Figure 1.
Diagram of butanol production in a stirred tank bioreactor integrated with a gas stripping system.
Figure 1.
Diagram of butanol production in a stirred tank bioreactor integrated with a gas stripping system.
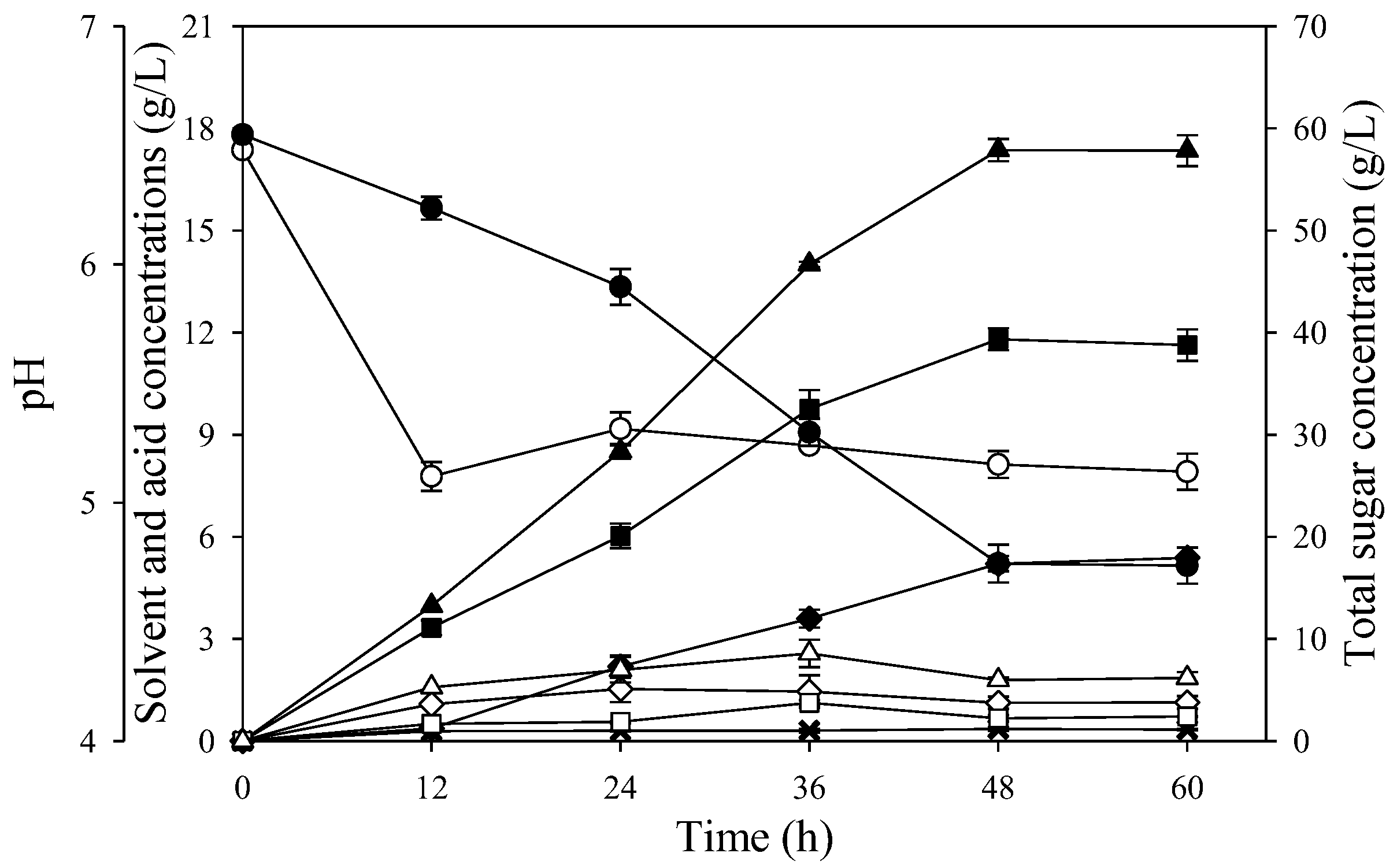
Figure 2.
Batch butanol fermentation profiles from the P2 medium (A) and SSJ medium with no nutrient supplementation (B) using free cells of C. beijerinckii TISTR 1461: acetone (♦), butanol (■), ethanol (×), ABE (▲), acetic acid (◊), butyric acid (□), total acid (Δ), pH (○), and total sugars (●).
Figure 2.
Batch butanol fermentation profiles from the P2 medium (A) and SSJ medium with no nutrient supplementation (B) using free cells of C. beijerinckii TISTR 1461: acetone (♦), butanol (■), ethanol (×), ABE (▲), acetic acid (◊), butyric acid (□), total acid (Δ), pH (○), and total sugars (●).
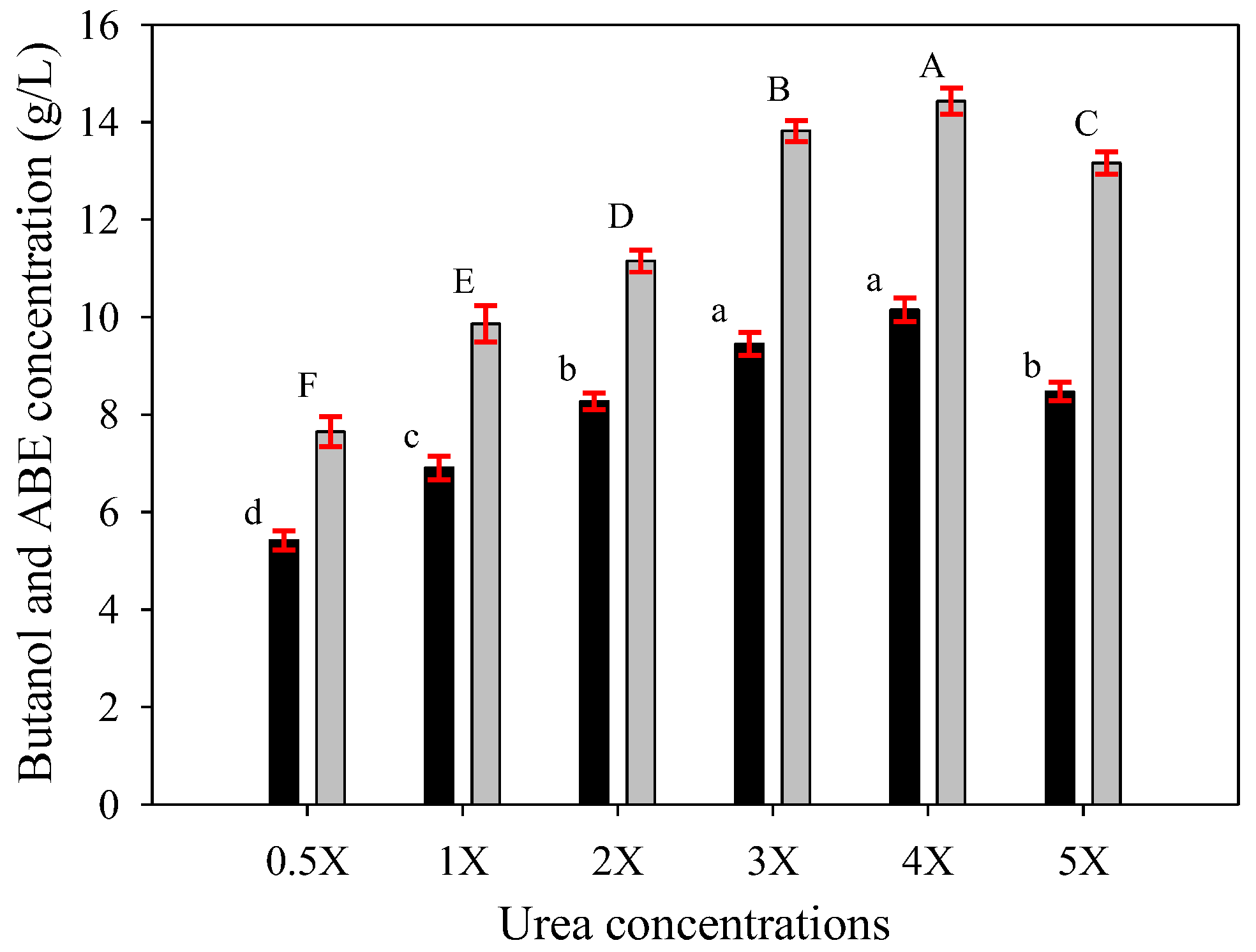
Figure 3.
Butanol (black bars) and ABE (gray bars) contents of butanol fermentation from SSJ medium supplemented with various urea concentrations by free cells (1X = 0.16 g/L of urea) at the fermentation time of 48 h. The error bars represent the standard deviation of the mean values. Bars associated with the same letter have no significant differences assessed by Duncan’s multiple range test with a critical value of 0.05.
Figure 3.
Butanol (black bars) and ABE (gray bars) contents of butanol fermentation from SSJ medium supplemented with various urea concentrations by free cells (1X = 0.16 g/L of urea) at the fermentation time of 48 h. The error bars represent the standard deviation of the mean values. Bars associated with the same letter have no significant differences assessed by Duncan’s multiple range test with a critical value of 0.05.
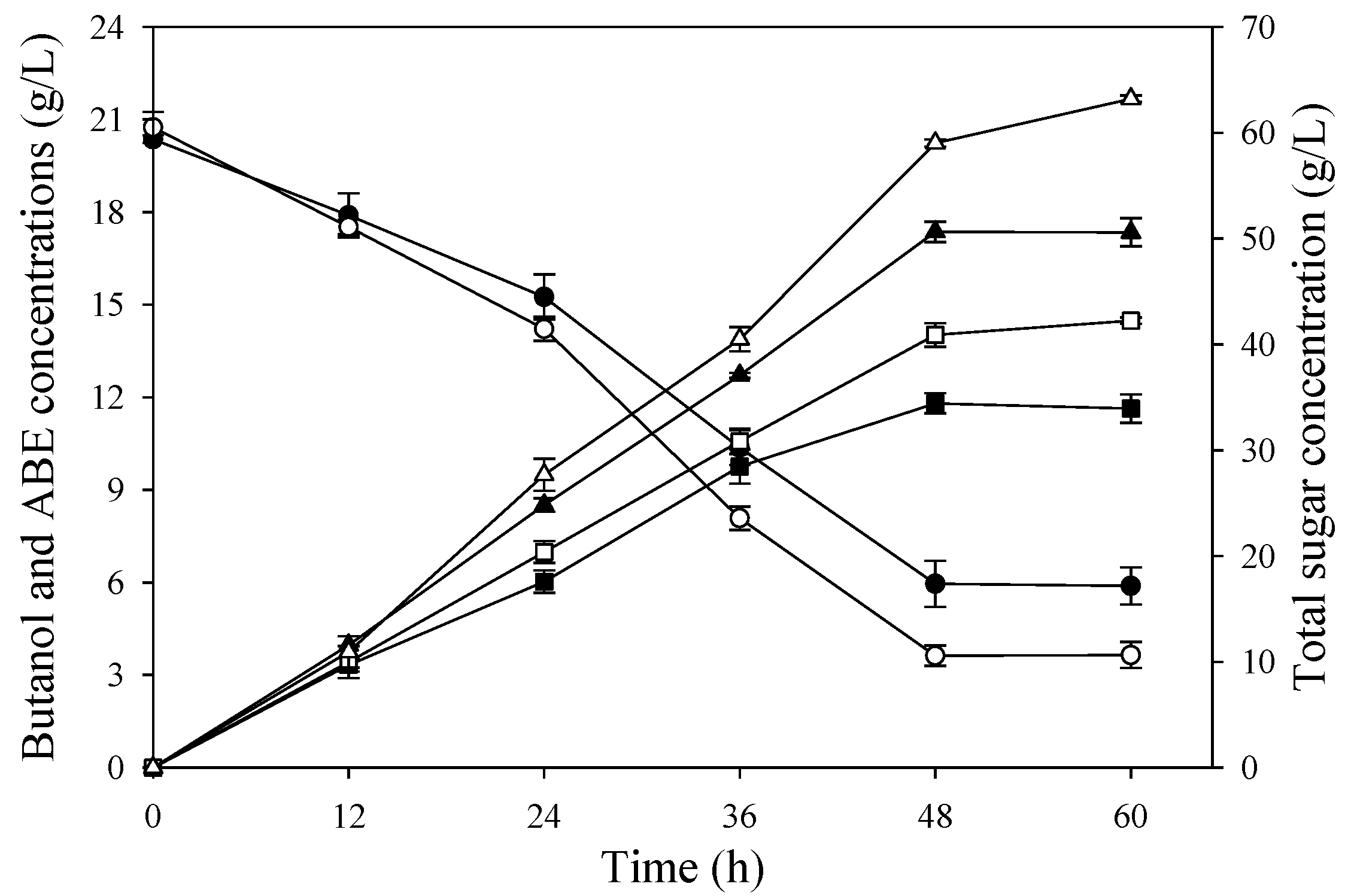
Figure 4.
Butanol fermentation profiles from SSJ medium supplemented with urea 4X (0.64 g/L) by free cells of C. beijerinckii TISTR 1461: acetone (♦), butanol (■), ethanol (×), ABE (▲), acetic acid (◊), butyric acid (□), total acid (Δ), pH (○), total sugars (●).
Figure 4.
Butanol fermentation profiles from SSJ medium supplemented with urea 4X (0.64 g/L) by free cells of C. beijerinckii TISTR 1461: acetone (♦), butanol (■), ethanol (×), ABE (▲), acetic acid (◊), butyric acid (□), total acid (Δ), pH (○), total sugars (●).
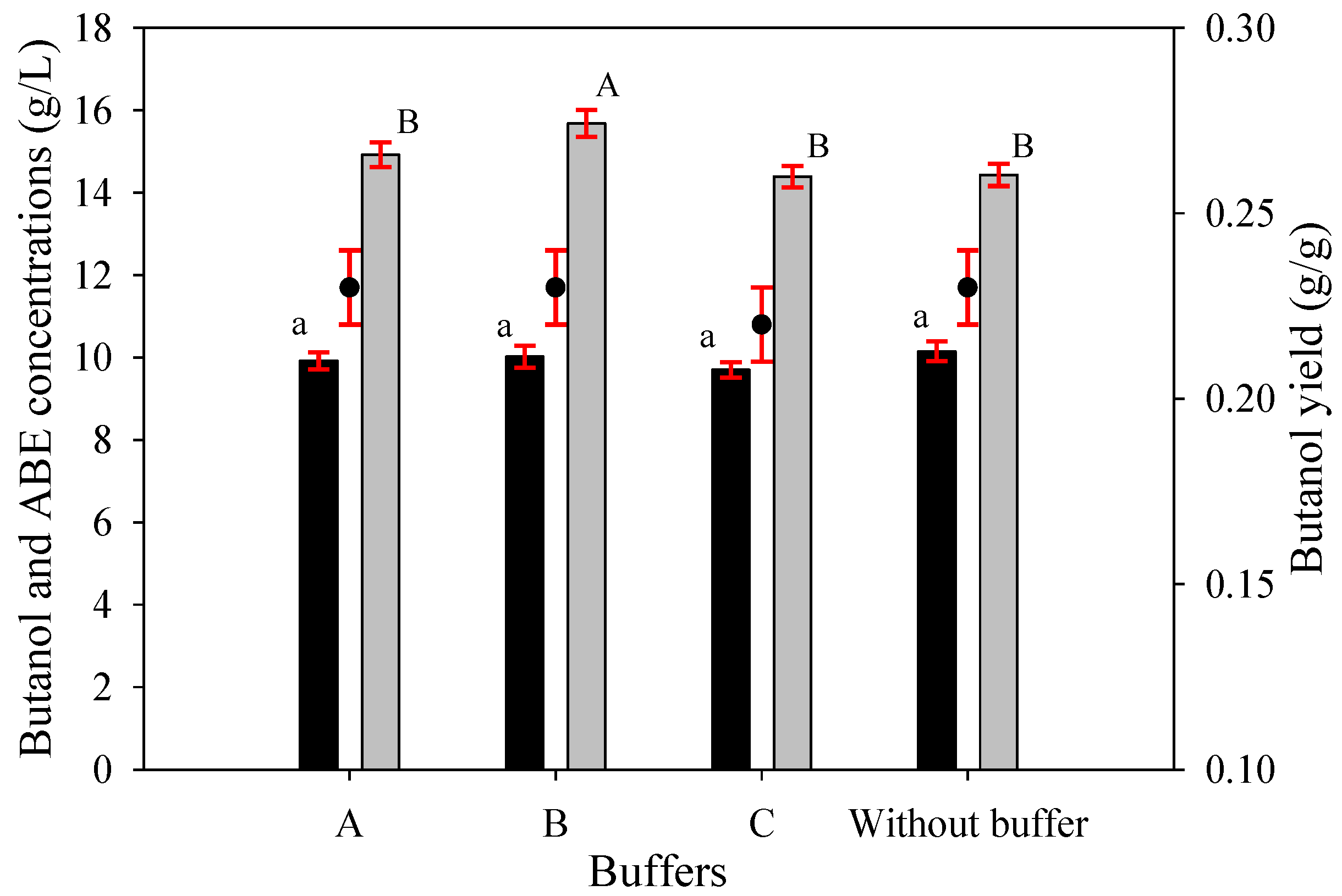
Figure 5.
Butanol concentration (black bars), ABE concentration (gray bars), and butanol yield (black dots) values of butanol fermentations from SSJ medium containing 0.64 g/L urea and various buffers with C. beijerinckii TISTR 1461 at 48 h. Buffer A: KH2PO4, K2HPO4, and ammonium acetate; buffer B: KH2PO4 and K2HPO4; buffer C: ammonium acetate and no buffer. The error bars represent the standard deviation of the mean values. Bars associated with the same letter have no significant differences assessed by Duncan’s multiple range test with a critical value of 0.05.
Figure 5.
Butanol concentration (black bars), ABE concentration (gray bars), and butanol yield (black dots) values of butanol fermentations from SSJ medium containing 0.64 g/L urea and various buffers with C. beijerinckii TISTR 1461 at 48 h. Buffer A: KH2PO4, K2HPO4, and ammonium acetate; buffer B: KH2PO4 and K2HPO4; buffer C: ammonium acetate and no buffer. The error bars represent the standard deviation of the mean values. Bars associated with the same letter have no significant differences assessed by Duncan’s multiple range test with a critical value of 0.05.
Figure 6.
The pH profiles during butanol fermentations from SSJ medium containing 0.64 g/L of urea and various buffers with C. beijerinckii TISTR 1461. Buffer A (○): KH2PO4, K2HPO4, and ammonium acetate; buffer B (□): KH2PO4 and K2HPO4; buffer C (∆): ammonium acetate; no buffer (◊).
Figure 6.
The pH profiles during butanol fermentations from SSJ medium containing 0.64 g/L of urea and various buffers with C. beijerinckii TISTR 1461. Buffer A (○): KH2PO4, K2HPO4, and ammonium acetate; buffer B (□): KH2PO4 and K2HPO4; buffer C (∆): ammonium acetate; no buffer (◊).
Figure 7.
Scanning electron microscope (SEM) images of cigarette filter tips (A) and bamboo chopsticks (B) at 100× magnification, as well as the physical characteristics of the cigarette filter tips (C) and bamboo chopstick pieces (D) before and after butanol fermentation in SSJ medium.
Figure 7.
Scanning electron microscope (SEM) images of cigarette filter tips (A) and bamboo chopsticks (B) at 100× magnification, as well as the physical characteristics of the cigarette filter tips (C) and bamboo chopstick pieces (D) before and after butanol fermentation in SSJ medium.
Figure 8.
Batch butanol fermentation profiles from SSJ medium supplemented with 0.64 g/L of urea and cocktail buffers with immobilized C. beijerinckii TISTR 1461 cells on cigarette filter tips (A) and bamboo chopstick pieces (B): acetone (♦), butanol (■), ethanol (×), ABE (▲), acetic acid (◊), butyric acid (□), total acid (Δ), pH (○), total sugars (●).
Figure 8.
Batch butanol fermentation profiles from SSJ medium supplemented with 0.64 g/L of urea and cocktail buffers with immobilized C. beijerinckii TISTR 1461 cells on cigarette filter tips (A) and bamboo chopstick pieces (B): acetone (♦), butanol (■), ethanol (×), ABE (▲), acetic acid (◊), butyric acid (□), total acid (Δ), pH (○), total sugars (●).
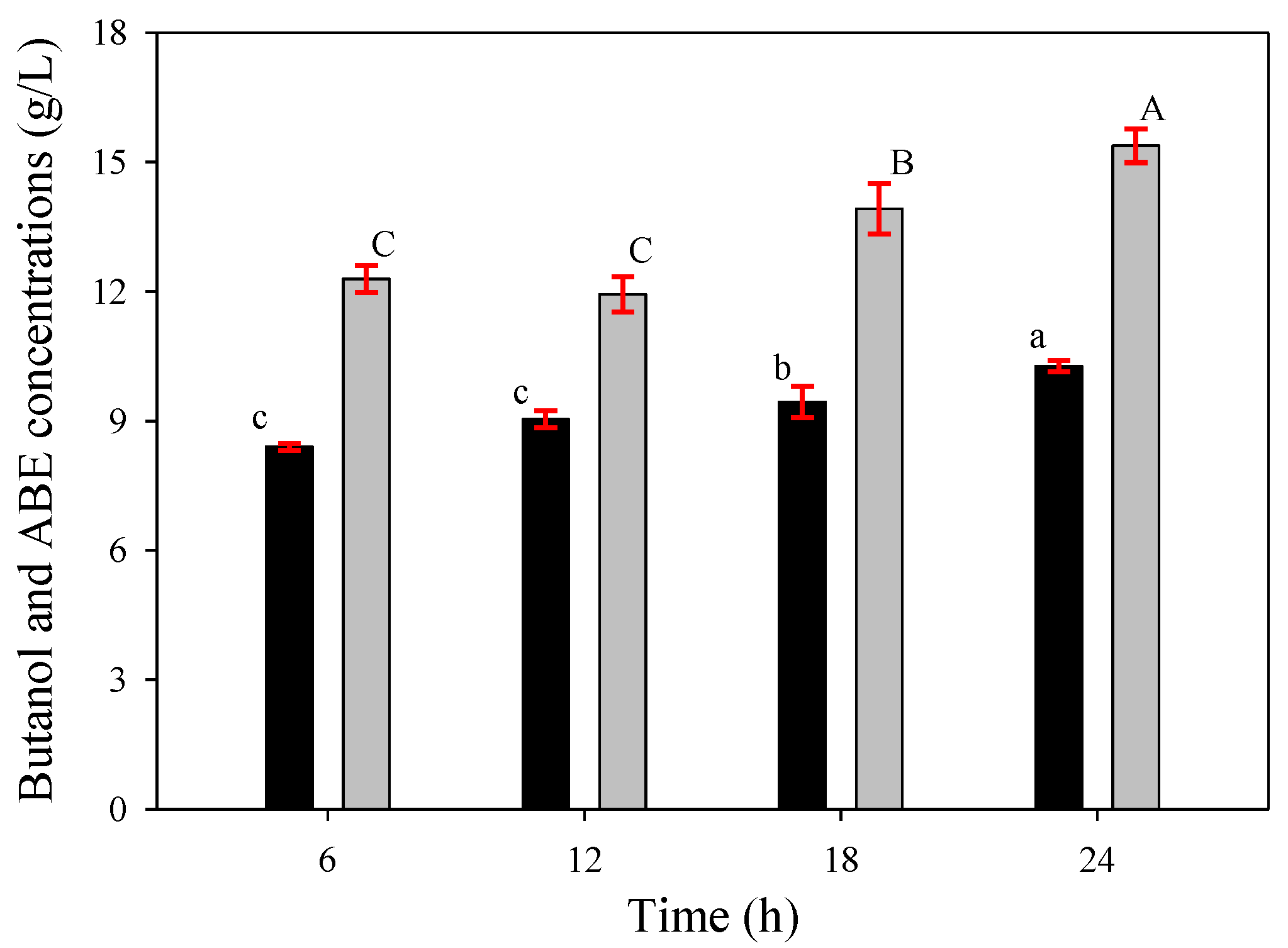
Figure 9.
Butanol (black bars) and ABE (gray bars) concentrations of butanol fermentation from SSJ by immobilized cells under various immobilization times. The error bars represent the standard deviation of the mean values. Bars associated with the same letter have no significant differences assessed by Duncan’s multiple range test with a critical value of 0.05.
Figure 9.
Butanol (black bars) and ABE (gray bars) concentrations of butanol fermentation from SSJ by immobilized cells under various immobilization times. The error bars represent the standard deviation of the mean values. Bars associated with the same letter have no significant differences assessed by Duncan’s multiple range test with a critical value of 0.05.
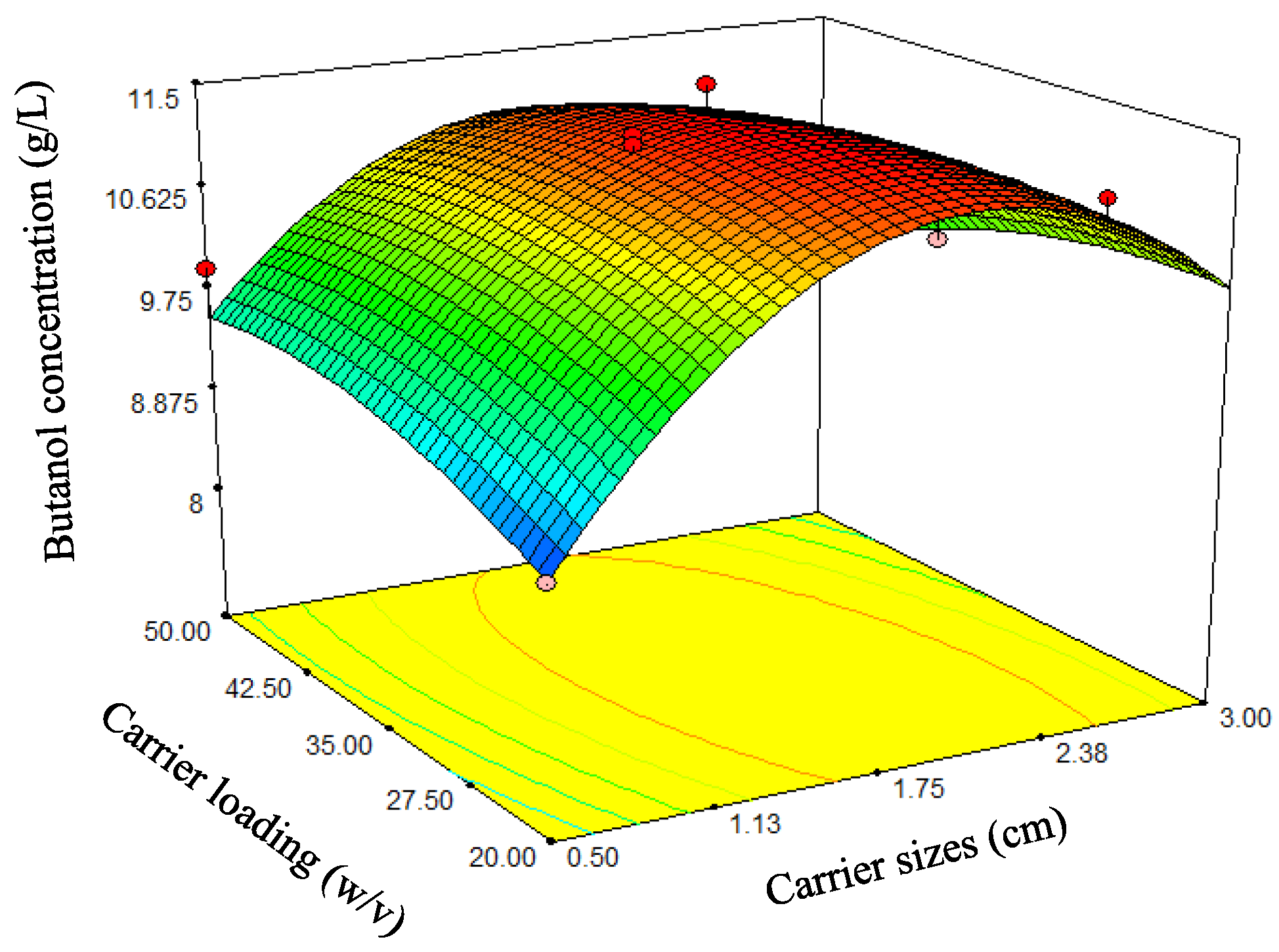
Figure 10.
Three-dimensional response surface plots for butanol production—effects of the carrier size and carrier loading.
Figure 10.
Three-dimensional response surface plots for butanol production—effects of the carrier size and carrier loading.
Figure 11.
Batch butanol fermentation profiles from the SSJ medium by immobilized C. beijerinckii TISTR 1461 cells on cigarette filter tips (carrier size, 1.9 cm and carrier loading, 1:32 (w/v)) in a 2 L STR: acetone (♦), butanol (■), ethanol (×), ABE (▲), acetic acid (◊), butyric acid (□), total acid (Δ), pH (○), total sugars (●).
Figure 11.
Batch butanol fermentation profiles from the SSJ medium by immobilized C. beijerinckii TISTR 1461 cells on cigarette filter tips (carrier size, 1.9 cm and carrier loading, 1:32 (w/v)) in a 2 L STR: acetone (♦), butanol (■), ethanol (×), ABE (▲), acetic acid (◊), butyric acid (□), total acid (Δ), pH (○), total sugars (●).
Figure 12.
Batch butanol fermentations from SSJ medium with immobilized C. beijerinckii TISTR 1461 with (○, □, ∆) and without (●, ■, ▲) a gas stripping system: total sugar (○, ●), butanol (□, ■) and ABE (∆, ▲) concentrations.
Figure 12.
Batch butanol fermentations from SSJ medium with immobilized C. beijerinckii TISTR 1461 with (○, □, ∆) and without (●, ■, ▲) a gas stripping system: total sugar (○, ●), butanol (□, ■) and ABE (∆, ▲) concentrations.
Table 1.
The composition of the sweet sorghum stem juice (cv. KKU 40).
Table 1.
The composition of the sweet sorghum stem juice (cv. KKU 40).
| Composition | Concentration |
|---|
| Total soluble solids (°Bx) | 18.0 |
| Sucrose (g/L) a | 71.32 |
| Glucose (g/L) a | 45.85 |
| Fructose (g/L) a | 51.42 |
| Total protein b,* (g/100 mL) | 0.82 |
| Total nitrogen * (g/L) | 1.31 |
| Sulfur c,* (mg/L) | 2.41 |
| Potassium d,* (mg/L) | 4800.50 |
| Phosphorus d,* (mg/L) | 380.40 |
| Calcium d,* (mg/L) | 888.95 |
| Magnesium d,* (mg/L) | 290.50 |
| Sodium d,* (mg/L) | 59.59 |
| Iron d,* (mg/L) | 0.351 |
| Manganese d,* (mg/L) | 0.167 |
| Zinc d,* (mg/L) | 0.078 |
| Copper d,* (mg/L) | 0.025 |
| Molybdenum d,* (mg/L) | 0.055 |
| Nickel d,* (mg/L) | 0.007 |
| Boron d,* (mg/L) | 0.060 |
Table 2.
The compositions of the dried spent yeast (DSY) and yeast extract (YE).
Table 2.
The compositions of the dried spent yeast (DSY) and yeast extract (YE).
| Composition a | Concentration (%, Dry Weight) |
|---|
| DSY | YE |
|---|
| Total carbohydrate | 17.25 | 26.86 |
| Protein | 65.84 | 46.11 |
| Total fat | 1.79 | 3.27 |
| Crude fiber | 0.06 | 0.02 |
| Ash | 6.01 | 13.24 |
| Moisture | 9.11 | 10.52 |
Table 3.
Experimental design of a 5-level and 2-variable central composition design.
Table 3.
Experimental design of a 5-level and 2-variable central composition design.
| Run | Coded Value | Actual Value |
|---|
| x1 | x2 | x1 | x2 |
|---|
| 1 | +1 | +1 | 2.50 | 1:50 |
| 2 | 0 | 0 | 1.50 | 1:35 |
| 3 | −1 | +1 | 0.50 | 1:50 |
| 4 | +α | 0 | 2.70 | 1:35 |
| 5 | 0 | 0 | 1.50 | 1:35 |
| 6 | −1 | −1 | 0.50 | 1:20 |
| 7 | 0 | +α | 1.50 | 1:53 |
| 8 | +1 | −1 | 2.50 | 1:20 |
| 9 | 0 | 0 | 1.50 | 1:35 |
| 10 | 0 | 0 | 1.50 | 1:35 |
| 11 | 0 | −α | 1.50 | 1:17 |
| 12 | −α | 0 | 0.30 | 1:35 |
| 13 | 0 | 0 | 1.50 | 1:35 |
Table 4.
Batch butanol fermentation efficiency profiles for the P2 medium and SSJ medium containing various nitrogen sources.
Table 4.
Batch butanol fermentation efficiency profiles for the P2 medium and SSJ medium containing various nitrogen sources.
| Medium | Nitrogen Supplement | Product (g/L) | SC (%) | t (h) | YB/S (g/g) | QB (g/L·h) |
|---|
| Acetone | Butanol | Ethanol | ABE | Total Acids |
|---|
| P2 | YE | 4.96 ± 0.17 a | 11.06 ± 0.24 a | 0.37 ± 0.04 a | 16.39 ± 0.45 a | 1.96 ± 0.21 a,b | 74.46 ± 1.11 a | 48 | 0.25 ± 0.01 a | 0.23 ± 0.01 a |
| SSJ | - | 2.36 ± 0.20 d,e | 4.19 ± 0.18 e | 0.32 ± 0.03 a,b | 6.82 ± 0.21 d | 2.13 ± 0.18 b | 31.45 ± 1.11 c,d | 36 | 0.22 ± 0.01 c | 0.08 ± 0.01 d |
| DSY | 3.01 ± 0.16 b | 6.60 ± 0.22 b | 0.35 ± 0.04 a | 9.96 ± 0.42 b | 2.46 ± 0.22 a | 37.02 ± 1.84 b | 48 | 0.23 ± 0.01 b,c | 0.14 ± 0.01 b |
| Urea | 2.63 ± 0.16 c | 6.91 ± 0.24 b | 0.31 ± 0.04 a | 9.86 ± 0.36 b | 1.94 ± 0.16 a,b | 39.65 ± 1.89 b | 48 | 0.24 ± 0.01 a,b | 0.14 ± 0.01 b |
| Ammonium acetate | 2.09 ± 0.13 e | 4.84 ± 0.18 d | 0.27 ± 0.05 b | 7.20 ± 0.26 d | 1.63 ± 0.11 c | 28.82 ± 1.56 d | 48 | 0.22 ± 0.01 c | 0.10 ± 0.01 c |
| Ammonium sulfate | 2.48 ± 0.19 c | 5.43 ± 0.15 c | 0.35 ± 0.02 a | 8.23 ± 0.23 c | 1.92 ± 0.19 a,b | 32.97 ± 1.42 c | 48 | 0.25 ± 0.01 a | 0.15 ± 0.01 b |
Table 5.
Batch butanol fermentation efficiency levels from SSJ medium supplemented with urea at various concentrations at 48 h of fermentation.
Table 5.
Batch butanol fermentation efficiency levels from SSJ medium supplemented with urea at various concentrations at 48 h of fermentation.
Urea
Concentration | Product (g/L) | SC (%) | YB/S (g/g) | QB (g/L·h) |
|---|
| Acetone | Butanol | Ethanol | ABE | Total Acids |
| 0.5X | 1.84 ± 0.17 e | 5.42 ± 0.20 e | 0.36 ± 0.03 a | 7.65 ± 0.31 f | 1.90 ± 0.18 d | 41.00 ± 1.46 e | 0.22 ± 0.01 a | 0.17 ± 0.01 c |
| 1X | 2.63 ± 0.15 d | 6.91 ± 0.24 d | 0.31 ± 0.04 a | 9.86 ± 0.35 e | 1.94 ± 0.16 d | 39.65 ± 1.89 e | 0.24 ± 0.01 a | 0.14 ± 0.01 d |
| 2X | 3.24 ± 0.17 c | 8.27 ± 0.17 c | 0.30 ± 0.05 a,b | 11.15 ± 0.23 d | 2.95 ± 0.21 b | 59.89 ± 1.37 d | 0.22 ± 0.01 a | 0.14 ± 0.01 d |
| 3X | 4.01 ± 0.19 b | 9.47 ± 0.23 b | 0.33 ± 0.05 a | 13.82 ± 0.26 b | 3.95 ± 0.15 a | 68.69 ± 1.68 b | 0.23 ± 0.01 a | 0.20 ± 0.01 a |
| 4X | 3.98 ± 0.18 b | 10.15 ± 0.24 a | 0.29 ± 0.04 a,b | 14.43 ± 0.27 a | 2.47 ± 0.23 c | 72.31 ± 1.42 a | 0.23 ± 0.01 a | 0.21 ± 0.01 a |
| 5X | 4.44 ± 0.13 a | 8.47 ± 0.19 c | 0.24 ± 0.03 b | 13.16 ± 0.23 c | 2.36 ± 0.25 c | 63.63 ± 1.56 c | 0.22 ± 0.01 a | 0.18 ± 0.01 b |
Table 6.
Batch butanol fermentation efficiency levels with immobilized C. beijerinckii TISTR 1461 on cigarette filter tips and bamboo chopstick pieces at 48 h of fermentation.
Table 6.
Batch butanol fermentation efficiency levels with immobilized C. beijerinckii TISTR 1461 on cigarette filter tips and bamboo chopstick pieces at 48 h of fermentation.
| Carrier | Product (g/L) | SC (%) | YB/S (g/g) | QB (g/L·h) |
|---|
| Acetone | Butanol | Ethanol | ABE | Total Acids |
|---|
| Cigarette filter tips | 2.93 ± 0.17 a | 8.18 ± 0.19 b | 0.28 ± 0.03 a | 11.39 ± 0.28 b | 5.00 ± 0.16 b | 60.09 ± 1.32 a | 0.22 ± 0.01 a | 0.17 ± 0.00 b |
| Bamboo chopstick pieces | 3.13 ± 0.16 a | 9.20 ± 0.08 a | 0.29 ± 0.03 a | 12.62 ± 0.27 a | 5.80 ± 0.12 a | 61.59 ± 1.01 a | 0.24 ± 0.01 a | 0.19 ± 0.00 a |
Table 7.
Butanol fermentation efficiencies from SSJ medium by immobilized C. beijerinckii TISTR 1461 using different immobilization media at 48 h of fermentation.
Table 7.
Butanol fermentation efficiencies from SSJ medium by immobilized C. beijerinckii TISTR 1461 using different immobilization media at 48 h of fermentation.
| Immobilization Medium | Product (g/L) | SC (%) | YB/S (g/g) | QB (g/L·h) |
|---|
| Acetone | Butanol | Ethanol | ABE | Total Acids |
|---|
| SSJ supplemented with urea | 2.62 ± 0.17 b | 9.04 ± 0.19 a | 0.26 ± 0.05 a | 11.93 ± 0.41 b | 3.21 ± 0.19 b | 60.30 ± 1.25 a | 0.24 ± 0.01 a | 0.18 ± 0.01 a |
| SSJ supplemented with urea and buffers | 3.13 ± 0.16 a | 9.20 ± 0.08 a | 0.29 ± 0.03 a | 12.62 ± 0.27 a | 5.80 ± 0.04 a | 61.59 ± 1.01 a | 0.24 ± 0.01 a | 0.19 ± 0.00 a |
| TGY | 2.34 ± 0.20 b | 7.53 ± 0.16 b | 0.27 ± 0.02 a | 10.14 ± 0.38 c | 2.83 ± 0.27 b | 52.02 ± 1.72 b | 0.24 ± 0.01 a | 0.16 ± 0.01 b |
Table 8.
Butanol fermentation efficiency levels at 48 h with the immobilized cells under different immobilization times.
Table 8.
Butanol fermentation efficiency levels at 48 h with the immobilized cells under different immobilization times.
| Immobilization Time (h) | Products (g/L) | SC (%) | YB/S (g/g) | QB (g/L·h) |
|---|
| Acetone | Butanol | Ethanol | ABE | Total Acids |
|---|
| 6 | 3.61 ± 0.21 c | 8.40 ± 0.08 c | 0.28 ± 0.02 a | 12.29 ± 0.31 c | 1.80 ± 0.45 b | 61.05 ± 1.62 c | 0.23 ± 0.00 a | 0.18 ± 0.01 c |
| 12 | 2.62 ± 0.17 d | 9.04 ± 0.19 b | 0.26 ± 0.05 a | 11.93 ± 0.41 c | 3.21 ± 0.19 a | 60.30 ± 1.25 c | 0.24 ± 0.01 a | 0.19 ± 0.01 b,c |
| 18 | 4.24 ± 0.20 b | 9.44 ± 0.37 b | 0.28 ± 0.01 a | 13.92 ± 0.58 b | 1.88 ± 0.88 b | 66.03 ± 4.60 b | 0.24 ± 0.01 a | 0.20 ± 0.01 a,b |
| 24 | 4.81 ± 0.08 a | 10.27 ± 0.13 a | 0.30 ± 0.02 a | 15.38 ± 0.39 a | 3.03 ± 0.44 a | 73.84 ± 2.85 a | 0.23 ± 0.01 a | 0.21 ± 0.00 a |
Table 9.
Response values (butanol concentration) of 13 experimental runs.
Table 9.
Response values (butanol concentration) of 13 experimental runs.
| Run | Actual Value | Response (Butanol Concentration, PB) |
|---|
| x1 | x2 | Experimental Value (g/L) | Predicted Value (g/L) |
|---|
| 1 | 2.50 | 1:50 | 11.01 ± 0.13 | 10.40 |
| 2 | 1.50 | 1:35 | 11.28 ± 0.35 | 11.20 |
| 3 | 0.50 | 1:50 | 9.94 ± 0.49 | 9.46 |
| 4 | 2.70 | 1:35 | 10.18 ± 0.32 | 10.73 |
| 5 | 1.50 | 1:35 | 11.28 ± 0.35 | 11.20 |
| 6 | 0.50 | 1:20 | 8.89 ± 0.40 | 8.89 |
| 7 | 1.50 | 1:53 | 10.06 ± 0.27 | 10.76 |
| 8 | 2.50 | 1:20 | 11.18 ± 0.46 | 11.03 |
| 9 | 1.50 | 1:35 | 11.28 ± 0.28 | 11.20 |
| 10 | 1.50 | 1:35 | 11.36 ± 0.27 | 11.20 |
| 11 | 1.50 | 1:17 | 10.75 ± 0.33 | 10.80 |
| 12 | 0.30 | 1:35 | 8.65 ± 0.15 | 8.88 |
| 13 | 1.50 | 1:35 | 11.36 ± 0.27 | 11.20 |
Table 10.
The analysis of variance (ANOVA) for the parameters of the RSM based on a CCD fitted to a quadratic response surface model.
Table 10.
The analysis of variance (ANOVA) for the parameters of the RSM based on a CCD fitted to a quadratic response surface model.
| Sources | Sum Squares | Df | Mean Square | F-Value
| p-Value
|
|---|
| Model | 9.13 | 5 | 1.83 | 8.26 | 0.0075 |
| x1 | 3.92 | 1 | 3.92 | 17.76 | 0.0040 |
| x2 | 0.0004 | 1 | 0.0004 | 0.0018 | 0.9675 |
| x1x2 | 0.37 | 1 | 0.37 | 1.68 | 0.2355 |
| x12 | 4.24 | 1 | 4.24 | 19.17 | 0.0032 |
| x22 | 0.36 | 1 | 0.36 | 1.65 | 0.2400 |
| Lack of fit | 1.54 | 3 | 0.51 | 267.23 | <0.0001 |
| Residual | 1.55 | 7 | 0.22 | | |
| Pure error | 0.0077 | 4 | 0.0019 | | |
| Total | 10.67 | 12 | | | |
| R-squared = 0.8551 | | | | |
Table 11.
Comparison of batch butanol fermentation efficiency levels with immobilized Clostridium cells.
Table 11.
Comparison of batch butanol fermentation efficiency levels with immobilized Clostridium cells.
| Substrate | Strain | Carrier | PB (g/L) | Improved PB Compared with Free Cells (%) | YB/S
(g/g) | QB
(g/L·h) | Reference |
|---|
| Glucose (60 g/L) | C. acetobutylicum ABE 1201 | Sweet sorghum bagasse | 14.02 | 17.62 | 0.25 | 0.22 | [40] |
| Glucose (60 g/L) | C. acetobutylicum XY 16 | Sugarcane bagasse | 12.91 | 19.31 | 0.21 | 0.13 | [52] |
| Glucose (70 g/L) | C. acetobutylicum ABE 1201 | Corn stalk bagasse | 14.28 | 2.29 | 0.23 | 0.25 | [53] |
SSJ
(60 g/L) | C. beijerinckii TISTR 1461 | Bamboo chopstick pieces | 11.62 | 14.71 | 0.26 | 0.24 | This study |
Table 12.
Batch butanol fermentation efficiency levels from SSJ medium with immobilized C. beijerinckii TISTR 1461 in 2 L STRs with and without a gas stripping system (GS) at 48 h of fermentation.
Table 12.
Batch butanol fermentation efficiency levels from SSJ medium with immobilized C. beijerinckii TISTR 1461 in 2 L STRs with and without a gas stripping system (GS) at 48 h of fermentation.
| System | PB (g/L) | SC (%) | YB/S (g/g) | QB (g/L·h) |
|---|
| No GS | 11.62 ± 0.15 b | 70.75 ± 3.50 b | 0.26 ± 0.01 a | 0.24 ± 0.01 b |
| With GS | 14.02 ± 0.19 a | 82.53 ± 1.18 a | 0.27 ± 0.01 a | 0.29 ± 0.00 a |