri in liquor production requires further study.
3.3. Clinical Application
With the deepening of antibiotic research, probiotics have been widely studied and applied in clinical medicine. Several studies have shown that the symbiosis of Lactobacillus can accelerate the restoration of the host’s intestinal microbiota to its original good state, thereby regulating intestinal flora homeostasis in patients with acute diarrhea [76]. It has been proven that L. reuteri can significantly improve chronic constipation, colic, and diarrhea in infants, without adverse side effects such as vomiting, bloating, and increased gas [84].
Studies have found that a variety of L. reuteri can stimulate immunomodulatory responses, reduce human intestinal inflammation (to varying degrees), and effectively prevent DSS-induced colitis in rats by reducing P-selectin-dependent leukocytes and platelet–endothelial cell interactions. Liu et al. showed that the L. reuteri DSM17938 strain may reduce intestinal oxidative stress and increase its antioxidant capacity, thereby reducing the intestinal inflammatory response and reducing the damage caused by small intestinal necrotizing colitis [64,85]. Research has brought new targets for the treatment of patients with enteritis, but the pathogenesis of intestinal inflammation has not been determined, and the specific molecular signaling pathways of L. reuteri involved in the inflammatory response by regulating oxidative stress still need to be further explored.
L. reuteri DSM17648 can recognize and adhere to the surface of the adhesion molecules of Helicobacter pylori, inhibiting the binding of Helicobacter pylori to glycolipid receptors and forming insoluble polymers with them, which are then excreted through the digestive tract [86], reducing gastric inflammation and cancer risk. Currently, the treatment of Helicobacter pylori by L. reuteri has released in the market, and there are strains (DSM17648, HN019, etc.) being sold on the market.
At present, L. reuteri has contributed to new research progress in the treatment of the oral cavity, the regulation of vaginal flora in women, liver failure treatments, and airway inflammation treatment. However, further clinical applications still have a long way to go.
4. Conclusions
L. reuteri, as a common lactic acid bacterium in the intestinal tracts of humans and most vertebrates, is widely used in daily life. L. reuteri can effectively improve the intestinal environment, promote intestinal health, and achieve immune function by affecting microbial flora, improving intestinal structures, and regulating the levels of factors such as TNF-α, IL-6, and IL-8. It has broad prospects and research potential in animal breeding and medical treatments.
At present, there are a number of studies on the influence of L. reuteri on intestinal flora and the regulation of immunity; however, research on the pathways of postbiotic generation and the physicochemical properties and functions of metabolites requires further study. L. reuteri can produce a variety of bacteriocins which require more research and discussion on the quorum sensing law and on the mechanisms of changes in gut microbiota. The pathogenesis of many diseases is complex and remains unclear, and the potential threat of active probiotics to some diseases is still controversial. The changes in intestinal flora after oral administration, especially the changes in intestinal metabolites, are also not well studied. Therefore, the duration, effectiveness, and safety of L. reuteri interventional therapy still requires considerable theoretical knowledge, large samples, and multi-center clinical trials. In the future, L. reuteri-related treatment may become a new and safer treatment for inflammatory diseases.
Author Contributions
Conceptualization, J.J. and Z.L; writing—original draft preparation and software, J.J.; writing—review and editing, Z.L., K.L., and Y.X.; suggestions on revision, A.Z., J.T., and Y.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research Program of Hunan Province (2022NK2040), Hunan Provincial Innovative Construction Special Fund (2021NK4260) and project support of Hunan Province Agricultural Industry Technology system (2022-67).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We wish to thank the Key Research Program of Hunan Province (2022NK2040), Hunan Provincial Innovative Construction Special Fund (2021NK4260) and project support of Hunan Province Agricultural Industry Technology system (2022-67).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Duar, R.M.; Frese, S.A.; Lin, X.B.; Fernando, S.C.; Burkey, T.E.; Tasseva, G.; Peterson, D.A.; Blom, J.; Wenzel, C.Q.; Szymanski, C.M.; et al. Experimental evaluation of host adaptation of Lactobacillus reuteri to different vertebrate species. Appl. Environ. Microbiol. 2017, 83, e00132-17. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, L.; Chen, L.; Ren, X.; Ge, H.; Li, B.; Ma, G.; Ke, X.; Zhu, J.; Li, L.; et al. Potential probiotic characterization of Lactobacillus reuteri from traditional Chinese highland barley wine and application for room-temperature-storage drinkable yogurt. J. Dairy Sci. 2018, 101, 5780–5788. [Google Scholar] [CrossRef] [PubMed]
- İspirli, H.; Şimşek, Ö.; Skory, C.; Sağdıç, O.; Dertli, E. Characterization of a 4, 6-α-glucanotransferase from Lactobacillus reuteri E81 and production of malto-oligosaccharides with immune-modulatory roles. Int. J. Biol. Macromol. 2019, 124, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tao, Q.; Teixeira, J.S.; Su, M.S.-W.; Gänzle, M.G. Contribution of glutaminases to glutamine metabolism and acid resistance in Lactobacillus reuteri and other vertebrate host adapted lactobacilli. Food Microbiol. 2020, 86, 103343. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Siepmann, F.B.; Tovar, L.E.R.; Chen, X.; Gänzle, M.G. Effect of copy number of the spoVA2mob operon, sourdough and reutericyclin on ropy bread spoilage caused by Bacillus spp. Food Microbiol. 2020, 91, 103507. [Google Scholar] [CrossRef]
- Hu, Z.; Jia, P.; Bai, Y.; Fan, T.-P.; Zheng, X.; Cai, Y. Characterisation of five alcohol dehydrogenases from Lactobacillus reuteri DSM20016. Process Biochem. 2019, 86, 73–79. [Google Scholar] [CrossRef]
- Zabed, H.M.; Zhang, Y.; Guo, Q.; Yun, J.; Yang, M.; Zhang, G.; Qi, X. Co-biosynthesis of 3-hydroxypropionic acid and 1,3-propanediol by a newly isolated Lactobacillus reuteri strain during whole cell biotransformation of glycerol. J. Clean. Prod. 2019, 226, 432–442. [Google Scholar] [CrossRef]
- Garg, S.; Singh, T.P.; Malik, R.K. In vivo implications of potential probiotic Lactobacillus reuteri lr6 on the gut and immunological parameters as an adjuvant against protein energy malnutrition. Probiotics Antimicrob. Proteins 2020, 12, 517–534. [Google Scholar] [CrossRef]
- Turco, R.; Russo, M.; Bruzzese, D.; Staiano, A. Efficacy of a partially hydrolysed formula, with reduced lactose content and with Lactobacillus reuteri DSM 17938 in infant colic: A double blind, randomised clinical trial. Clin. Nutr. 2021, 40, 412–419. [Google Scholar] [CrossRef]
- Morita, H.; Toh, H.; Fukuda, S.; Horikawa, H.; Oshima, K.; Suzuki, T.; Murakami, M.; Hisamatsu, S.; Kato, Y.; Takizawa, T.; et al. Comparative genome analysis of Lactobacillus reuteri and Lactobacillus fermentum reveal a genomic island for reuterin and cobalamin production. DNA Res. 2008, 15, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, S.H.; Kim, Y.J.; Jeong, H.J.; Ryu, J.S.; Lee, H.J.; Kim, T.W.; Im, S.-H.; Oh, J.Y.; Kim, M.K. Clinical effect of IRT-5 probiotics on immune modulation of autoimmunity or alloimmunity in the eye. Nutrients 2017, 9, 1166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Cai, W.; Li, D.; Zheng, W.; Xiao, Y.; Zhao, H.; Pan, S. Effect of oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on vaginal Group B Streptococcus colonization and vaginal microbiome in late pregnancy. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med. Univ. 2020, 40, 1753–1759. [Google Scholar] [CrossRef]
- Yamato, M.; Nakada, R.; Nakamura, Y. Release of spirosin associated with potassium phosphate-induced autolysis in Lactobacillus reuteri DSM 20016. Microbiol. Res. 1998, 153, 29–35. [Google Scholar] [CrossRef]
- Jones, M.L.; Martoni, C.J.; Prakash, S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: A post hoc analysis of a randomized controlled trial. J. Clin. Endocrinol. Metab. 2013, 98, 2944–2951. [Google Scholar] [CrossRef]
- Wells, J.G.; Davis, B.; Wachsmuth, I.; Riley, L.W.; Remis, R.S.; Sokolow, R.; Morris, G.K. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J. Clin. Microbiol. 1983, 18, 512–520. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Wang, S.; Zhang, W.; Wang, J.; Tian, H.; Wang, Y.; Ji, H. Fecal microbiota and its correlation with fatty acids and free amino acids metabolism in piglets after a Lactobacillus strain oral administration. Front. Microbiol. 2019, 10, 785. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Chandra, V.; Kim, N.-H.; Rai, R.; Kumar, P.; Kim, K.; Aeron, A.; Kang, S.C.; Maheshwari, D.; Na, M.; et al. Ghost probiotics with a combined regimen: A novel therapeutic approach against the Zika virus, an emerging world threat. Crit. Rev. Biotechnol. 2018, 38, 438–454. [Google Scholar] [CrossRef]
- Li, X.; Yue, L.; Guan, X.; Qiao, S. The adhesion of putative probiotic lactobacilli to cultured epithelial cells and porcine intestinal mucus. J. Appl. Microbiol. 2008, 104, 1082–1091. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Fei, S.; Xie, C.; Xia, Y.; Ai, L. Colonisation with endogenous Lactobacillus reuteri R28 and exogenous Lactobacillus plantarum AR17-1 and the effects on intestinal inflammation in mice. Food Funct. 2021, 12, 2481–2488. [Google Scholar] [CrossRef]
- Leonard, M.T.; Valladares, R.B.; Ardissone, A.; Gonzalez, C.F.; Lorca, G.L.; Triplett, E.W. Complete genome sequences of Lactobacillus johnsonii strain N6.2 and Lactobacillus reuteri strain TD1. Genome Announc. 2014, 2, e00397-14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kang, Z.; Zhou, J.; Chen, J.; Du, G. High-level expression and characterization of recombinant acid urease for enzymatic degradation of urea in rice wine. Appl. Microbiol. Biotechnol. 2015, 99, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Klein, G. Antibiotic resistance and molecular characterization of probiotic and clinical Lactobacillus strains in relation to safety aspects of probiotics. Foodborne Pathog. Dis. 2011, 8, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Jatuponwiphat, T.; Namrak, T.; Supataragul, A.; Nitisinprasert, S.; Nakphaichit, M.; Vongsangnak, W. Comparative genome analysis reveals metabolic traits associated with probiotics properties in Lactobacillus reuteri KUB-AC5. Gene Rep. 2019, 17, 100536. [Google Scholar] [CrossRef]
- Kim, D.; Cho, M.-J.; Cho, S.; Lee, Y.; Byun, S.J.; Lee, S. Complete genome sequence of Lactobacillus reuteri SKKU-OGDONS-01, isolated from a chicken’s small intestine. Microbiol. Resour. Announc. 2018, 7, e01251-18. [Google Scholar] [CrossRef]
- Hu, R.; Lin, H.; Wang, M.; Zhao, Y.; Liu, H.; Min, Y.; Yang, X.; Gao, Y.; Yang, M. Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J. Anim. Sci. Biotechnol. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Lv, M.; Lei, Q.; Yin, H.; Hu, T.; Wang, S.; Dong, K.; Pan, H.; Liu, Y.; Lin, Q.; Cao, Z. Effects of Prebiotics and Synbiotics on Gut Microbiota. Pol. J. Microbiol. 2021, 70, 511–520. [Google Scholar] [CrossRef]
- Chen, L.-H.; Chen, Y.-H.; Cheng, K.-C.; Chien, T.-Y.; Chan, C.-H.; Tsao, S.-P.; Huang, H.-Y. Antiobesity effect of Lactobacillus reuteri 263 associated with energy metabolism remodeling of white adipose tissue in high-energy-diet-fed rats. J. Nutr. Biochem. 2018, 54, 87–94. [Google Scholar] [CrossRef]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N.; McCabe, L.R. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef]
- Ding, Y.-H.; Qian, L.-Y.; Pang, J.; Lin, J.-Y.; Xu, Q.; Wang, L.-H.; Huang, D.-S.; Zou, H. The regulation of immune cells by Lactobacilli: A potential therapeutic target for anti-atherosclerosis therapy. Oncotarget 2017, 8, 59915. [Google Scholar] [CrossRef]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteribiofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Xiao, H.; Shi, Y.; Le, G.W.; Sun, J. Isolation of Lactobacillus reuteri from Peyer’s patches and their effects on sIgA production and gut microbiota diversity. Mol. Nutr. Food Res. 2016, 60, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tran, D.Q.; Fatheree, N.Y.; Marc Rhoads, J. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G177–G186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Q.; Wang, J.; Qiu, X.; Qi, R.; Huang, J. Identification of differentially expressed miRNAs after Lactobacillus reuteri treatment in the ileum mucosa of piglets. Genes Genom. 2020, 42, 1327–1338. [Google Scholar] [CrossRef]
- Yang, F.; Wang, A.; Zeng, X.; Hou, C.; Liu, H.; Qiao, S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Liu, H.; Hou, C.; Wang, G.; Jia, H.; Yu, H.; Zeng, X.; Thacker, P.A.; Zhang, G.; Qiao, S. Lactobacillus reuteri I5007 modulates intestinal host defense peptide expression in the model of IPEC-J2 cells and neonatal piglets. Nutrients 2017, 9, 559. [Google Scholar] [CrossRef]
- Zheng, J.; Ruan, L.; Sun, M.; Gänzle, M. A genomic view of lactobacilli and pediococci demonstrates that phylogeny matches ecology and physiology. Appl. Environ. Microbiol. 2015, 81, 7233–7243. [Google Scholar] [CrossRef]
- El-Ziney, M.; Van Den Tempel, T.; Debevere, J.; Jakobsen, M. Application of reuterin produced by Lactobacillus reuteri 12002 for meat decontamination and preservation. J. Food Prot. 1999, 62, 257–261. [Google Scholar] [CrossRef]
- Talarico, T.; Casas, I.; Chung, T.C.; Dobrogosz, W. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 1988, 32, 1854–1858. [Google Scholar] [CrossRef]
- Vollenweider, S.; Evers, S.; Zurbriggen, K.; Lacroix, C. Unraveling the hydroxypropionaldehyde (HPA) system: An active antimicrobial agent against human pathogens. J. Agric. Food Chem. 2010, 58, 10315–10322. [Google Scholar] [CrossRef]
- Chung, T.; Axelsson, L.; Lindgren, S.; Dobrogosz, W. In vitro studies on reuterin synthesis by Lactobacillus reuteri. Microb. Ecol. Health Dis. 1989, 2, 137–144. [Google Scholar] [CrossRef]
- Greifová, G.; Májeková, H.; Greif, G.; Body, P.; Greifová, M.; Dubničková, M. Analysis of antimicrobial and immunomodulatory substances produced by heterofermentative Lactobacillus reuteri. Folia Microbiol. 2017, 62, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Ganzle, M.G.; Holtzel, A.; Walter, J.; Jung, G.N.; Hammes, W.P. Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl. Environ. Microbiol. 2000, 66, 4325–4333. [Google Scholar] [CrossRef]
- Kabuki, T.; Saito, T.; Kawai, Y.; Uemura, J.; Itoh, T. Production, purification and characterization of reutericin 6, a bacteriocin with lytic activity produced by Lactobacillus reuteri LA6. Int. J. Food Microbiol. 1997, 34, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Toba, T.; Samant, S.; Yoshioka, E.; Itoh, T. Reutericin 6, a new bacteriocin produced by Lactobacillus reuteri LA 6. Lett. Appl. Microbiol. 1991, 13, 281–286. [Google Scholar] [CrossRef]
- Arakawa, K.; Kawai, Y.; Ito, Y.; Nakamura, K.; Chujo, T.; Nishimura, J.; Kitazawa, H.; Saito, T. HPLC purification and re-evaluation of chemical identity of two circular bacteriocins, gassericin A and reutericin 6. Lett. Appl. Microbiol. 2010, 50, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Maqueda, M.; Sánchez-Hidalgo, M.; Fernández, M.; Montalbán-López, M.; Valdivia, E.; Martínez-Bueno, M. Genetic features of circular bacteriocins produced by Gram-positive bacteria. FEMS Microbiol. Rev. 2008, 32, 2–22. [Google Scholar] [CrossRef]
- Bøhle, L.A.; Brede, D.A.; Diep, D.B.; Holo, H.; Nes, I.F. Specific degradation of the mucus adhesion-promoting protein (MapA) of Lactobacillus reuteri to an antimicrobial peptide. Appl. Environ. Microbiol. 2010, 76, 7306–7309. [Google Scholar] [CrossRef]
- Seo, B.J.; Mun, M.R.; Kim, C.-J.; Lee, I.; Chang, Y.-H.; Park, Y.-H. Bile tolerant Lactobacillus reuteri isolated from pig feces inhibits enteric bacterial pathogens and porcine rotavirus. Vet. Res. Commun. 2010, 34, 323–333. [Google Scholar] [CrossRef]
- Moussavi, M.; Adams, M.C. An in vitro study on bacterial growth interactions and intestinal epithelial cell adhesion characteristics of probiotic combinations. Curr. Microbiol. 2010, 60, 327–335. [Google Scholar] [CrossRef]
- Saulnier, D.M.; Santos, F.; Roos, S.; Mistretta, T.-A.; Spinler, J.K.; Molenaar, D.; Teusink, B.; Versalovic, J. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS ONE 2011, 6, e18783. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Kaur, G.; Kapila, S.; Malik, R.K. Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Front. Microbiol. 2017, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.S.; Ki, S.J.; Thanavel, R.; Kim, J.J.; Lee, M.O.; Kim, J.; Jung, C.R.; Han, T.S.; Cho, H.S.; Ryu, C.M.; et al. Maturation of human intestinal organoids in vitro facilitates colonization by commensal lactobacilli by reinforcing the mucus layer. FASEB J. 2020, 34, 9899–9910. [Google Scholar] [CrossRef]
- Wang, G.; Huang, S.; Cai, S.; Yu, H.; Wang, Y.; Zeng, X.; Qiao, S. Lactobacillus reuteri ameliorates intestinal inflammation and modulates gut microbiota and metabolic disorders in dextran sulfate sodium-induced colitis in mice. Nutrients 2020, 12, 2298. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 263–278. [Google Scholar] [CrossRef]
- Li, L.; Fang, Z.; Liu, Z.; Zhao, J.; Zhang, H.; Wang, S.; He, J.; Lu, W.; Chen, W. Lactobacillus reuteri CCFM1072 and CCFM1040 with the role of Treg cells regulation alleviate airway inflammation through modulating gut microbiota in allergic asthma mice. J. Funct. Foods 2021, 76, 104286. [Google Scholar] [CrossRef]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NF-κB signaling in the intestine. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G608–G617. [Google Scholar] [CrossRef]
- Lu, P.; Sodhi, C.P.; Hackam, D.J. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology 2014, 21, 81–93. [Google Scholar] [CrossRef]
- Zegarra-Ruiz, D.F.; El Beidaq, A.; Iñiguez, A.J.; Di Ricco, M.L.; Vieira, S.M.; Ruff, W.E.; Mubiru, D.; Fine, R.L.; Sterpka, J.; Greiling, T.M.; et al. A diet-sensitive commensal Lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe 2019, 25, 113–127.e6. [Google Scholar] [CrossRef]
- Shornikova, A.-V.; Casas, I.A.; Mykkänen, H.; Salo, E.; Vesikari, T. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. Pediatr. Infect. Dis. J. 1997, 16, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Anukam, K.C.; Hayes, K.; Summers, K.; Reid, G. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 may help downregulate TNF-Alpha, IL-6, IL-8, IL-10 and IL-12 (p70) in the neurogenic bladder of spinal cord injured patient with urinary tract infections: A two-case study. Adv. Urol. 2009, 2009, 680363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsieh, F.-C.; Lan, C.-C.E.; Huang, T.-Y.; Chen, K.-W.; Chai, C.-Y.; Chen, W.-T.; Fang, A.-H.; Chen, Y.-H.; Wu, C.-S. Heat-killed and live Lactobacillus reuteri GMNL-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2374–2388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G1087–G1096. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, E.; Serata, M.; Sako, T. Suppressive effect on activation of macrophages by Lactobacillus casei strain Shirota genes determining the synthesis of cell wall-associated polysaccharides. Appl. Environ. Microbiol. 2008, 74, 4746–4755. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Connell, T.D. Type II heat-labile enterotoxins: Structure, function, and immunomodulatory properties. Vet. Immunol. Immunopathol. 2013, 152, 68–77. [Google Scholar] [CrossRef]
- Kšonžeková, P.; Bystrický, P.; Vlčková, S.; Pätoprstý, V.; Pulzová, L.; Mudroňová, D.; Kubašková, T.; Csank, T.; Tkáčiková, Ľ. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19. [Google Scholar] [CrossRef]
- Tkáčiková, Ľ.; Mochnáčová, E.; Tyagi, P.; Kiššová, Z.; Bhide, M. Comprehensive mapping of the cell response to E. coli infection in porcine intestinal epithelial cells pretreated with exopolysaccharide derived from Lactobacillus reuteri. Vet. Res. 2020, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sims, I.M.; Frese, S.A.; Walter, J.; Loach, D.; Wilson, M.; Appleyard, K.; Eason, J.; Livingston, M.; Baird, M.; Cook, G. Structure and functions of exopolysaccharide produced by gut commensal Lactobacillus reuteri 100-23. ISME J. 2011, 5, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Hoffmann, M.; Rath, E.; Hölzlwimmer, G.; Quintanilla-Martinez, L.; Loach, D.; Tannock, G.; Haller, D. Lactobacillus reuteri 100-23 transiently activates intestinal epithelial cells of mice that have a complex microbiota during early stages of colonization. J. Nutr. 2008, 138, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, S.R.; Smidt, H.; de Vos, W.M.; Bruijns, S.C.; Singh, S.K.; Valence, F.; Molle, D.; Lortal, S.; Altermann, E.; Klaenhammer, T.R.; et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. USA 2008, 105, 19474–19479. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Ladero, V.; Del Rio, B.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Biofilm-forming capacity in biogenic amine-producing bacteria isolated from dairy products. Front. Microbiol. 2016, 7, 591. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Koay, K.-P.; Tsai, B.C.-K.; Kuo, C.-H.; Kuo, W.-W.; Luk, H.-N.; Day, C.H.; Chen, R.-J.; Chen, M.Y.-C.; Padma, V.V.; Huang, C.-Y. Hyperglycemia-Induced Cardiac Damage Is Alleviated by Heat-Inactivated Lactobacillus reuteri GMNL-263 via Activation of the IGF1R Survival Pathway. Probiotics Antimicrob. Proteins 2021, 13, 1044–1053. [Google Scholar] [CrossRef]
- Ortiz-Rivera, Y.; Sánchez-Vega, R.; Gutiérrez-Méndez, N.; León-Félix, J.; Acosta-Muñiz, C.; Sepulveda, D. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Kim, J.-K.; Kim, H.-J.; Kim, W.-Y.; Kim, Y.-B.; Park, Y.-H. Selection of a potential probiotic Lactobacillus strain and subsequent in vivo studies. Antonie Van Leeuwenhoek 2001, 80, 193–199. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Kim, I.H. Effect of direct-fed microbial on growth performance, nutrient digestibility, fecal noxious gas emission, fecal microbial flora and diarrhea score in weanling pigs. Anim. Feed. Sci. Technol. 2015, 200, 86–92. [Google Scholar] [CrossRef]
- Shi, S.; Dong, J.; Cheng, X.; Hu, J.; Liu, Y.; He, G.; Zhang, J.; Yu, H.; Jia, L.; Zhou, D. Biological characteristics and whole-genome analysis of the potential probiotic, Lactobacillus reuteri S5. Lett. Appl. Microbiol. 2022, 74, 593–603. [Google Scholar] [CrossRef]
- Bao, X.; Xiang, S.; Chen, J.; Shi, Y.; Chen, Y.; Wang, H.; Zhu, X. Effect of Lactobacillus reuteri on vitamin B12 content and microbiota composition of furu fermentation. LWT 2019, 100, 138–143. [Google Scholar] [CrossRef]
- Chai, L.-J.; Qiu, T.; Lu, Z.-M.; Deng, Y.-J.; Zhang, X.-J.; Shi, J.-S.; Xu, Z.-H. Modulating microbiota metabolism via bioaugmentation with Lactobacillus casei and Acetobacter pasteurianus to enhance acetoin accumulation during cereal vinegar fermentation. Food Res. Int. 2020, 138, 109737. [Google Scholar] [CrossRef]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of potentially probiotic lactic acid bacteria on the physicochemical composition and acceptance of fermented cereal beverages. J. Funct. Foods 2015, 15, 106–115. [Google Scholar] [CrossRef]
- Kakimoto, S.; Sumino, Y.; ichi Akiyama, S.; Nakao, Y. Purification and characterization of acid urease from Lactobacillus reuteri. Agric. Biol. Chem. 1989, 53, 1119–1125. [Google Scholar] [CrossRef][Green Version]
- Savino, F.; Garro, M.; Montanari, P.; Galliano, I.; Bergallo, M. Crying time and RORγ/FOXP3 expression in Lactobacillus reuteri DSM17938-treated infants with colic: A randomized trial. J. Pediatr. 2018, 192, 171–177.E1. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Guo, C.; Gong, F. Protective effect of Lactobacillus reuteri against oxidative stress in neonatal mice with necrotizing enterocolitis. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med. Univ. 2019, 39, 1221–1226. [Google Scholar] [CrossRef]
- Buckley, M.; Lacey, S.; Doolan, A.; Goodbody, E.; Seamans, K. The effect of Lactobacillus reuteri supplementation in Helicobacter pylori infection: A placebo-controlled, single-blind study. BMC Nutr. 2018, 4, 48. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).