Improvement of Rumen Fermentation Efficiency Using Different Energy Sources: In Vitro Comparison between Buffalo and Cow
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. In Vitro Fermentation
2.3. End-Products Measurement
2.4. Data Processing
2.5. Statistical Analysis
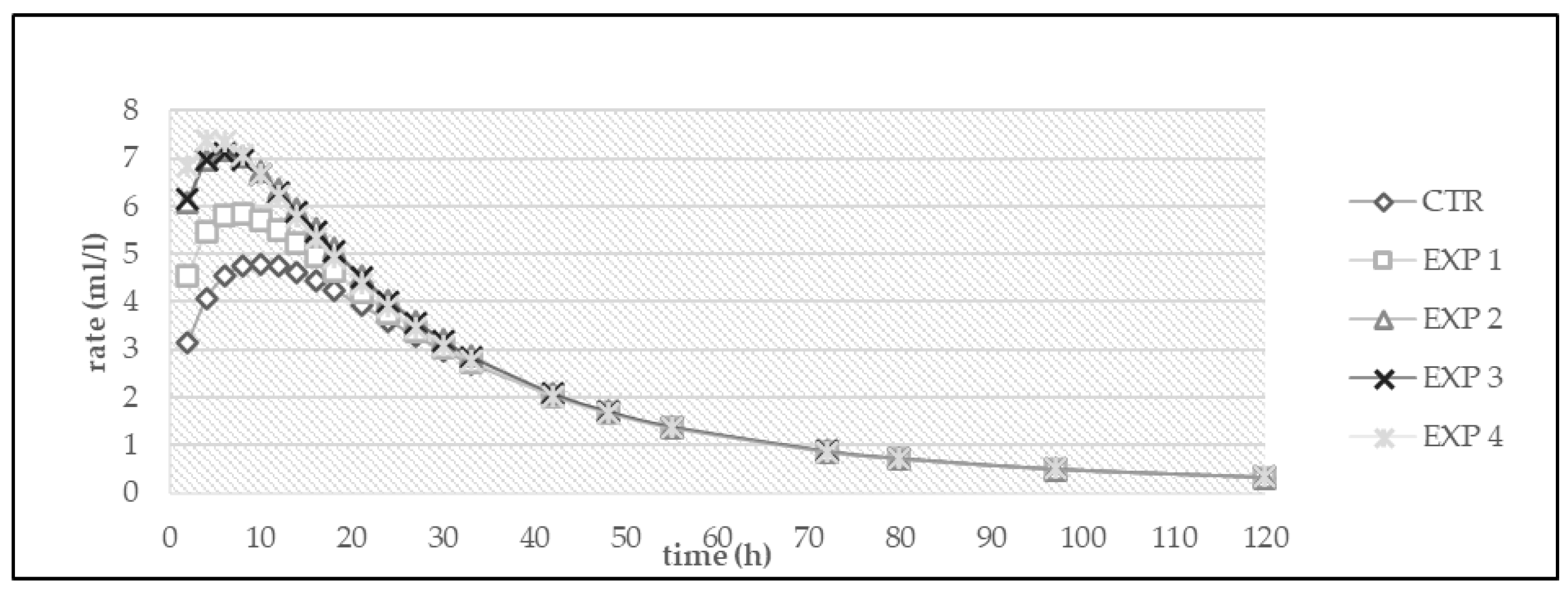
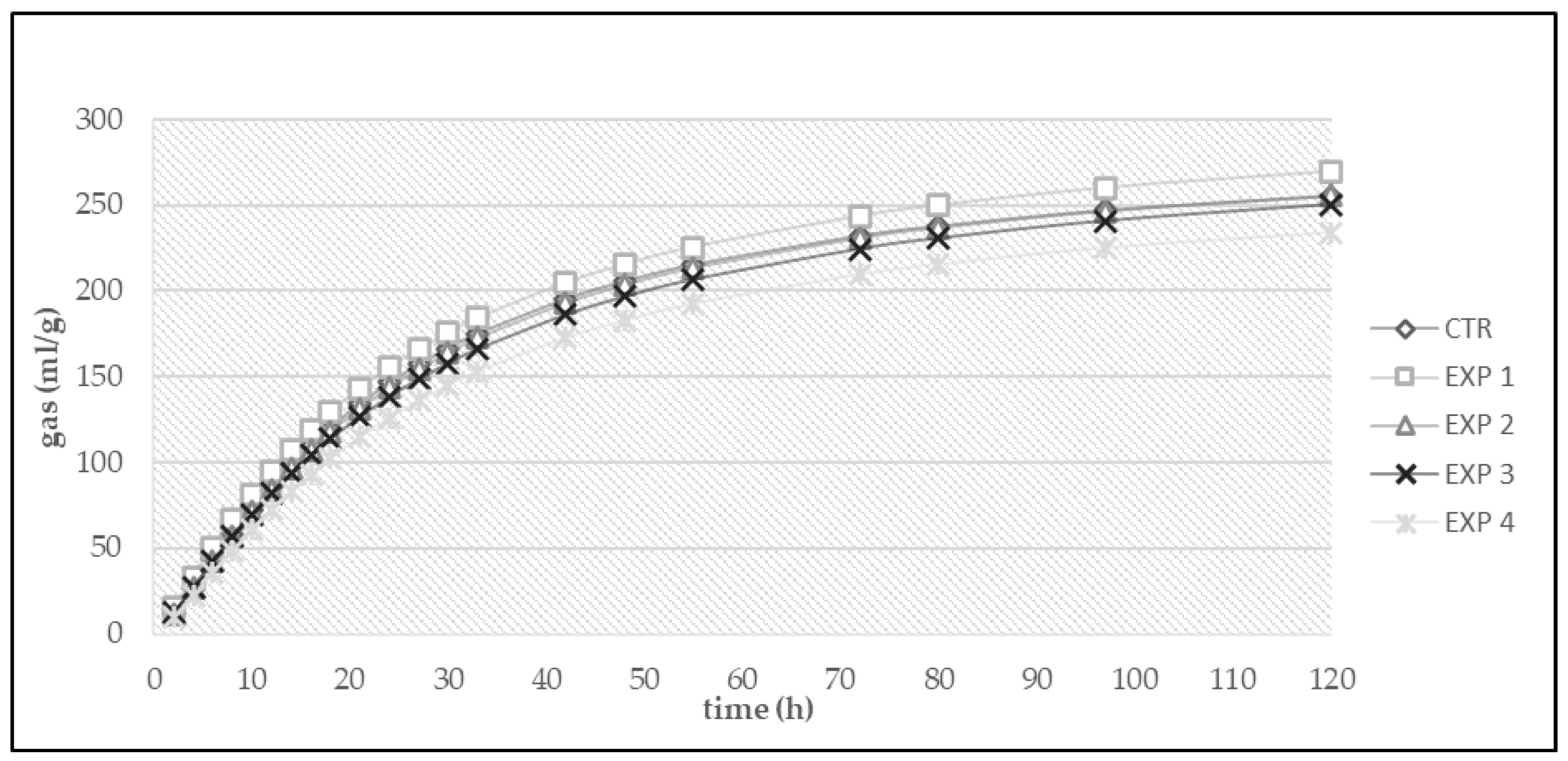
3. Results
3.1. In Vitro Fermentation
3.2. End-Product Measurement
4. Discussion
4.1. Liquid Feed Effects on In Vitro Fermentation
4.2. Buffalo vs. Bovine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azarpajouh, S.; Calderón Díaz, J.A.; Bueso Quan, S.; Taheri, H. Farm 4.0: Innovative smart dairy technologies and their applications as tools for welfare assessment in dairy cattle. CAB Rev. 2021, 16, 045. [Google Scholar] [CrossRef]
- Licitra, F.; Perillo, L.; Antoci, F.; Piccione, G.; Giannetto, C.; Salonia, R.; Giudice, E.; Monteverde, V.; Cascone, G. Management Factors Influence Animal Welfare and the to Infectious Diseases in Dairy Cows. Animals 2021, 11, 3321. [Google Scholar] [CrossRef] [PubMed]
- Crump, A.; Jenkins, K.; Bethell, E.J.; Ferris, C.P.; Arnott, G. Pasture Access Affects Behavioral Indicators of Wellbeing in Dairy Cows. Animals 2019, 9, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheller, L.S.; Ghizzi, L.G.; Marques, J.A.; Takiya, C.S.; Grigoletto, N.T.S.; Dias, M.S.S.; Silva, T.B.P.; Nunes, A.T.; da Silva, G.G.; Fernandes, L.G.X.; et al. Effects of organic acid-based products added to total mixed ration on performance and ruminal fermentation of dairy cows. Anim. Feed Sci. Technol. 2020, 261, 114406. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Research Council: Washington, DC, USA, 2001. [Google Scholar]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Pearson Education Limited: London, UK, 2011. [Google Scholar]
- Morales, J.U.; Alatorre, A.H.; Ascalante, A.A.; Lopez, S.B.; Vasquez, H.G.; Gomez, M.O.D. Nutritional Characteristics of Silage and Hay of Pearl Millet at Four Phenological Stages. J. Anim. Vet. Adv. 2011, 10, 1378–1382. [Google Scholar] [CrossRef] [Green Version]
- Udén, P. Plant organic acids in fresh and ensiled forage plants. Grass Forage Sci. 2018, 73, 583–587. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A Review Regarding the Use of Molasses in Animal Nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef]
- DeVries, T.J.; Gill, R.M. Adding liquid feed to a total mixed ration reduces feed sorting behavior and improves productivity of lactating dairy cows. J. Dairy Sci. 2011, 95, 2648–2655. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Khan, S.; Mobashar, M.; Inam, M.; Ahmed, I.; Khan N., A.; Ali, M.; Khan, H. Effect of Different Levels of Organic Acids Supplementation on Feed Intake, Milk Yield and Milk Composition of Dairy Cows during Thermal Stress. Greener J. Agric. Sci. 2013, 3, 762–768. [Google Scholar]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fusaro, I.; Giammarco, M.; Formigoni, A.; Mammi, L.M.E. In vitro evaluation of sugar digestibility in molasses. Ital. J. Anim. Sci. 2021, 20, 571–577. [Google Scholar] [CrossRef]
- Cesarani, A.; Biffani, S.; Garcia, A.; Lourenco, D.; Bertolini, G.; Neglia, G.; Misztal, I.; Macciotta, N.P.P. Genomic investigation of milk production in Italian buffalo. Ital. J. Anim. Sci. 2021, 20, 539–547. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Gonzalez, O.J.; Chiofalo, B.; Grossi, M.; Tudisco, R.; Panetta, C.; Infascelli, F. Meat quality of buffalo young bulls fed faba bean as protein source. Meat Sci. 2014, 96, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J.A. Simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Raso, G.; Todaro, M. Silage of Prickly Pears (Opuntia spp.) Juice By-Products. Animals 2020, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Musco, N.; Trotta, N.; Cutrignelli, M.I.; Di Francia, A.; Infascelli, F.; Tudisco, R.; Lombardi, P.; Vastolo, A.; Calabrò, S. Chemical Characterisation and in Vitro Gas Production Kinetics of Eight Faba Bean Varieties. Animals 2020, 10, 398. [Google Scholar] [CrossRef] [Green Version]
- Groot, J.C.J.; Cone, J.W.; William, B.A.; Debersaque, F.M.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feedstuff. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Bauer, E.; Williams, B.A.; Voigt, C.; Mosenthin, R.; Verstegen, M.W.A. Microbial activities of faeces from unweaned and adult pigs, in relation to selected fermentable carbohydrates. J. Anim. Sci. 2001, 73, 313–322. [Google Scholar] [CrossRef]
- da Costa, D.A.; de Souza, C.L.; Eloísa de Oliveira, S.S.; da Costa Carneiro, J. By-products of sugar cane industry in ruminant nutrition. Int. J. Adv. Agric. Res. 2015, 1–9. [Google Scholar] [CrossRef]
- Piquer, O.; Ródenas, L.C.; Casado Blas, E.; Pascual, J.J. Whole citrus fruits as an alternative to wheat grain or citrus pulp in sheep diet: Effect on the evolution of ruminal parameters. Small Rumin 2009, 83, 14–21. [Google Scholar] [CrossRef]
- Wing, J.M.; Van Horn, H.H.; Sklare, S.D.; Harris, B., Jr. Effects of Citrus Molasses Distillers Solubles and Molasses on Rumen Parameters and Lactation. J. Dairy Sci. 1988, 71, 414–420. [Google Scholar] [CrossRef]
- Ravelo, A.D.; Calvo Agustinho, B.; Arce-Cordero, J.; Monterio, H.F.; Bennet, S.L.; Sarmikasoglou, E.; Vinyard, J.; Vieira, E.R.Q.; Lobo, R.R.; Ferraretto, L.F.; et al. Effects of partially replacing dietary corn with molasses, condensed whey permeate, or treated condensed whey permeate on ruminal microbial fermentation. J Dairy Sci 2021, 105, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ma, C.; Fan, X.; Shah, A.M.; Mao, J. Use of condensed molasses fermentation solubles as an alternative source of concentrates in dairy cows. Anim. Biosci. 2021, 34, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Khattab, M.S.A. Glycerol as Feedstuff for Ruminant. Sci. Int. 2015, 3, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.; Shen, C.; Liu, Q.; Zhang, S.-L.; Shao, T.; Wang, C.; Wang, Y.X.; Xu, Q.-F.; Huo, W.-J. The effect of lactic acid bacteria inoculums on in vitro rumen fermentation, methane production, ruminal cellulolytic bacteria populations and cellulase activities of corn stover silage. J. Integr. Agric. 2020, 19, 838–847. [Google Scholar]
- Petri, R.M.; Münnich, M.; Zebeli, Q.; Klevenhusen, F. Graded replacement of corn grain with molassed sugar beet pulp modulates the fecal microbial community and hindgut fermentation profile in lactating dairy cows. J. Dairy Sci. 2019, 102, 5019–5030. [Google Scholar] [CrossRef]
- Bartocci, S.; Amici, A.; Verna, M.; Terramoccia, S.; Martillotti, F. Solid and fluid passage rate in buffalo, cattle and sheep fed diets with different forage to concentrate ratios. Livest Prod. Sci. 1997, 52, 201–208. [Google Scholar] [CrossRef]
- Bartocci, S.; Terramoccia, S.; Puppo, S. New acquisitions on the digestive physiology of the Mediterranean buffalo. In Buffalo Production and Research, REU Technical Series 67; FAO: Rome, Italy, 2005; pp. 161–172. [Google Scholar]
- Vastolo, A.; Calabrò, S.; Pacifico, S.; Koura, B.I.; Cutrignelli, M.I. Chemical and nutritional characteristics of Cannabis sativa L. co-products. J. Anim. Physiol. Anim. Nutr. 2021, 1–9. [Google Scholar] [CrossRef]
- Calabrò, S.; Williams, B.A.; Piccolo, V.; Infascelli, F.; Tamminga, S.A. Comparison between buffalo (Bubalus bubalis) and cow (Bos taurus) rumen fluids in terms of the in vitro fermentation characteristics of three fibrous feedstuffs. J. Sci. Food Agric. 2004, 84, 645–652. [Google Scholar] [CrossRef]
- Zicarelli, L. Alimentazione Della Bufala da Latte. In II Simposio Paulista de Bubalinocultura. Anais’; Franzolin Neto, R., Baruselli, P.S., Eds.; FZEA: Pirassununga, Brazil, 2001; pp. 1–61. Available online: https://repositorio.usp.br/item/001282892 (accessed on 29 June 2022).
- Tong, F.; Wang, T.; Gao, N.L.; Liu, Z.; Cui, K.; Duan, Y.; Wu, S.; Luo, Y.; Li, Z.; Yang, C.; et al. The microbiome of the buffalo digestive tract. Nat. Commun. 2022, 13, 823. [Google Scholar] [CrossRef]
- Bandarupalli, V.V.K.; St-Pierre, B. Identification of a candidate starch utilizing strain of Prevotella albensis from bovine Rumen. Microorganisms 2020, 8, 2005. [Google Scholar] [CrossRef]


| Liquid Feed_1 | Liquid Feed_2 | Liquid Feed_3 | Liquid Feed_4 |
|---|---|---|---|
| Cane molasses | Cane molasses | Cane molasses | Cane molasses |
| - | - | Soluble condensed molasses | - |
| Beet molasses | Beet molasses | Beet molasses | |
| - | - | Glycerol | |
| - | Glucose syrup | Glucose syrup | Glucose syrup |
| - | Citrus molasses | - | - |
| - | Isomaltulose molasses | Isomaltulose molasses | Isomaltulose molasses |
| - | Barley malt | Barley malt | Barley malt |
| - | Sucrose | Sucrose | Sucrose |
| - | Sodium chloride | Sodium chloride | Sodium chloride |
| - | acetic and propionic acids | acetic and propionic acids | acetic and propionic acids |
| Liquid Feed_1 | Liquid Feed_2 | Liquid Feed_3 | Liquid Feed_4 | Control Diet | |
|---|---|---|---|---|---|
| Moisture | 30.0 | 32.0 | 33.0 | 30.0 | 55.0 |
| CP | 8.00 | 7.50 | 12.5 | 5.00 | 13.7 |
| EE | 0.10 | 0.10 | 0.10 | 0.10 | 4.42 |
| CF | 0.10 | 0.10 | 0.10 | 0.10 | 19.9 |
| NDF | - | - | - | - | 44.2 |
| Ash | 8.50 | 7.00 | 8.00 | 5.50 | 9.00 |
| NSC | 40.0 | 41.0 | 32.0 | 32.0 | 28.7 |
| NEl (Mcal/kg) | 1.30 | 1.35 | 1.28 | 1.40 | 14.38 |
| Diet | OMD (%) | OMCV (mL/g) | Tmax (h) | Rmax (mL/h) |
|---|---|---|---|---|
| CTR | 71.0 | 252 | 4.50 | 7.92 |
| EXP 1 | 67.2 | 265 | 1.70 | 9.47 |
| EXP 2 | 66.2 | 255 | 5.22 | 8.56 |
| EXP 3 | 67.7 | 256 | 4.04 | 8.37 |
| EXP 4 | 65.4 | 226 | 4.82 | 6.44 |
| CTR vs. | ||||
| EXP 1 | ** | *** | *** | *** |
| EXP 2 | ** | NS | ** | NS |
| EXP 3 | * | NS | * | NS |
| EXP 4 | *** | *** | NS | *** |
| MSE | 1.14 | 2.59 | 0.03 | 0.10 |
| Diet | OMD (%) | OMCV (mL/g) | Tmax (h) | Rmax (mL/h) |
|---|---|---|---|---|
| CTR | 70.6 | 209 | 10.8 | 4.81 |
| EXP 1 | 70.8 | 235 | 7.93 | 6.12 |
| EXP 2 | 69.9 | 253 | 4.89 | 7.32 |
| EXP 3 | 70.8 | 255 | 5.72 | 7.40 |
| EXP 4 | 70.9 | 249 | 4.13 | 7.27 |
| CTR vs. | ||||
| EXP 1 | NS | *** | ** | *** |
| EXP 2 | NS | *** | *** | *** |
| EXP 3 | NS | *** | *** | *** |
| EXP 4 | NS | *** | *** | *** |
| MSE | 0.97 | 8.37 | 0.25 | 0.08 |
| Items | OMD (%) | OMCV (mL/g) | Tmax (h) | Rmax (mL/h) |
|---|---|---|---|---|
| Inoculum effect | ||||
| Buffalo | 70.6 | 241 | 6.69 | 6.59 |
| Bovine | 67.4 | 251 | 4.06 | 8.15 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 |
| Substrate effect | ||||
| p value | 0.0070 | <0.001 | <0.001 | <0.001 |
| Interaction Substrate x Inoculum | ||||
| p value | 0.0080 | <0.001 | <0.001 | <0.001 |
| MSE | 1.24 | 5.67 | 0.14 | 0.09 |
| CTR vs. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Items | Units | CTR | EXP1 | EXP2 | EXP3 | EXP4 | EXP1 | EXP2 | EXP3 | EXP4 | MSE |
| pH | 6.54 | 6.53 | 6.52 | 6.51 | 6.49 | NS | NS | NS | ** | 3e-3 | |
| VFA | mmol/l | 98.7 | 109 | 99.2 | 112 | 108 | *** | NS | *** | *** | 1.29 |
| Ace | %VFA | 59.1 | 60.1 | 60.1 | 61.3 | 58.8 | NS | NS | ** | NS | 0.51 |
| Prop | %VFA | 19.4 | 19.5 | 18.3 | 17.7 | 17.7 | NS | *** | *** | *** | 0.09 |
| Iso-But | %VFA | 1.68 | 1.40 | 2.12 | 1.56 | 1.51 | * | ** | NS | NS | 0.01 |
| But | %VFA | 14.2 | 13.9 | 14.5 | 13.6 | 15.6 | NS | NS | * | *** | 0.05 |
| Iso-Val | %VFA | 2.99 | 2.57 | 3.11 | 3.62 | 4.20 | NS | NS | NS | ** | 0.11 |
| Val | %VFA | 2.61 | 2.39 | 2.13 | 2.07 | 2.36 | NS | ** | ** | NS | 0.03 |
| BCFA | %VFA | 4.66 | 3.98 | 5.01 | 5.18 | 5.51 | NS | NS | NS | * | 0.16 |
| A/P | 3.05 | 3.08 | 3.29 | 3.48 | 3.11 | NS | NS | ** | NS | 0.02 | |
| CTR vs. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Items | Units | CTR | EXP1 | EXP2 | EXP3 | EXP4 | EXP1 | EXP2 | EXP3 | EXP4 | MSE |
| pH | 6.52 | 6.48 | 6.51 | 6.49 | 6.50 | *** | NS | ** | ** | 4.2 × 10−4 | |
| VFA | mmol/l | 107 | 108 | 111 | 95.5 | 98.9 | NS | ** | *** | *** | 0.74 |
| Ace | %VFA | 62.5 | 62.6 | 60.8 | 64.7 | 63.5 | NS | *** | *** | * | 0.12 |
| Prop | %VFA | 19.4 | 19.4 | 20.3 | 20.2 | 19.4 | NS | * | * | NS | 0.09 |
| Iso-But | %VFA | 1.12 | 1.15 | 1.13 | 1.04 | 0.94 | NS | NS | NS | * | 0.005 |
| But | %VFA | 12.4 | 14.07 | 13.5 | 11.6 | 12.5 | ** | * | NS | NS | 0.32 |
| Iso-Val | %VFA | 2.99 | 2.06 | 2.22 | 2.05 | 1.90 | *** | *** | *** | *** | 0.002 |
| Val | %VFA | 1.73 | 1.70 | 1.53 | 1.56 | 1.99 | NS | NS | NS | NS | 0.01 |
| BCFA | %VFA | 3.96 | 3.18 | 3.22 | 2.98 | 2.82 | *** | *** | *** | *** | 0.03 |
| A/P | 3.25 | 3.17 | 2.97 | 3.23 | 3.30 | NS | ** | NS | NS | 0.009 | |
| pH | VFA | Ace | Prop | Iso-But | But | Iso-Val | Val | BCFA | A/P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Inoculum effect | ||||||||||
| Buffalo | 6.50 | 104 | 62.8 | 19.7 | 1.06 | 12.8 | 2.24 | 1.70 | 3.23 | 3.20 |
| Bovine | 6.52 | 105 | 59.9 | 18.5 | 1.65 | 14.4 | 3.30 | 2.31 | 4.87 | 3.18 |
| p value | 0.0007 | 0.0015 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.710 |
| Substrate effect | ||||||||||
| p value | 0.0008 | <0.001 | <0.001 | 0.0002 | <0.001 | <0.001 | 0.0014 | 0.0006 | 0.0008 | 0.0040 |
| Interaction Substrate x Inoculum | ||||||||||
| p value | 0.0142 | <0.001 | 0.0001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.0890 | <0.001 | <0.001 |
| MSE | 2e-4 | 1.00 | 0.34 | 0.09 | 0.008 | 0.21 | 0.08 | 0.027 | 0.09 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vastolo, A.; Matera, R.; Serrapica, F.; Cutrignelli, M.I.; Neglia, G.; Kiatti, D.d.; Calabrò, S. Improvement of Rumen Fermentation Efficiency Using Different Energy Sources: In Vitro Comparison between Buffalo and Cow. Fermentation 2022, 8, 351. https://doi.org/10.3390/fermentation8080351
Vastolo A, Matera R, Serrapica F, Cutrignelli MI, Neglia G, Kiatti Dd, Calabrò S. Improvement of Rumen Fermentation Efficiency Using Different Energy Sources: In Vitro Comparison between Buffalo and Cow. Fermentation. 2022; 8(8):351. https://doi.org/10.3390/fermentation8080351
Chicago/Turabian StyleVastolo, Alessandro, Roberta Matera, Francesco Serrapica, Monica I. Cutrignelli, Gianluca Neglia, Dieu donné Kiatti, and Serena Calabrò. 2022. "Improvement of Rumen Fermentation Efficiency Using Different Energy Sources: In Vitro Comparison between Buffalo and Cow" Fermentation 8, no. 8: 351. https://doi.org/10.3390/fermentation8080351
APA StyleVastolo, A., Matera, R., Serrapica, F., Cutrignelli, M. I., Neglia, G., Kiatti, D. d., & Calabrò, S. (2022). Improvement of Rumen Fermentation Efficiency Using Different Energy Sources: In Vitro Comparison between Buffalo and Cow. Fermentation, 8(8), 351. https://doi.org/10.3390/fermentation8080351








