Conversion of Syngas from Entrained Flow Gasification of Biogenic Residues with Clostridium carboxidivorans and Clostridium autoethanogenum
Abstract
1. Introduction
| ΔG0 = −154.6 kJ mol−1 | |
| ΔG0 = −217.4 kJ mol−1 | |
| ΔG0 = −420.8 kJ mol−1 | |
| ΔG0 = −486.4 kJ mol−1 | |
| ΔG0 = −656.0 kJ mol−1 | |
| ΔG0 = −753.0 kJ mol−1 | |
| ΔG0 = −361.8 kJ mol−1 | |
| ΔG0 = −74.3 kJ mol−1 | |
| ΔG0 = −97.0 kJ mol−1 | |
| ΔG0 = −220.2 kJ mol−1 | |
| ΔG0 = −245.6 kJ mol−1 | |
| ΔG0 = −341.0 kJ mol−1 | |
| ΔG0 = −395.0 kJ mol−1 | |
| ΔG0 = −140.7 kJ mol−1 | |
| ΔG0 = −114.5 kJ mol−1 | |
| ΔG0 = −137.1 kJ mol−1 | |
| ΔG0 = −317.0 kJ mol−1 | |
| ΔG0 = −334.0 kJ mol−1 | |
| ΔG0 = −540.0 kJ mol−1 | |
| ΔG0 = −514.0 kJ mol−1 | |
| ΔG0 = −221.1 kJ mol−1 |
2. Materials and Methods
2.1. Microorganisms, Media, and Preculture Conditions
2.2. Conditions for Batch Processes in Stirred-Tank Bioreactors
2.3. Syngas Production and Purification
2.3.1. Biomass Gasification-BOOSTER Test Rig
2.3.2. Syngas Cleaning Test Rig
2.3.3. Catalytic Oxygen Reduction in a Tube Reactor
2.4. Analytical Methods
2.4.1. Liquid Product Analysis
2.4.2. Synthesis Gas Analysis
3. Results
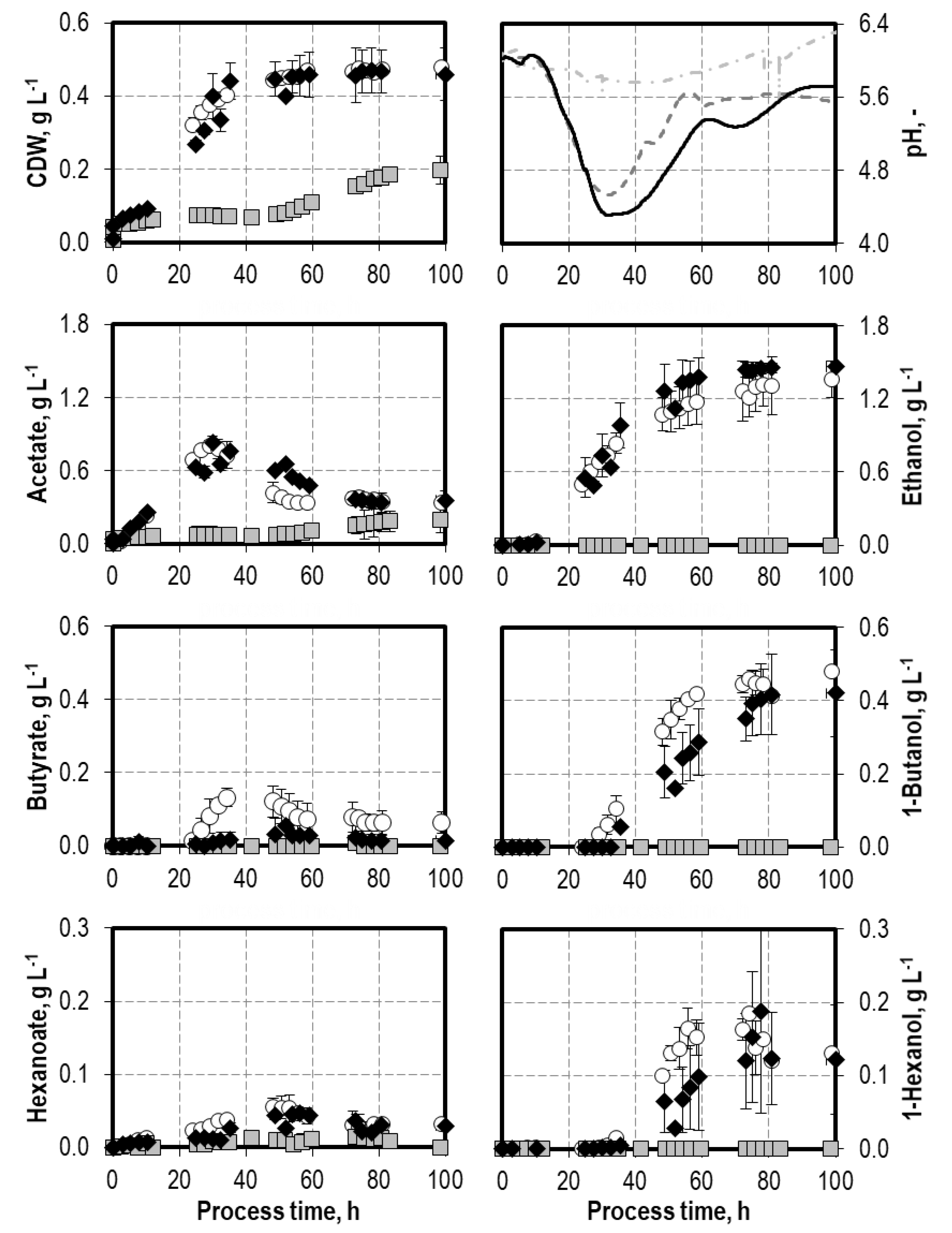
3.1. Batch Process Performances of C. carboxidivorans with Artificial and Biogenic Syngas
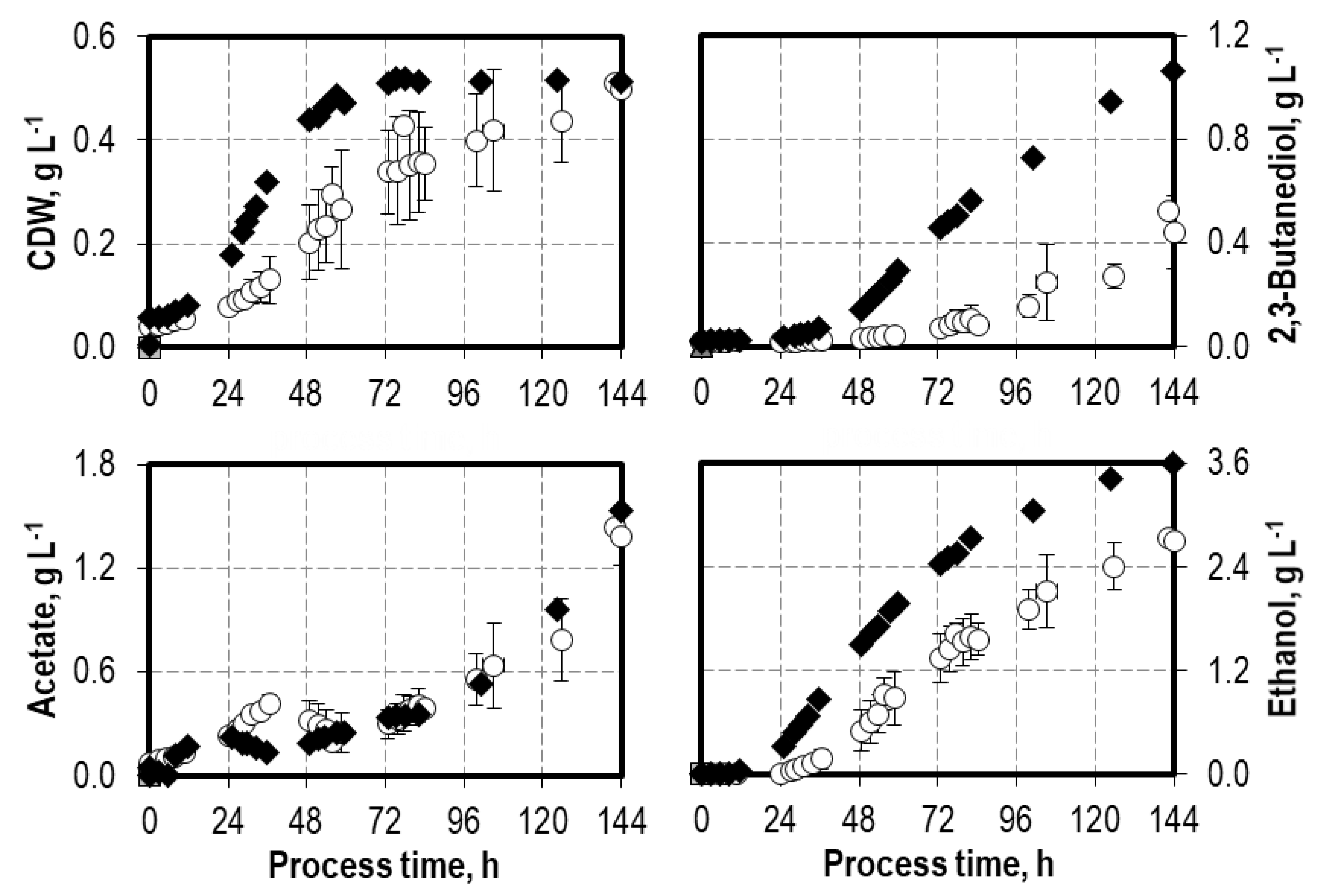
3.2. Batch Process Performances of C. autoethanogenum with Artificial and Biogenic Syngas
4. Discussion
4.1. Influence of Oxygen Impurities on Syngas Fermentation Processes with C. carboxidivorans
4.2. Comparison to Other Syngas Fermentation Processes with Pure and Biogenic Syngas
4.3. Challenges and Opportunities on High Syngas Conversion in Industrial Scale Bioreactors
4.4. Optimizing the Composition of Syngases from Gasification of Biomass
4.5. Outlook on Future Syngas Fermentation Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kremling, M.; Briesemeister, L.; Gaderer, M.; Fendt, S.; Spliethoff, H. Oxygen-blown entrained flow gasification of biomass: Impact of fuel parameters and oxygen stoichiometric ratio. Energy Fuels 2017, 31, 3949–3959. [Google Scholar] [CrossRef]
- Kremling, M.B. Experimentelle Untersuchungen zur Sauerstoffgeblasenen Flugstromvergasung von Staubförmiger Biomasse. Ph.D. Thesis, Technical University of Munich, Verlag Dr. Hut. Munich, Germany, 2018. [Google Scholar]
- Xu, D.; Tree, D.R.; Lewis, R.S. The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass Bioenergy 2011, 35, 2690–2696. [Google Scholar] [CrossRef]
- Infantes, A.; Kugel, M.; Raffelt, K.; Neumann, A. Side-by-side Comparison of clean and biomass-derived, impurity-containing syngas as substrate for acetogenic fermentation with Clostridium ljungdahlii. Fermentation 2020, 6, 84. [Google Scholar] [CrossRef]
- Ahmed, A.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Effects of biomass-generated producer gas constituents on cell growth, product distribution and hydrogenase activity of Clostridium carboxidivorans P7T. Biomass Bioenergy 2006, 30, 665–672. [Google Scholar] [CrossRef]
- Liakakou, E.T.; Infantes, A.; Neumann, A.; Vreugdenhil, B.J. Connecting gasification with syngas fermentation: Comparison of the performance of lignin and beech wood. Fuel 2021, 290, 120054. [Google Scholar] [CrossRef]
- Benevenuti, C.; Amaral, P.; Ferreira, T.; Seidl, P. Impacts of syngas composition on anaerobic fermentation. Reactions 2021, 2, 391–407. [Google Scholar] [CrossRef]
- Broer, K.M.; Brown, R.C. Effect of equivalence ratio on partitioning of nitrogen during biomass gasification. Energy Fuels 2016, 30, 407–413. [Google Scholar] [CrossRef]
- Schneider, J.; Grube, C.; Herrmann, A.; Rönsch, S. Atmospheric entrained-flow gasification of biomass and lignite for decentralized applications. Fuel Process. Technol. 2016, 152, 72–82. [Google Scholar] [CrossRef]
- Briesemeister, L.; Kremling, M.; Fendt, S.; Spliethoff, H. Air-blown entrained-flow gasification of biomass: Influence of operating conditions on tar generation. Energy Fuels 2017, 31, 10924–10932. [Google Scholar] [CrossRef]
- Dürre, P. Biobutanol: An attractive biofuel. Biotechnol. J. 2007, 2, 1525–1534. [Google Scholar] [CrossRef]
- Köpke, M.; Mihalcea, C.; Bromley, J.C.; Simpson, S.D. Fermentative production of ethanol from carbon monoxide. Curr. Opin. Biotechnol. 2011, 22, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.E.; Nogle, R.; Abdalla, T.; Rasor, B.J.; Canter, C.; Jensen, R.O.; Wang, L.; Strutz, J.; Chirania, P.; de Tissera, S.; et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nat. Biotechnol. 2022, 40, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation. Biochim. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed]
- Doll, K.; Rückel, A.; Kämpf, P.; Wende, M.; Weuster-Botz, D. Two stirred-tank bioreactors in series enable continuous production of alcohols from carbon monoxide with Clostridium carboxidivorans. Bioprocess Biosyst. Eng. 2018, 41, 1403–1416. [Google Scholar] [CrossRef]
- Liou, J.S.-C.; Balkwill, D.L.; Drake, G.R.; Tanner, R.S. Clostridium carboxidivorans sp. nov., a solvent-producing Clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 2085–2091. [Google Scholar] [CrossRef]
- Rückel, A.; Hannemann, J.; Maierhofer, C.; Fuchs, A.; Weuster-Botz, D. Studies on syngas fermentation with Clostridium carboxidivorans in stirred-tank reactors with defined gas impurities. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Hurst, K.M.; Lewis, R.S. Carbon monoxide partial pressure effects on the metabolic process of syngas fermentation. Biochem. Eng. J. 2010, 48, 159–165. [Google Scholar] [CrossRef]
- Abrini, J.; Naveau, H.; Nyns, E.-J. Clostridium autoethanogenum, sp. nov., an anaerobic bacterium that produces ethanol from carbon monoxide. Arch. Microbiol. 1994, 161, 345–351. [Google Scholar] [CrossRef]
- Oliveira, L.; Rückel, A.; Nordgauer, L.; Schlumprecht, P.; Hutter, E.; Weuster-Botz, D. Comparison of syngas-fermenting Clostridia in stirred-tank bioreactors and the effects of syngas impurities. Microorganisms 2022, 10, 681. [Google Scholar] [CrossRef]
- Köpke, M.; Mihalcea, C.; Liew, F.; Tizard, J.H.; Ali, M.S.; Conolly, J.J.; Al-Sinawi, B.; Simpson, S.D. 2,3-butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl. Environ. Microbiol. 2011, 77, 5467–5475. [Google Scholar] [CrossRef] [PubMed]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Carbon monoxide fermentation to ethanol by Clostridium autoethanogenum in a bioreactor with no accumulation of acetic acid. Bioresour. Technol. 2015, 186, 122–127. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Biological conversion of carbon monoxide: Rich syngas or waste gases to bioethanol. Biofuels Bioprod. Bioref. 2011, 5, 93–114. [Google Scholar] [CrossRef]
- Li, N.; Yang, J.; Chai, C.; Yang, S.; Jiang, W.; Gu, Y. Complete genome sequence of Clostridium carboxidivorans P7(T), a syngas-fermenting bacterium capable of producing long-chain alcohols. J. Biotechnol. 2015, 211, 44–45. [Google Scholar] [CrossRef]
- Humphreys, C.M.; McLean, S.; Schatschneider, S.; Millat, T.; Henstra, A.M.; Annan, F.J.; Breitkopf, R.; Pander, B.; Piatek, P.; Rowe, P.; et al. Whole genome sequence and manual annotation of Clostridium autoethanogenum, an industrially relevant bacterium. BMC Genom. 2015, 16, 1085. [Google Scholar] [CrossRef] [PubMed]
- Bruant, G.; Lévesque, M.-J.; Peter, C.; Guiot, S.R.; Masson, L. Genomic analysis of carbon monoxide utilization and butanol production by Clostridium carboxidivorans strain P7. PLoS ONE 2010, 5, e13033. [Google Scholar] [CrossRef]
- Fernández-Naveira, Á.; Veiga, M.C.; Kennes, C. H-B-E (hexanol-butanol-ethanol) fermentation for the production of higher alcohols from syngas/waste gas. J. Chem. Technol. Biotechnol. 2017, 92, 712–731. [Google Scholar] [CrossRef]
- Ganigué, R.; Sánchez-Paredes, P.; Bañeras, L.; Colprim, J. Low fermentation pH is a trigger to alcohol production, but a killer to chain elongation. Front. Microbiol. 2016, 7, 702. [Google Scholar] [CrossRef]
- Ramió-Pujol, S.; Ganigué, R.; Bañeras, L.; Colprim, J. How can alcohol production be improved in carboxydotrophic Clostridia? Process Biochem. 2015, 50, 1047–1055. [Google Scholar] [CrossRef]
- Ukpong, M.N.; Atiyeh, H.K.; de Lorme, M.J.M.; Liu, K.; Zhu, X.; Tanner, R.S.; Wilkins, M.R.; Stevenson, B.S. Physiological response of Clostridium carboxidivorans during conversion of synthesis gas to solvents in a gas-fed bioreactor. Biotechnol. Bioeng. 2012, 109, 2720–2728. [Google Scholar] [CrossRef]
- Liew, F.; Henstra, A.M.; Köpke, M.; Winzer, K.; Simpson, S.D.; Minton, N.P. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metab. Eng. 2017, 40, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Bao, T.; Yang, S.-T. Engineering Clostridium for improved solvent production: Recent progress and perspective. Appl. Microbiol. Biotechnol. 2019, 103, 5549–5566. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781315380476. [Google Scholar]
- Xu, D.; Lewis, R.S. Syngas fermentation to biofuels: Effects of ammonia impurity in raw syngas on hydrogenase activity. Biomass Bioenergy 2012, 45, 303–310. [Google Scholar] [CrossRef]
- Datar, R.P.; Shenkman, R.M.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Fermentation of biomass-generated producer gas to ethanol. Biotechnol. Bioeng. 2004, 86, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Hirota, A.; Hirayama, K.; Shin, T.; Murao, S. Properties of Ascorbate Oxidase Produced by Acremonium sp. HI-25. Biosci. Biotechnol. Biochem. 1995, 59, 1052–1056. [Google Scholar] [CrossRef][Green Version]
- Picton, R.; Eggo, M.C.; Merrill, G.A.; Langman, M.J.S.; Singh, S. Mucosal protection against sulphide: Importance of the enzyme rhodanese. Gut 2002, 50, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Allais, J.J.; Louktibi, A.; Baratti, J. Oxidation of Methanol by the Yeast Pichia pastoris. Purification and Properties of the Formate Dehydrogenase. Agric. Biol. Chem. 1983, 47, 2547–2554. [Google Scholar] [CrossRef]
- Meakin, G.E.; Jepson, B.J.N.; Richardson, D.J.; Bedmar, E.J.; Delgado, M.J. The role of Bradyrhizobium japonicum nitric oxide reductase in nitric oxide detoxification in soya bean root nodules. Biochem. Soc. Trans. 2006, 34, 195–196. [Google Scholar] [CrossRef]
- Emerson, D.F.; Woolston, B.M.; Liu, N.; Donnelly, M.; Currie, D.H.; Stephanopoulos, G. Enhancing hydrogen-dependent growth of and carbon dioxide fixation by Clostridium ljungdahlii through nitrate supplementation. Biotechnol. Bioeng. 2019, 116, 294–306. [Google Scholar] [CrossRef]
- Fröstl, J.M.; Seifritz, C.; Drake, H.L. Effect of nitrate on the autotrophic metabolism of the acetogens Clostridium thermoautotrophicum and Clostridium thermoaceticum. J. Bacteriol. 1996, 178, 4597–4603. [Google Scholar] [CrossRef]
- Seifritz, C.; Drake, H.L.; Daniel, S.L. Nitrite as an energy-conserving electron sink for the acetogenic bacterium Moorella thermoacetica. Curr. Microbiol. 2003, 46, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Oswald, F.; Zwick, M.; Omar, O.; Hotz, E.N.; Neumann, A. Growth and Product Formation of Clostridium ljungdahlii in Presence of Cyanide. Front. Microbiol. 2018, 9, 1213. [Google Scholar] [CrossRef] [PubMed]
- Hyman, M.R.; Ensign, S.A.; Arp, D.J.; Ludden, P.W. Carbonyl sulfide inhibition of CO dehydrogenase from Rhodospirillum rubrum. Biochemistry 1989, 28, 6821–6826. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Aufurth, S.; Rahlfs, S. The Na+ cycle in Acetobacterium woodii: Identification and characterization of a Na+ translocating F1F0-ATPase with a mixed oligomer of 8 and 16 kDa proteolipids. Biochim. Biophys. Acta (BBA) Bioenerg. 2001, 1505, 108–120. [Google Scholar] [CrossRef]
- Maden, B.E.H. Tetrahydrofolate and tetrahydromethanopterin compared: Functionally distinct carriers in C1 metabolism. Biochem. J. 2000, 350, 609–629. [Google Scholar] [CrossRef]
- Vanoni, M.A.; Matthews, R.G. Kinetic isotope effects on the oxidation of reduced nicotinamide adenine dinucleotide phosphate by the flavoprotein methylenetetrahydrofolate reductase. Biochemistry 1984, 23, 5272–5279. [Google Scholar] [CrossRef]
- Liu, C.-G.; Xue, C.; Lin, Y.-H.; Bai, F.-W. Redox potential control and applications in microaerobic and anaerobic fermentations. Biotechnol. Adv. 2013, 31, 257–265. [Google Scholar] [CrossRef]
- Karnholz, A.; Küsel, K.; Gössner, A.; Schramm, A.; Drake, H.L. Tolerance and metabolic response of acetogenic bacteria toward oxygen. Appl. Environ. Microbiol. 2002, 68, 1005–1009. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Syngas fermentation in a 100-L pilot scale fermentor: Design and process considerations. J. Biosci. Bioeng. 2010, 109, 492–498. [Google Scholar] [CrossRef]
- Groher, A.; Weuster-Botz, D. Comparative reaction engineering analysis of different acetogenic bacteria for gas fermentation. J. Biotechnol. 2016, 228, 82–94. [Google Scholar] [CrossRef]
- Daniell, J.; Köpke, M.; Simpson, S. Commercial biomass syngas fermentation. Energies 2012, 5, 5372–5417. [Google Scholar] [CrossRef]
- Battino, R.; Seybold, P.G.; Campanell, F.C. Correlations involving the solubility of gases in water at 298.15 K and 101325 Pa. J. Chem. Eng. Data 2011, 56, 727–732. [Google Scholar] [CrossRef]
- Liew, F.; Kopke, M.; Dennis, S. Gas fermentation for commercial biofuels production. In Liquid, Gaseous and Solid Biofuels—Conversion Techniques; Fang, Z., Ed.; InTech: Houston, TX, USA, 2013; ISBN 978-953-51-1050-757. [Google Scholar]
- Liu, X.; Fabos, V.; Taylor, S.; Knight, D.W.; Whiston, K.; Hutchings, G.J. One-Step Production of 1,3-Butadiene from 2,3-Butanediol Dehydration. Chemistry 2016, 22, 12290–12294. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Butanol fermentation research: Upstream and downstream manipulations. Chem. Rec. 2004, 4, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Bowen, S.H.; Lewis, R.S. A thermodynamic analysis of electron production during syngas fermentation. Bioresour. Technol. 2011, 102, 8071–8076. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, J.K.; Valgepea, K.; de Souza Pinto Lemgruber, R.; Casini, I.; Plan, M.; Tappel, R.; Simpson, S.D.; Köpke, M.; Nielsen, L.K.; Marcellin, E. Enhancing CO2-valorization using Clostridium autoethanogenum for sustainable fuel and chemicals production. Front. Bioeng. Biotechnol. 2020, 8, 204. [Google Scholar] [CrossRef]
- Riegler, P.; Chrusciel, T.; Mayer, A.; Doll, K.; Weuster-Botz, D. Reversible retrofitting of a stirred-tank bioreactor for gas-lift operation to perform synthesis gas fermentation studies. Biochem. Eng. J. 2019, 141, 89–101. [Google Scholar] [CrossRef]
- Weuster-Botz, D. Process Engineering Aspects for the Microbial Conversion of C1 Gases. Adv. Biochem. Eng. Biotechnol. 2022, 180, 33–56. [Google Scholar] [CrossRef]
- Youssef, A.A. Fluid Dynamics and Scale-Up of Bubble Columns with Internals. Ph.D. Thesis, Washington University in St. Louis, St. Louis, MO, USA, 2010. All Theses and Dissertations (ETDs). [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda, G.; Barba, J.; Mendoza, J.M. Effect of steam content in the air–steam flow on biomass entrained flow gasification. Fuel Processing Technology 2012, 99, 43–55. [Google Scholar] [CrossRef]
- Vishwajeet; Pawlak-Kruczek, H.; Baranowski, M.; Czerep, M.; Chorążyczewski, A.; Krochmalny, K.; Ostrycharczyk, M.; Ziółkowski, P.; Madejski, P.; Mączka, T.; et al. Entrained flow plasma gasification of sewage sludge–Proof-of-concept and fate of inorganics. Energies 2022, 15, 1948. [Google Scholar] [CrossRef]
- Mazzoni, L.; Janajreh, I.; Elagroudy, S.; Ghenai, C. Modeling of plasma and entrained flow co-gasification of MSW and petroleum sludge. Energy 2020, 196, 117001. [Google Scholar] [CrossRef]
- Agon, N.; Hrabovský, M.; Chumak, O.; Hlína, M.; Kopecký, V.; Masláni, A.; Bosmans, A.; Helsen, L.; Skoblja, S.; van Oost, G.; et al. Plasma gasification of refuse derived fuel in a single-stage system using different gasifying agents. Waste Manag. 2016, 47, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Kawamoto, K.; Fukushima, R.; Tanaka, S. Woody biomass and RPF gasification using reforming catalyst and calcium oxide. Chemosphere 2011, 83, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
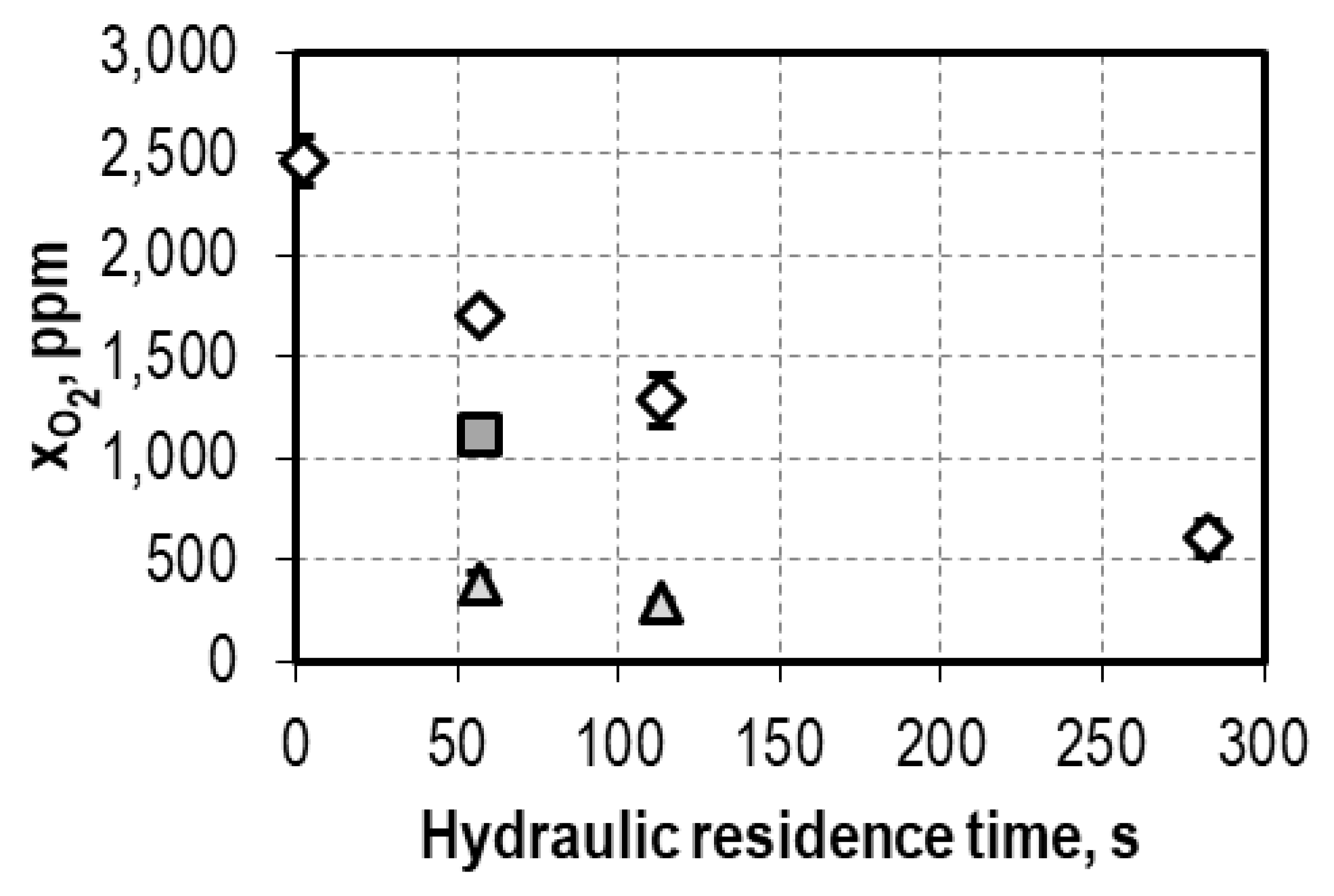
| Syngas Component | Artificial Syngas | Biogenic Syngas after Syngas Cleaning | Biogenic Syngas after Syngas Cleaning with Additional Oxygen Reduction |
|---|---|---|---|
| N2 | 36.7 ± 0.3% | 34.4 ± 2.9% | 30.2 ± 3.8% |
| CO | 30.1 ± 0.4% | 30.3 ± 1.7% | 31.6 ± 3.4% |
| CO2 | 10.3 ± 0.2% | 9.4 ± 0.7% | 10.0 ± 1.1% |
| H2 | 18.5 ± 0.1% | 22.2 ± 0.4% | 21.9 ± 1.7% |
| CH4 | <<0.1% | 0.46 ± 0.009% | 0.51 ± 0.034% |
| O2 | <<50 ppm | 2459 ± 122 ppm | 293 ± 5 ppm |
| NH3 | <1 ppm | 3099 ± 181 ppm | 766 ± 344 ppm |
| H2S | <1 ppm | <50 ppm | <50 ppm |
| NOX | <1 ppm | <1 ppm | <1 ppm |
| HCN | <1 ppm | <1 ppm | <1 ppm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rückel, A.; Oppelt, A.; Leuter, P.; Johne, P.; Fendt, S.; Weuster-Botz, D. Conversion of Syngas from Entrained Flow Gasification of Biogenic Residues with Clostridium carboxidivorans and Clostridium autoethanogenum. Fermentation 2022, 8, 465. https://doi.org/10.3390/fermentation8090465
Rückel A, Oppelt A, Leuter P, Johne P, Fendt S, Weuster-Botz D. Conversion of Syngas from Entrained Flow Gasification of Biogenic Residues with Clostridium carboxidivorans and Clostridium autoethanogenum. Fermentation. 2022; 8(9):465. https://doi.org/10.3390/fermentation8090465
Chicago/Turabian StyleRückel, Anton, Anne Oppelt, Philipp Leuter, Philipp Johne, Sebastian Fendt, and Dirk Weuster-Botz. 2022. "Conversion of Syngas from Entrained Flow Gasification of Biogenic Residues with Clostridium carboxidivorans and Clostridium autoethanogenum" Fermentation 8, no. 9: 465. https://doi.org/10.3390/fermentation8090465
APA StyleRückel, A., Oppelt, A., Leuter, P., Johne, P., Fendt, S., & Weuster-Botz, D. (2022). Conversion of Syngas from Entrained Flow Gasification of Biogenic Residues with Clostridium carboxidivorans and Clostridium autoethanogenum. Fermentation, 8(9), 465. https://doi.org/10.3390/fermentation8090465





