The Implications of Composite Dark Purple Rice Malt on Phenolic Acid Profiles, 4-Vinyl Guaiacol Reduction and Enhancing the Antioxidation of Beer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Malts and Yeast Strain
2.2. Wort Production and Characterisation
2.3. Bioreactor Configuration for Fermentation
2.4. Bottle Maturation of Beer
2.5. Analysis of Fermented Wort and Matured Beer
2.5.1. Extraction of Phenolic Acids in Wort and Beer via the Non-Hydrolysed Method
2.5.2. Analysis of Phenolic Acids in Composite Malt Wort and Beer
2.5.3. Quantification of 4-Vinylguaiacol in Wort and Beer
2.5.4. Total Anthocyanin by the pH Differential Method
2.5.5. Antioxidant Activity via 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2-2′-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) Assays
2.5.6. Statistical Analysis
3. Results and Discussion
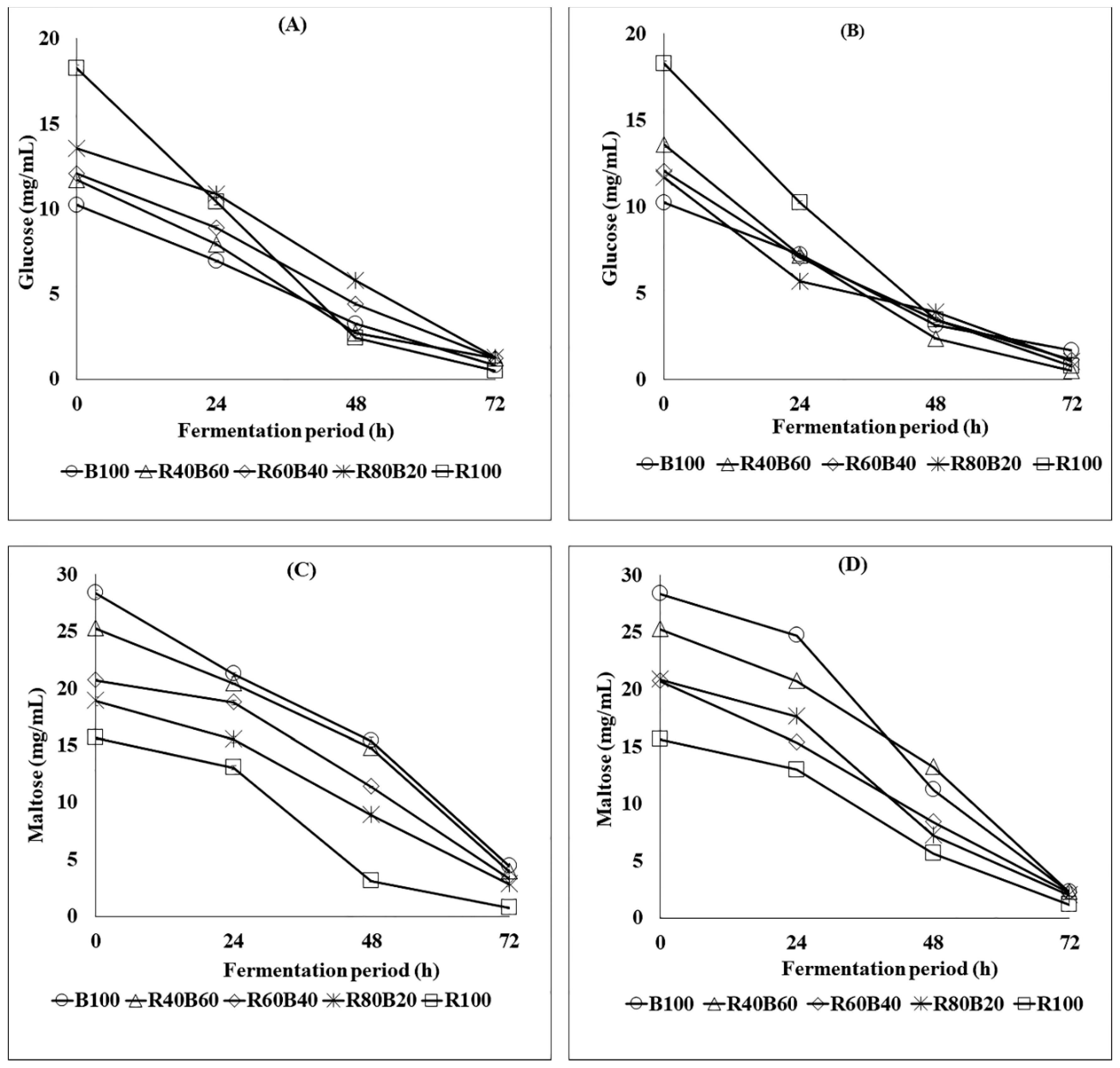
3.1. Glucose and Maltose Dynamics during Fermentation and Beer Maturation
3.2. Ethanol Production
3.3. Colour Unit of Cast Wort and Matured Beer
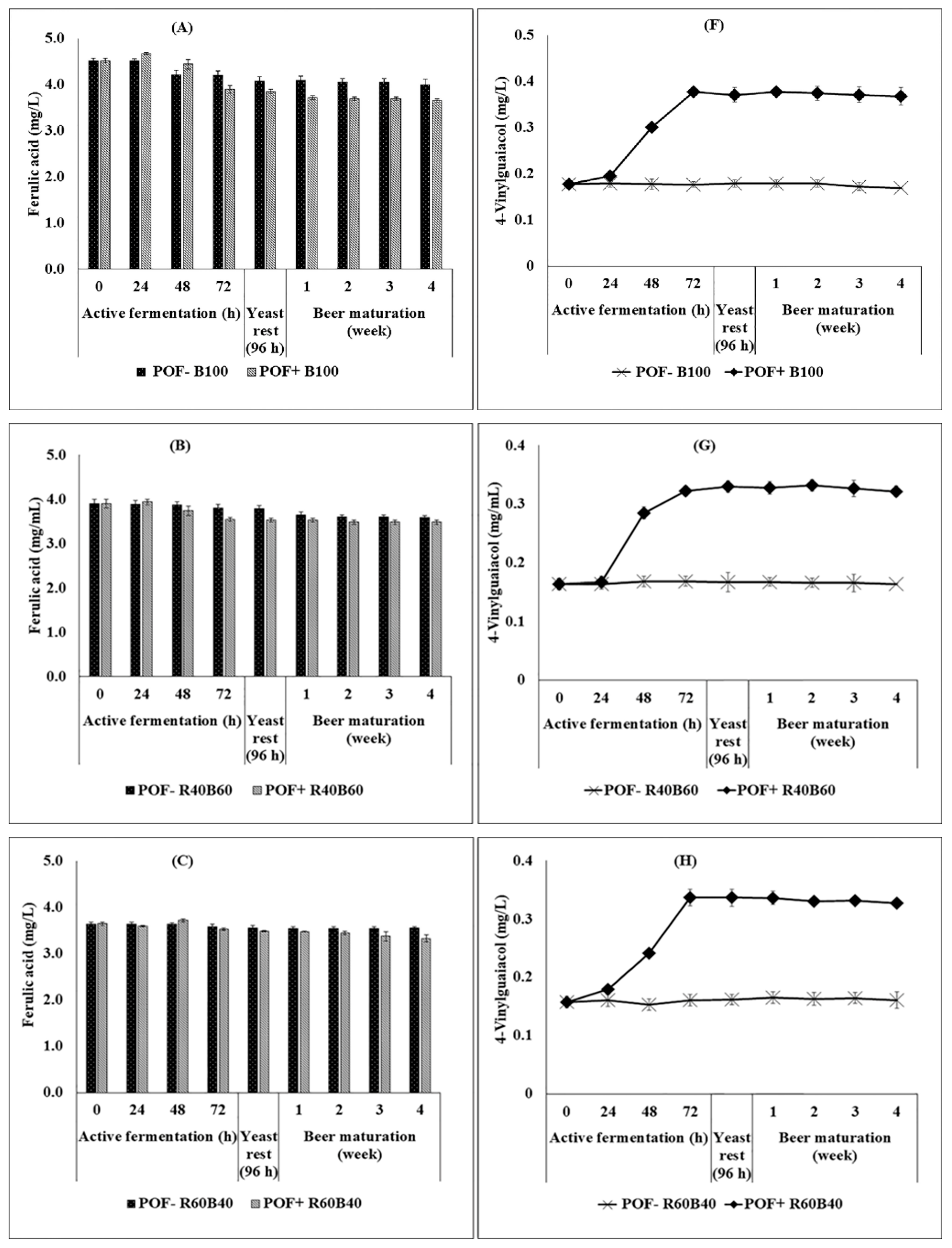
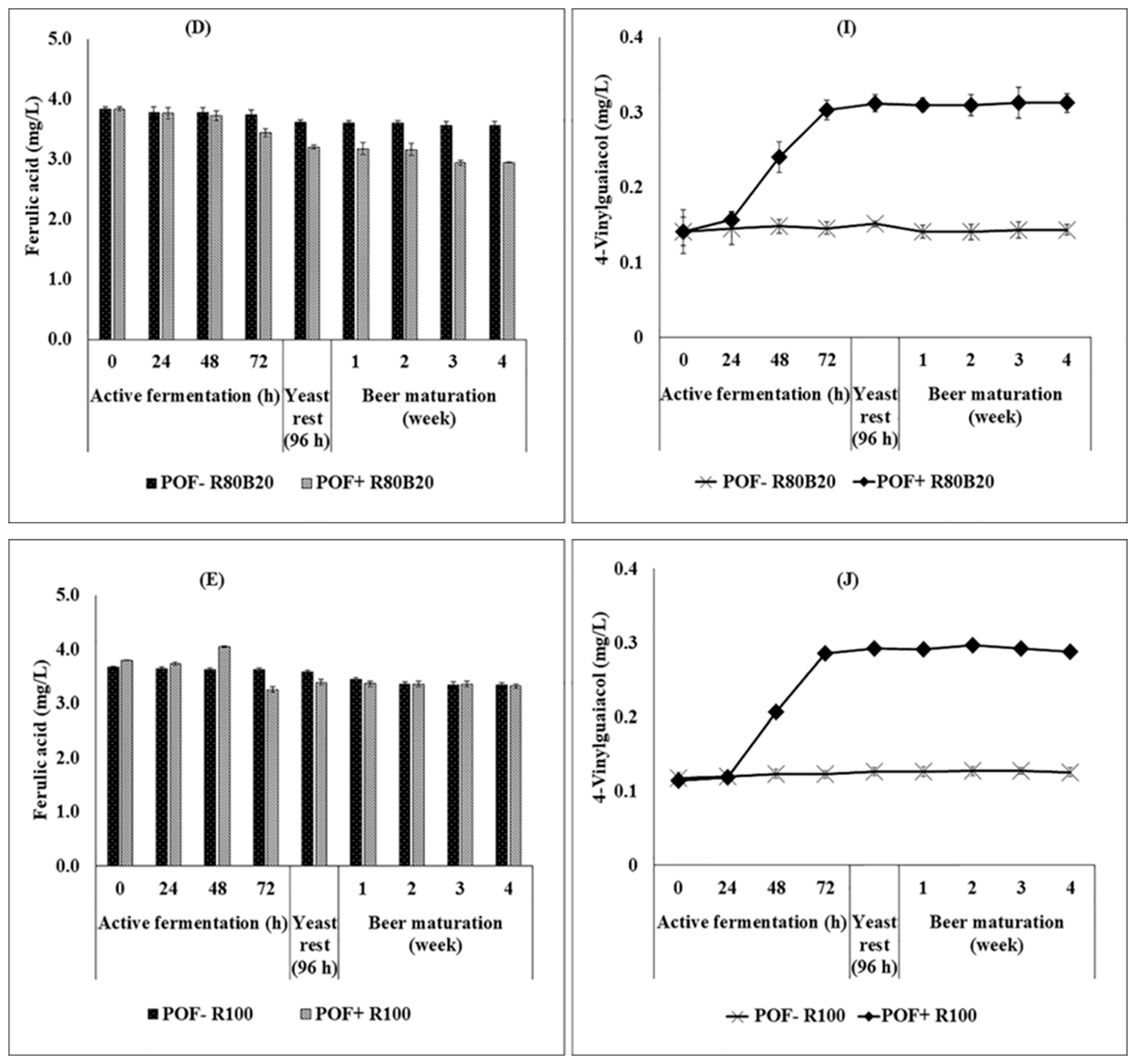
3.4. Ferulic Acid Changes and 4-Vinylguaiacol Production during the Brewing Process as an Impact of Composite Riceberry Rice Malt–Barley Malt and Yeast Type
3.5. p-Coumaric Acid Kinetic in Fermented Wort and Beer
3.6. Sinapic Acid (SA) Kinetics in Fermented Wort and Beer
3.7. Vanillic Acid Dynamics in Fermented Wort and Matured Beer
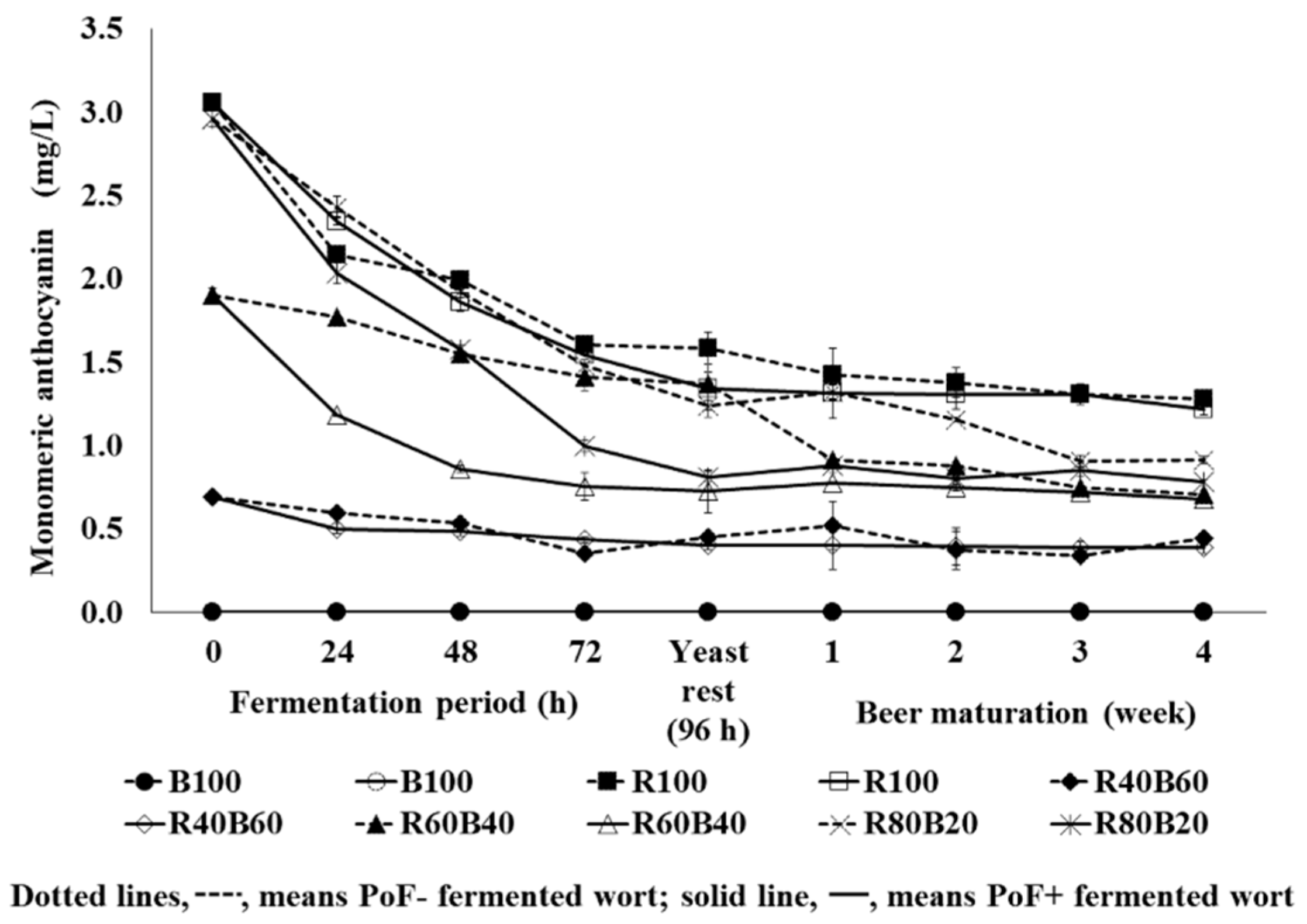
3.8. Dynamics of Monomeric Anthocyanin in Fermented Wort and Beer
3.9. Antioxidant Activity (AOA) during Wort Fermentation and in Beer via DPPH and ABTS Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butsat, S.; Weerapreeyakul, N.; Siriamornpun, S. Changes in phenolic acids and antioxidant activity in thai rice husk at five growth stages during grain development. J. Agric. Food Chem. 2009, 57, 4566–4571. [Google Scholar] [CrossRef] [PubMed]
- Vanbeneden, N.; Van Roey, T.; Willems, F.; Delvaux, F.; Delvaux, F.R. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008, 111, 83–91. [Google Scholar] [CrossRef]
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, A.B.; Delvaux, F.R. Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: Indications for feruloyl esterase activity in Saccharomyces cerevisiae. J. Agric. Food Chem. 2004, 52, 602–608. [Google Scholar] [CrossRef]
- Mukai, N.; Masaki, K.; Fujii, T.; Kawamukai, M.; Iefuji, H. PAD1 and FDC1 are essential for the decarboxylation of phenylacrylic acids in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2010, 109, 564–569. [Google Scholar] [CrossRef]
- Vanbeneden, N.; Gils, F.; Delvaux, F.; Delvaux, F.R. Formation of 4-vinyl and 4-ethyl derivatives from hydroxycinnamic acids: Occurrence of volatile phenolic flavour compounds in beer and distribution of Pad1-activity among brewing yeasts. Food Chem. 2008, 107, 221–230. [Google Scholar] [CrossRef]
- Maeda, M.; Motosoko, M.; Tokashiki, T.; Tokashiki, J.; Mizutani, O.; Uechi, K.; Goto, M.; Taira, T. Phenolic acid decarboxylase of Aspergillus luchuensis plays a crucial role in 4-vinylguaiacol production during awamori brewing. J. Biosci. Bioeng. 2020, 130, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Yamada, R.; Ayuzawa, R.; Nakamura, K. Mutation and deletion of pad1 and/or fdc1 and absence of phenolic off-flavor production in top and bottom-fermenting yeasts. J. Am. Soc. Brew. Chem. 2020, 78, 74–79. [Google Scholar] [CrossRef]
- Usansa, U.; Sompong, N.; Wanapu, C.; Boonkerd, N.; Teaumroong, N. The influences of steeping duration and temperature on the α- and β-amylase activities of six thai rice malt cultivars (Oryza sativa L. Indica). J. Inst. Brew. 2009, 115, 140–147. [Google Scholar] [CrossRef]
- Mayer, H.; Ceccaroni, D.; Marconi, O.; Sileoni, V.; Perretti, G.; Fantozzi, P. Development of an all rice malt beer: A gluten free alternative. LWT Food Sci. Technol. 2016, 67, 67–73. [Google Scholar] [CrossRef]
- Dos Santos, J.P.; Acunha, T.d.S.; Prestes, D.N.; Rombaldi, C.V.; El Halal, S.L.M.; Vanier, N.L. From brown, red, and black rice to beer: Changes in phenolics, γ-aminobutyric acid, and physicochemical attributes. Cereal Chem. 2020, 97, 1148–1157. [Google Scholar] [CrossRef]
- Gonu, H.; Withayagiat, U. Composite malt with dark-purple rice malt improves the phenolic profile and antioxidant activity of malt extract. Int. J. Food Sci. Technol. 2021, 56, 5833–5842. [Google Scholar] [CrossRef]
- Moirangthem, K.; Jenkins, D.; Ramakrishna, P.; Rajkumari, R.; Cook, D. Indian black rice: A brewing raw material with novel functionality. J. Inst. Brew. 2020, 126, 35–45. [Google Scholar] [CrossRef]
- Gonu, H.; Wanapu, C.; Withayagiat, U. The Correlation of Free and Bound Phenolic Acid with Antioxidant Activity Accelerated by Germination Period and Temperature. J. Am. Soc. Brew. Chem. 2022, 80, 305–315. [Google Scholar] [CrossRef]
- European Brewery Convention. Analytica-EBC; Verlag Hans Carl: Nürnberg, Germany, 2000. [Google Scholar]
- Štulíková, K.; Novák, J.; Vlček, J.; Šavel, J.; Košin, P.; Dostálek, P. Bottle Conditioning: Technology and Mechanisms Applied in Refermented Beers. Beverages 2020, 6, 56. [Google Scholar] [CrossRef]
- Suriano, S.; Iannucci, A.; Codianni, P.; Fares, C.; Russo, M.; Pecchioni, N.; Marciello, U.; Savino, M. Phenolic acids profile, nutritional and phytochemical compounds, antioxidant properties in colored barley grown in southern Italy. Food Res. Int. 2018, 113, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Bao, J. Rice starch. In Rice Chemistry and Technology; Bao, J., Ed.; Woodhead Publishing and AACC International Press: Duxford, UK, 2018; pp. 55–108. [Google Scholar] [CrossRef]
- Usansa, U.; Burberg, F.; Geiger, E.; Back, W.; Wanapu, C.; Arendt, E.K.; Kreisz, S.; Boonkerd, N.; Teaumroong, N.; Zarnkow, M. Optimization of malting conditions for two black rice varieties, black non-waxy rice and black waxy rice (Oryza sativa L. indica). J. Inst. Brew. 2011, 117, 39–46. [Google Scholar] [CrossRef]
- Meier-Dörnberg, T.; Kory, O.I.; Jacob, F.; Michel, M.; Hutzler, M. Saccharomyces cerevisiae variety diastaticus friend or foe?—spoilage potential and brewing ability of different Saccharomyces cerevisiae variety diastaticus yeast isolates by genetic, phenotypic and physiological characterization. FEMS Yeast Res. 2018, 18, 1–27. [Google Scholar] [CrossRef]
- Henson, C.A.; Duke, S.H.; Vinje, M.A. Comparison of Barley Malt Amylolytic Enzyme Thermostabilities and Wort Sugars Produced During Mashing. J. Am. Soc. Brew. Chem. 2014, 72, 51–65. [Google Scholar] [CrossRef]
- McMurrough, I.; Madigan, D.; Donnelly, D.; Hurley, J.; Doyle, A.-M.; Hennigan, G.; McNulty, N.; Smyth, M.R. Control of Ferulic Acid and 4-Vinyl Guaiacol in Brewing. J. Inst. Brew. 1996, 102, 327–332. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, T.; Li, X.; Cai, G.; Lu, J. POD promoted oxidative gelation of water-extractable arabinoxylan through ferulic acid dimers. Evidence for its negative effect on malt filterability. Food Chem. 2016, 197, 422–426. [Google Scholar] [CrossRef]
- Szwajgier, D.; Pielecki, J.; Targoński, Z. The Release of Ferulic Acid and Feruloylated Oligosaccharides During Wort and Beer Production. J. Inst. Brew. 2005, 111, 372–379. [Google Scholar] [CrossRef]
- Pulvirenti, A.; De Vero, L.; Blaiotta, G.; Sidari, R.; Iosca, G.; Gullo, M.; Caridi, A. Selection of Wine Saccharomyces cerevisiae Strains and Their Screening for the Adsorption Activity of Pigments, Phenolics and Ochratoxin A. Fermentation 2020, 6, 80. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, Y.; He, Z.; Zhai, W.; Gong, H.; Yang, Z. Effect of Ferulic Acid on the Formation of Pyranoanthocyanins from Purple Corn (Zea mays L.) Cob in a Model System and Their Effects on Color. Int. J. Food Prop. 2015, 19, 847–858. [Google Scholar] [CrossRef]
- Echeverrigaray, S.; Scariot, F.J.; Menegotto, M.; Delamare, A.P.L. Anthocyanin adsorption by Saccharomyces cerevisiae during wine fermentation is associated to the loss of yeast cell wall/membrane integrity. Int. J. Food Microbiol. 2020, 314, 108383. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef]
- Outtrup, H. Proanthocyanidins, The Brewing Process, and The Quality of Beer. In Basic Life Science; Plant Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., LAKS, P.E., Eds.; Springer: Boston, MA, USA, 1992; Volume 59, pp. 849–858. [Google Scholar] [CrossRef]
- Ditrych, M.; Kordialik-Bogacka, E.; Czyżowska, A. Antiradical and reducing potential of commercial beers. Czech J. Food Sci. 2016, 33, 261–266. [Google Scholar] [CrossRef]
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Antioxidant activity and the most abundant phenolics in commercial dark beers. Int. J. Food Prop. 2017, 20 (Suppl. 1), S595–S609. [Google Scholar] [CrossRef]
- Ceccaroni, D.; Sileoni, V.; Marconi, O.; De Francesco, G.; Lee, E.G.; Perretti, G. Specialty rice malt optimization and improvement of rice malt beer aspect and aroma. LWT 2019, 99, 299–305. [Google Scholar] [CrossRef]



| Samples | Composite Malt Ratio | Wort Characteristics | ||
|---|---|---|---|---|
| Barley Malt (B) (%w/w) | Riceberry Rice Malt (R) (%w/w) | Free Amino Nitrogen (mg/L) | pH | |
| B100 | 100 | 0 | 282.00 ± 1.92 f | 5.36± 0.01 c |
| R40B60 | 60 | 40 | 195.90 ± 0.72 d | 5.36 ± 0.01 c |
| R60B40 | 40 | 60 | 171.70 ± 1.32 c | 5.28 ± 0.01 b |
| R80B20 | 20 | 80 | 145.70 ± 0.39 b | 5.24 ± 0.00 c |
| R100 | 0 | 100 | 127.70 ± 1.00 a | 5.21 ± 0.01 a |
| B100 | R40B60 | R60B40 | R80B20 | R100 | |
|---|---|---|---|---|---|
| Cast wort | 10.36 ± 0.11 | 11.89± 0.29 | 16.43 ± 0.24 | 26.27 ± 0.18 | 29.49 ± 0.15 |
| POF− | 8.36 ± 0.12 | 7.36 ± 0.23 | 13.28 ± 0.11 | 22.02 ± 0.16 | 24.46 ± 0.20 |
| POF+ | 7.36 ± 0.10 | 6.97 ± 0.18 | 12.36 ± 0.05 | 20.36 ± 0.09 | 22.15 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonu, H.; Withayagiat, U. The Implications of Composite Dark Purple Rice Malt on Phenolic Acid Profiles, 4-Vinyl Guaiacol Reduction and Enhancing the Antioxidation of Beer. Fermentation 2022, 8, 392. https://doi.org/10.3390/fermentation8080392
Gonu H, Withayagiat U. The Implications of Composite Dark Purple Rice Malt on Phenolic Acid Profiles, 4-Vinyl Guaiacol Reduction and Enhancing the Antioxidation of Beer. Fermentation. 2022; 8(8):392. https://doi.org/10.3390/fermentation8080392
Chicago/Turabian StyleGonu, Hellie, and Ulaiwan Withayagiat. 2022. "The Implications of Composite Dark Purple Rice Malt on Phenolic Acid Profiles, 4-Vinyl Guaiacol Reduction and Enhancing the Antioxidation of Beer" Fermentation 8, no. 8: 392. https://doi.org/10.3390/fermentation8080392






