Production and Potential Genetic Pathways of Three Different Siderophore Types in Streptomyces tricolor Strain HM10
Abstract
1. Introduction
2. Materials and Methods
2.1. Media and Growth Condition
2.2. Detection of Siderophore Production
2.3. Catechol-Type Siderophore
2.4. Hydroxamate-Type Siderophore
2.5. Carboxylate-Type Siderophore
2.6. Growth Pattern and Siderophore Production
2.7. Whole-Genome Sequencing
2.8. Genome Sequence Analysis of Siderophore Pathways
2.9. Phylogenetic Tree Construction
2.10. Statistical Analysis
3. Results
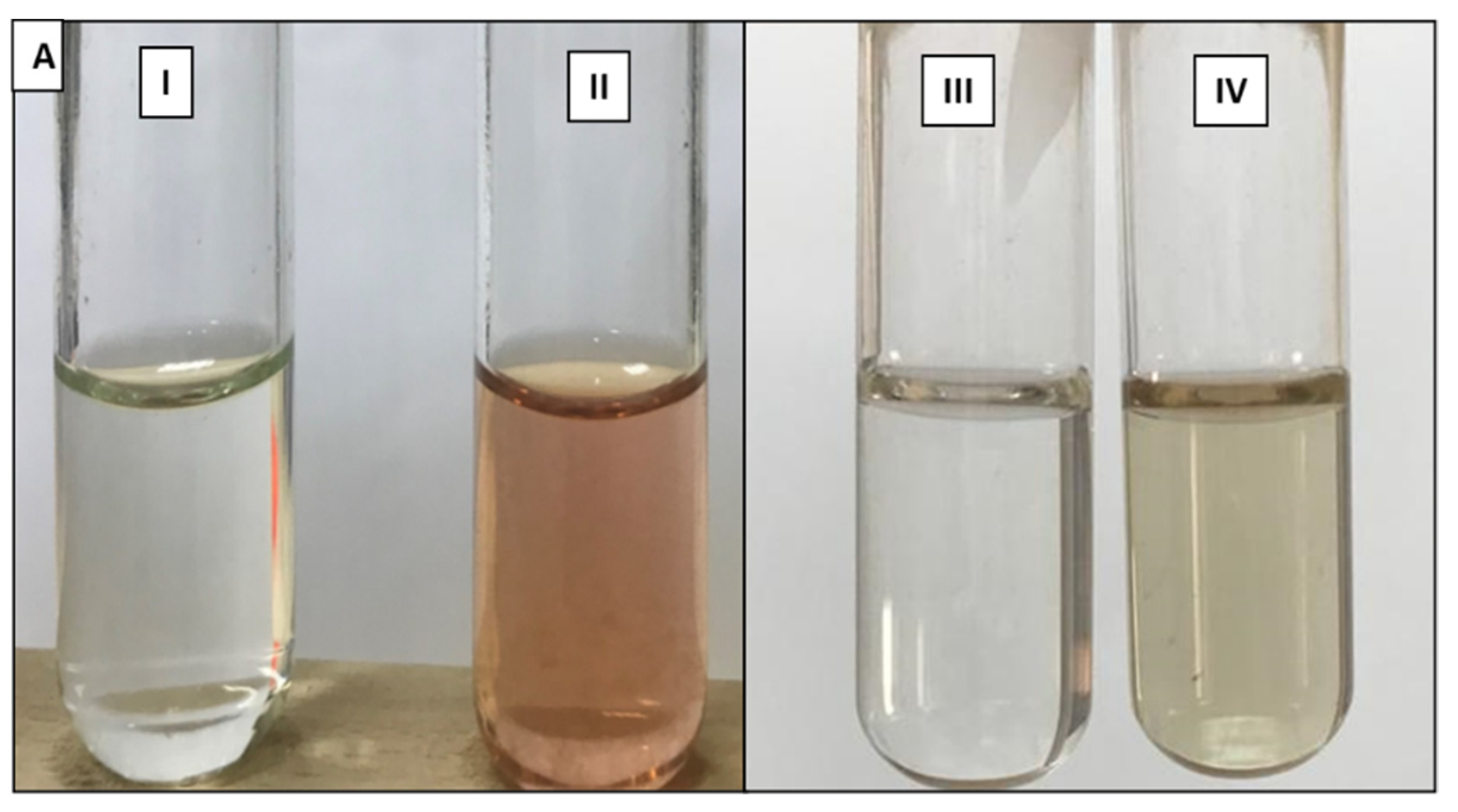
3.1. Siderophore Detection in Solid and Liquid Medium
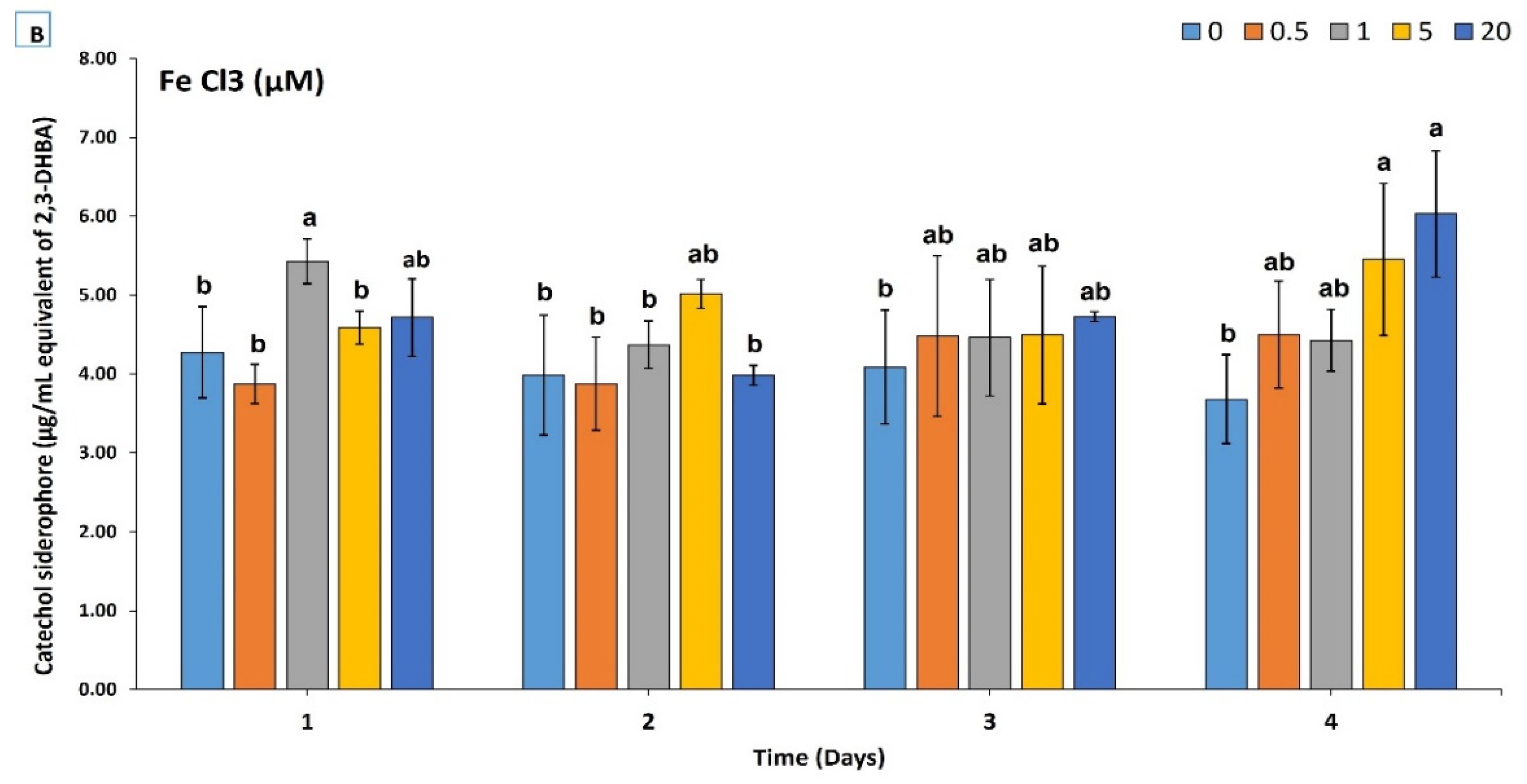
3.2. Chemical and Production Characterization of Siderophore
3.3. Genome Analysis for Siderophores Pathways
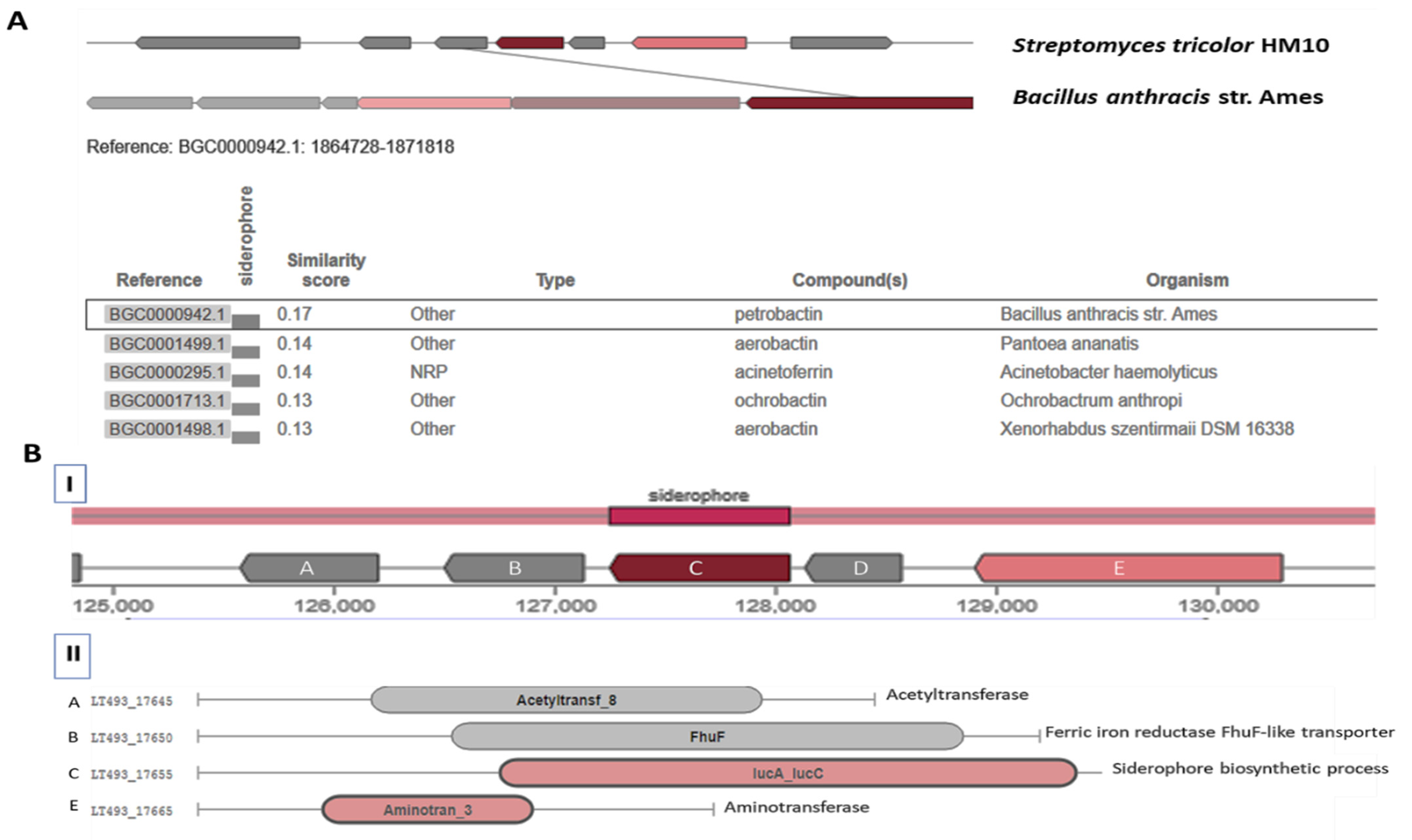
3.3.1. Catechol-Type Siderophore Pathway
3.3.2. Hydroximate-Type Siderophore Pathway
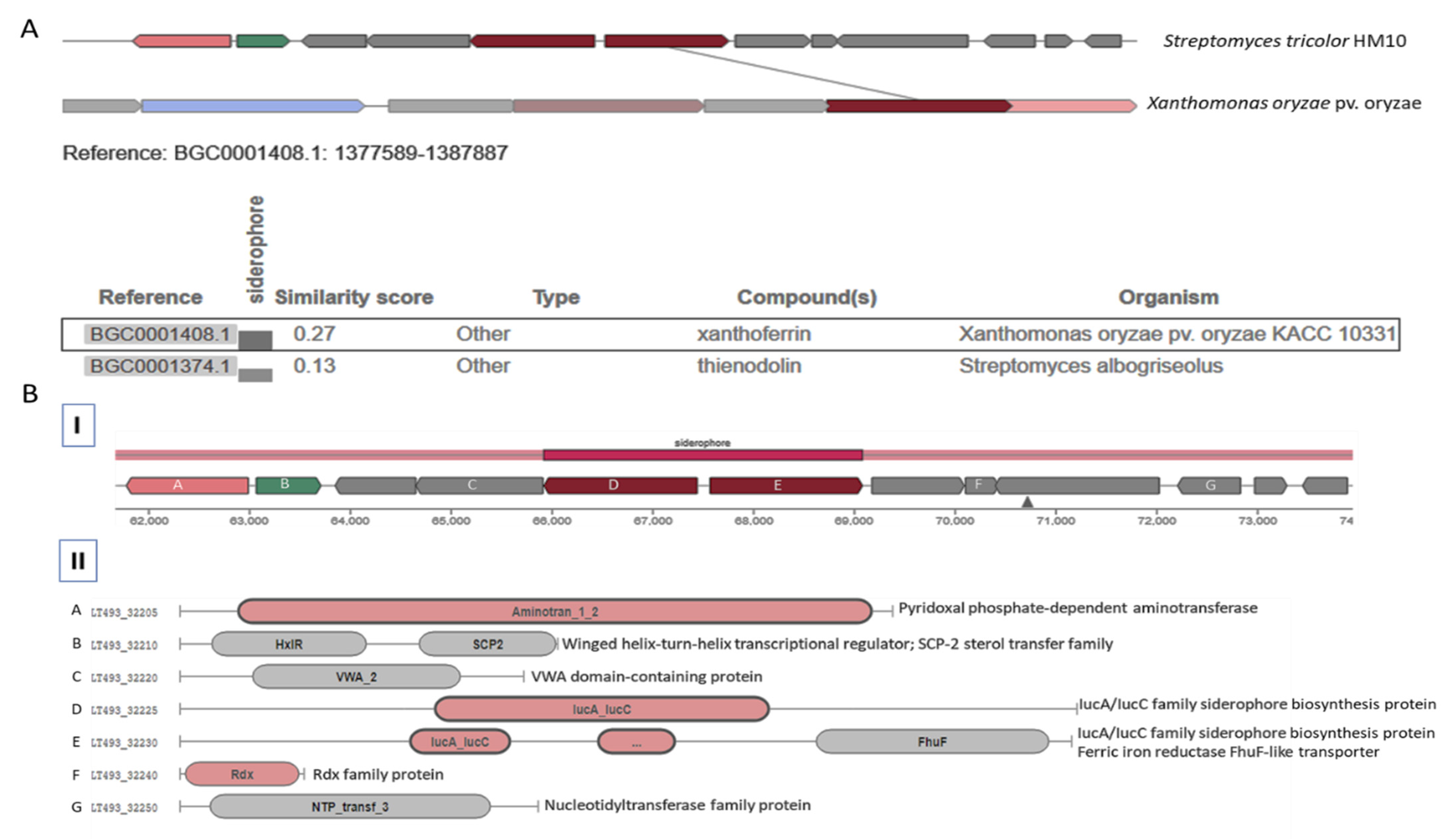
3.3.3. Carboxylate-Type Siderophore Pathway
3.4. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, B.; Chakrabartty, P.K. Siderophore Biosynthesis Genes of Rhizobium sp. Isolated from Cicer arietinum L. 3 Biotech 2014, 4, 391–401. [Google Scholar] [CrossRef]
- Searle, L.J.; Méric, G.; Porcelli, I.; Sheppard, S.K.; Lucchini, S. Variation in Siderophore Biosynthetic Gene Distribution and Production across Environmental and Faecal Populations of Escherichia coli. PLoS ONE 2015, 10, e0117906. [Google Scholar] [CrossRef]
- Neilands, J.B. Siderophores: Structure and Function of Microbial Iron Transport Compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef]
- Kodani, S.; Bicz, J.; Song, L.; Deeth, R.; Ohnishi-Kameyama, M.; Yoshida, M.; Ochi, K.; Challis, G. ChemInform Abstract: Structure and Biosynthesis of Scabichelin, a Novel Tris-Hydroxamate Siderophore Produced by the Plant Pathogen Streptomyces scabies 87.22. Org. Biomol. Chem. 2013, 11, 4686–4694. [Google Scholar] [CrossRef]
- Crosa, J.H.; Walsh, C.T. Genetics and Assembly Line Enzymology of Siderophore Biosynthesis in Bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 223–249. [Google Scholar] [CrossRef]
- Winkelmann, G. Microbial Siderophore-Mediated Transport. Biochem. Soc. Trans. 2002, 30, 691–696. [Google Scholar] [CrossRef]
- O’Brien, I.G.; Gibson, F. The Structure of Enterochelin and Related 2,3-dihydroxy-N-benzoyne Conjugates from Eschericha coli. Biochim. Biophys. Acta Gen. Subj. 1970, 215, 393–402. [Google Scholar] [CrossRef]
- Moynié, L.; Milenkovic, S.; Mislin, G.L.A.; Gasser, V.; Malloci, G.; Baco, E.; McCaughan, R.P.; Page, M.G.P.; Schalk, I.J.; Ceccarelli, M.; et al. The Complex of Ferric-Enterobactin with its Transporter from Pseudomonas aeruginosa Suggests a Two-Site Model. Nat. Commun. 2019, 10, 3673. [Google Scholar] [CrossRef]
- Yamanaka, K.; Oikawa, H.; Ogawa, H.-o.; Hosono, K.; Shinmachi, F.; Takano, H.; Sakuda, S.; Beppu, T.; Ueda, K. Desferrioxamine E produced by Streptomyces griseus stimulates growth and development of Streptomyces tanashiensis. Microbiology 2005, 151, 2899–2905. [Google Scholar] [CrossRef]
- Tunca, S.; Barreiro, C.; Sola-Landa, A.; Coque, J.J.R.; Martín, J.F. Transcriptional regulation of the desferrioxamine gene cluster of Streptomyces coelicolor is mediated by binding of DmdR1 to an iron box in the promoter of the desA gene. FEBS J. 2007, 274, 1110–1122. [Google Scholar] [CrossRef]
- Cheung, J.; Beasley, F.C.; Liu, S.; Lajoie, G.A.; Heinrichs, D.E. Molecular Characterization of Staphyloferrin B Biosynthesis in Staphylococcus aureus. Mol. Microbiol. 2009, 74, 594–608. [Google Scholar] [CrossRef]
- Hagan, A.K.; Plotnick, Y.M.; Dingle, R.E.; Mendel, Z.I.; Cendrowski, S.R.; Sherman, D.H.; Tripathi, A.; Hanna, P.C. Petrobactin Protects against Oxidative Stress and Enhances Sporulation Efficiency in Bacillus anthracis Sterne. MBio 2018, 9, e02079-18. [Google Scholar] [CrossRef]
- Najimi, M.; Lemos, M.L.; Osorio, C.R. Identification of Siderophore Biosynthesis Genes Essential for Growth of Aeromonas salmonicida under Iron Limitation Conditions. Appl. Environ. Microbiol. 2008, 74, 2341–2348. [Google Scholar] [CrossRef]
- Silva, M.G.; de Curcio, J.S.; Silva-Bailão, M.G.; Lima, R.M.; Tomazett, M.V.; de Souza, A.F.; Cruz-Leite, V.R.M.; Sbaraini, N.; Bailão, A.M.; Rodrigues, F.; et al. Molecular Characterization of Siderophore Biosynthesis in Paracoccidioides brasiliensis. IMA Fungus 2020, 11, 11. [Google Scholar] [CrossRef]
- Gärdes, A.; Triana, C.; Amin, S.A.; Green, D.H.; Romano, A.; Trimble, L.; Carrano, C.J. Detection of Photoactive Siderophore Biosynthetic Genes in the Marine Environment. BioMetals 2013, 26, 507–516. [Google Scholar] [CrossRef]
- Storey, E.P.; Boghozian, R.; Little, J.L.; Lowman, D.W.; Chakraborty, R. Characterization of ‘Schizokinen’; A Dihydroxamate-type Siderophore Produced by Rhizobium leguminosarum IARI 917. Biometals 2006, 19, 637–649. [Google Scholar] [CrossRef]
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in Environmental Research: Roles and Applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial Siderophores and their Potential Applications: A Review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef]
- Górska, A.; Sloderbach, A.; Marszałł, M.P. Siderophore-Drug Complexes: Potential Medicinal Applications of the ′Trojan horse′ Strategy. Trends Pharmacol. Sci. 2014, 35, 442–449. [Google Scholar] [CrossRef]
- De Serrano, L.O. Biotechnology of Siderophores in High-Impact Scientific Fields. Biomol. Concepts 2017, 8, 169–178. [Google Scholar] [CrossRef]
- Kurth, C.; Kage, H.; Nett, M. Siderophores as Molecular Tools in Medical and Environmental Applications. Org. Biomol. Chem. 2016, 14, 8212–8227. [Google Scholar] [CrossRef]
- Fan, D.; Fang, Q. Siderophores for Medical Applications: Imaging, Sensors, and Therapeutics. Int. J. Pharm. 2021, 597, 120306. [Google Scholar] [CrossRef]
- Albelda-Berenguer, M.; Monachon, M.; Joseph, E. Chapter Five—Siderophores: From Natural Roles to Potential Applications; Gadd, G.M., Sariaslani, S.B.T.A.i.A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 106, pp. 193–225. [Google Scholar]
- Dimkpa, C. Microbial Siderophores: Production, Detection and Application in Agriculture and Environment. Endocytobiosis Cell Res. 2016, 27, 7–16. [Google Scholar]
- Rapti, S.; Boyatzis, S.C.; Rivers, S.; Pournou, A. Siderophores and their Applications in Wood, Textile, and Paper Conservation BT—Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 301–339. [Google Scholar]
- Hofmann, M.; Heine, T.; Malik, L.; Hofmann, S.; Joffroy, K.; Senges, C.H.; Bandow, J.E.; Tischler, D. Screening for Microbial Metal-Chelating Siderophores for the Removal of Metal Ions from Solutions. Microorganisms 2021, 9, 111. [Google Scholar] [CrossRef]
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef]
- Bannerman, R.M.; Callender, S.T.; Williams, D.L. Effect of Desferrioxamine and D.T.P.A. in Iron Overload. Br. Med. J. 1962, 2, 1573–1577. [Google Scholar] [CrossRef][Green Version]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, Nature and Utility of Universal Iron Chelator—Siderophore: A review. Microbiol. Res. 2018, 212, 103–111. [Google Scholar] [CrossRef]
- Boyce, J.H.; Dang, B.; Ary, B.; Edmondson, Q.; Craik, C.S.; DeGrado, W.F.; Seiple, I.B. Platform to Discover Protease-Activated Antibiotics and Application to Siderophore–Antibiotic Conjugates. J. Am. Chem. Soc. 2020, 142, 21310–21321. [Google Scholar] [CrossRef]
- Saha, P.; Yeoh, B.S.; Xiao, X.; Golonka, R.M.; Kumarasamy, S.; Vijay-Kumar, M. Enterobactin, an iron chelating bacterial siderophore, arrests cancer cell proliferation. Biochem. Pharmacol. 2019, 168, 71–81. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M. Advances in the Antimicrobial and Therapeutic Potential of Siderophores. Environ. Chem. Lett. 2019, 17, 1485–1494. [Google Scholar] [CrossRef]
- Zumla, A.; Rao, M.; Wallis, R.S.; Kaufmann, S.H.E.; Rustomjee, R.; Mwaba, P.; Vilaplana, C.; Yeboah-Manu, D.; Chakaya, J.; Ippolito, G.; et al. Host-Directed Therapies for Infectious Diseases: Current Status, Recent Progress, and Future Prospects. Lancet Infect. Dis. 2016, 16, e47–e63. [Google Scholar] [CrossRef]
- Dimkpa, C.; Svatoš, A.; Merten, D.; Büchel, G.; Kothe, E. Hydroxamate Siderophores Produced by Streptomyces acidiscabies E13 Bind Nickel and Promote Growth in Cowpea (Vigna unguiculata L.) under Nickel Stress. Can. J. Microbiol. 2008, 54, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Imbert, M.; Béchet, M.; Blondeau, R. Comparison of the Main Siderophores Produced by some Species of Streptomyces. Curr. Microbiol. 1995, 31, 129–133. [Google Scholar] [CrossRef]
- Patel, P.; Song, L.; Challis, G.L. Distinct Extracytoplasmic Siderophore Binding Proteins Recognize Ferrioxamines and Ferricoelichelin in Streptomyces coelicolor A3(2). Biochemistry 2010, 49, 8033–8042. [Google Scholar] [CrossRef]
- Armin, R.; Zühlke, S.; Grunewaldt-Stöcker, G.; Mahnkopp-Dirks, F.; Kusari, S. Production of Siderophores by an Apple Root-Associated Streptomyces ciscaucasicus Strain GS2 Using Chemical and Biological OSMAC Approaches. Molecules 2021, 26, 562. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Y.; Lu, Y.; Wang, B.; Sun, J.; Zhang, H.; Wang, H. Chemistry and Biology of Siderophores from Marine Microbes. Mar. Drugs 2019, 17, 562. [Google Scholar] [CrossRef]
- Matsuo, Y.; Kanoh, K.; Jang, J.-H.; Adachi, K.; Matsuda, S.; Miki, O.; Kato, T.; Shizuri, Y. Streptobactin, a Tricatechol-Type Siderophore from Marine-Derived Streptomyces sp. YM5-799. J. Nat. Prod. 2011, 74, 2371–2376. [Google Scholar] [CrossRef]
- Rai, V.; Fisher, N.; Duckworth, O.W.; Baars, O. Extraction and Detection of Structurally Diverse Siderophores in Soil. Front. Microbiol. 2020, 11, 2165. [Google Scholar] [CrossRef]
- Rehan, M.; Alsohim, A.S.; Abidou, H.; Rasheed, Z.; Al Abdulmonem, W. Isolation, Identification, Biocontrol Activity, and Plant Growth Promoting Capability of a Superior Streptomyces tricolor Strain HM10. Pol. J. Microbiol. 2021, 70, 245–256. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Arnow, L.E. Colorimetric Determination of the Components of 3,4-dihydroxyphenylalaninetyrosine Mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar] [CrossRef]
- Atkin, C.L.; Neilands, J.B.; Phaff, H.J. Rhodotorulic Acid from Species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and A New Alanine-Containing Ferrichrome from Cryptococcus melibiosum. J. Bacteriol. 1970, 103, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Shenker, M.; Chen, Y.; Ghirlando, R.; Oliver, I.; Helmann, M.; Hadar, Y. Chemical Structure and Biological Activity of A Siderophore Produced by Rhizopus arrhizus. Soil Sci. Soc. Am. J. 1995, 59, 837–843. [Google Scholar] [CrossRef]
- Aboul-Maaty, N.A.-F.; Oraby, H.A.-S. Extraction of High-Quality Genomic DNA from different Plant Orders Applying A Modified CTAB-Based Method. Bull. Natl. Res. Cent. 2019, 43, 25. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of Long, Error-prone Reads Using Repeat Graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. AntiSMASH 6.0: Improving Cluster Detection and Comparison Capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Navarro-Muñoz, J.C.; Terlouw, B.R.; van der Hooft, J.J.J.; van Santen, J.A.; Tracanna, V.; Suarez Duran, H.G.; Pascal Andreu, V.; et al. MIBiG 2.0: A repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2020, 48, D454–D458. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- France, S. STATISTICA (Logiciel D’analyse de Données), Version 7.1. 2005. Available online: www.statsoft.fr (accessed on 16 June 2022).
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and Interactions in Plant Growth Promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- van Bergeijk, D.A.; Elsayed, S.S.; Du, C.; Santiago, I.N.; Roseboom, A.M.; Zhang, L.; Carrión, V.J.; Spaink, H.P.; van Wezel, G.P. The Ubiquitous Catechol Moiety Elicits Siderophore and Angucycline Production in Streptomyces. Commun. Chem. 2022, 5, 14. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Vilas-Boas, Â.; Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Comparison of Five Bacterial Strains Producing Siderophores with Ability to Chelate Iron Under Alkaline Conditions. AMB Express 2019, 9, 78. [Google Scholar] [CrossRef]
- Sasirekha, B.; Srividya, S. Siderophore Production by Pseudomonas aeruginosa FP6, a Biocontrol Strain for Rhizoctonia solani and Colletotrichum gloeosporioides Causing Diseases in Chilli. Agric. Nat. Resour. 2016, 50, 250–256. [Google Scholar] [CrossRef]
- Gull, M.; Hafeez, F.Y. Characterization of siderophore producing bacterial strain Pseudomonas fluorescens Mst 8.2 as plant growth promoting and biocontrol agent in wheat. Afr. J. Microbiol. Res. 2012, 6, 6308–6318. [Google Scholar] [CrossRef]
- Rondon, M.R.; Ballering, K.S.; Thomas, M.G. Identification and Analysis of A Siderophore Biosynthetic Gene Cluster from Agrobacterium tumefaciens C58. Microbiology 2004, 150, 3857–3866. [Google Scholar] [CrossRef]
- Clark, B.L. Characterization of A Catechol-Type Siderophore and the Detection of A Possible Outer Membrane Receptor Protein from Rhizobium leguminosarum Strain IARI 312; East Tennessee State University: Ann Arbor, MI, USA, 2004. [Google Scholar]
- Sumei, Y.; Chunying, T.; Xin, B.; Jinsong, L.; Tao, S.; Liying, D.; Yu, J. Optimization of Siderophore Production by Bacillus sp. PZ-1 and Its Potential Enhancement of Phytoextration of Pb from Soi. J. Microbiol. Biotechnol. 2017, 27, 1500–1512. [Google Scholar] [CrossRef]
- Sridevi, M.; Mallaiah, K. Production of Catechol-Type of Siderophores by Rhizobium Strains from Sesbania sesban (L.) Merr. Asian J. Bio Sci. 2008, 3, 187–194. [Google Scholar]
- Barona-Gómez, F.; Lautru, S.; Francou, F.-X.; Leblond, P.; Pernodet, J.-L.; Challis, G. Multiple Biosynthetic and Uptake Systems Mediate Siderophore-Dependent Iron Acquisition in Streptomyces coelicolor A3(2) and Streptomyces ambofaciens ATCC 23877. Microbiology 2006, 152, 3355–3366. [Google Scholar] [CrossRef] [PubMed]
- Patel, P. Investigations of Streptomyces coelicolor A3 (2) Siderophore Sinding Proteins; University of Warwick: Coventry, UK, 2009. [Google Scholar]
- Wali, U.M.; Mori, Y.; Maenaka, R.; Kai, K.; Tanaka, M.; Ohnishi, K.; Kiba, A.; Hikichi, Y. The N-acetyltransferase Gene-implicated Iron Acquisition Contributes to Host Specificity of Pseudomonas cichorii Strain SPC9018 and its Virulence. Physiol. Mol. Plant Pathol. 2015, 92, 14–21. [Google Scholar] [CrossRef]
- Vandenende, C.S.; Vlasschaert, M.; Seah, S.Y.K. Functional Characterization of an Aminotransferase Required for Pyoverdine Siderophore Biosynthesis in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2004, 186, 5596–5602. [Google Scholar] [CrossRef][Green Version]
- Trindade, I.B.; Hernandez, G.; Lebègue, E.; Barrière, F.; Cordeiro, T.; Piccioli, M.; Louro, R.O. Conjuring up A Ghost: Structural and Functional Characterization of FhuF, A Ferric Siderophore Reductase from E. coli. J. Biol. Inorg. Chem. 2021, 26, 313–326. [Google Scholar] [CrossRef]
- Fox, D.T.; Hotta, K.; Kim, C.-Y.; Koppisch, A.T. The Missing Link in Petrobactin Biosynthesis: AsbF Encodes a (−)-3-Dehydroshikimate Dehydratase. Biochemistry 2008, 47, 12251–12253. [Google Scholar] [CrossRef]
- Hagan, A.; Tripathi, A.; Berger, D.; Sherman, D.; Hanna, P. Petrobactin Is Exported from Bacillus anthracis by the RND-Type Exporter ApeX. mBio 2017, 8, e01238-17. [Google Scholar] [CrossRef] [PubMed]
- Koppisch, A.T.; Browder, C.C.; Moe, A.L.; Shelley, J.T.; Kinkel, B.A.; Hersman, L.E.; Iyer, S.; Ruggiero, C.E. Petrobactin is the Primary Siderophore Synthesized by Bacillus anthracis Str. Sterne under Conditions of Iron Starvation. Biometals 2005, 18, 577–585. [Google Scholar] [CrossRef]
- Lee, J.Y.; Janes, B.K.; Passalacqua, K.D.; Pfleger, B.F.; Bergman, N.H.; Liu, H.; Håkansson, K.; Somu, R.V.; Aldrich, C.C.; Cendrowski, S.; et al. Biosynthetic Analysis of the Petrobactin Siderophore Pathway from Bacillus anthracis. J. Bacteriol. 2007, 189, 1698–1710. [Google Scholar] [CrossRef]
- Lee, J.Y.; Passalacqua, K.D.; Hanna, P.C.; Sherman, D.H. Regulation of Petrobactin and Bacillibactin Biosynthesis in Bacillus anthracis under Iron and Oxygen Variation. PLoS ONE 2011, 6, e20777. [Google Scholar] [CrossRef] [PubMed]
- Nusca, T.; Kim, Y.; Maltseva, N.; Lee, J.; Eschenfeldt, W.; Stols, L.; Schofield, M.; Scaglione, J.; Dixon, S.; Oves-Costales, D.; et al. Functional and Structural Analysis of the Siderophore Synthetase AsbB through Reconstitution of the Petrobactin Biosynthetic Pathway from Bacillus anthracis. J. Biol. Chem. 2012, 287, 16058–16072. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, B.F.; Kim, Y.; Nusca, T.D.; Maltseva, N.; Lee, J.Y.; Rath, C.M.; Scaglione, J.B.; Janes, B.K.; Anderson, E.C.; Bergman, N.H.; et al. Structural and Functional Analysis of AsbF: Origin of the Stealth 3,4-dihydroxybenzoic Acid Subunit for Petrobactin Biosynthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 17133–17138. [Google Scholar] [CrossRef]
- Pfleger, B.F.; Lee, J.Y.; Somu, R.V.; Aldrich, C.C.; Hanna, P.C.; Sherman, D.H. Characterization and Analysis of Early Enzymes for Petrobactin Biosynthesis in Bacillus anthracis. Biochemistry 2007, 46, 4147–4157. [Google Scholar] [CrossRef] [PubMed]
- Barona-Gómez, F.; Wong, U.; Giannakopulos, A.E.; Derrick, P.J.; Challis, G.L. Identification of a Cluster of Genes that Directs Desferrioxamine Biosynthesis in Streptomyces coelicolor M145. J. Am. Chem. Soc. 2004, 126, 16282–16283. [Google Scholar] [CrossRef] [PubMed]
- Challis, G.L. Exploitation of the Streptomyces coelicolor A3(2) Genome Sequence for Discovery of New Natural Products and Biosynthetic Pathways. J. Ind. Microbiol. Biotechnol. 2014, 41, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Günter, K.; Toupet, C.; Schupp, T. Characterization of an Iron-Regulated Promoter involved in Desferrioxamine B Synthesis in Streptomyces pilosus: Repressor-Binding Site and Homology to the Diphtheria Toxin Gene Promoter. J. Bacteriol. 1993, 175, 3295–3302. [Google Scholar] [CrossRef]
- Ronan, J.L.; Kadi, N.; McMahon, S.A.; Naismith, J.H.; Alkhalaf, L.M.; Challis, G.L. Desferrioxamine Biosynthesis: Diverse Hydroxamate Assembly by Substrate-Tolerant Acyl Transferase DesC. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170068. [Google Scholar] [CrossRef]
- Drechsel, H.; Tschierske, M.; Thieken, A.; Jung, G.; Zähner, H.; Winkelmann, G. The Carboxylate Type Siderophore Rhizoferrin and its Analogs Produced by Directed Fermentation. J. Ind. Microbiol. 1995, 14, 105–112. [Google Scholar] [CrossRef]
- Li, B.; Deng, X.; Kim, S.H.; Buhrow, L.; Tomchick, D.R.; Phillips, M.A.; Michael, A.J. Alternative Pathways Utilize or Circumvent Putrescine for Biosynthesis of Putrescine-Containing Rhizoferrin. J. Biol. Chem 2021, 296, 100146. [Google Scholar] [CrossRef]
- Pandey, A.; Sonti, R.V. Role of the FeoB Protein and Siderophore in Promoting Virulence of Xanthomonas oryzae pv. oryzae on Rice. J. Bacteriol. 2010, 192, 3187–3203. [Google Scholar] [CrossRef]
- Pandey, S.S.; Patnana, P.K.; Rai, R.; Chatterjee, S. Xanthoferrin, the α-hydroxycarboxylate-Type Siderophore of Xanthomonas campestris pv. campestris, is Required for Optimum Virulence and Growth Inside Cabbage. Mol. Plant Pathol. 2017, 18, 949–962. [Google Scholar] [CrossRef]
- Thode, S.K.; Rojek, E.; Kozlowski, M.; Ahmad, R.; Haugen, P. Distribution of Siderophore Gene Systems on a Vibrionaceae phylogeny: Database Searches, Phylogenetic Analyses and Evolutionary Perspectives. PLoS ONE 2018, 13, e0191860. [Google Scholar] [CrossRef]
- Crowley, D.E. Microbial Siderophores in the Plant Rhizosphere BT—Iron Nutrition in Plants and Rhizospheric Microorganisms; Barton, L.L., Abadia, J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 169–198. [Google Scholar]
- Masalha, J.; Kosegarten, H.; Elmaci, Ö.; Mengel, K. The Central Role of Microbial Activity for Iron Acquisition in Maize and Sunflower. Biol. Fertil. Soils 2000, 30, 433–439. [Google Scholar] [CrossRef]
- O’Brien, S.; Hodgson, D.J.; Buckling, A. Social Evolution of Toxic Metal Bioremediation in Pseudomonas aeruginosa. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140858. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, E.; Yu, Z.; Pei, X.; Chen, S.; Zhang, P.; Shin, M.-C.; Gong, J.; He, H.; Yang, V.C. CPP-Assisted Intracellular Drug Delivery, What Is Next? Int. J. Mol. Sci. 2016, 17, 1892. [Google Scholar] [CrossRef] [PubMed]
- Tsafack, A.; Libman, J.; Shanzer, A.; Cabantchik, Z.I. Chemical Determinants of Antimalarial Activity of Reversed Siderophores. Antimicrob. Agents Chemother. 1996, 40, 2160–2166. [Google Scholar] [CrossRef]
- Lovejoy, B.D.; Richardson, R.D. Iron Chelators as Anti-Neoplastic Agents: Current Developments and Promise of the PIH Class of Chelators. Curr. Med. Chem. 2003, 10, 1035–1049. [Google Scholar] [CrossRef][Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehan, M.; Barakat, H.; Almami, I.S.; Qureshi, K.A.; Alsohim, A.S. Production and Potential Genetic Pathways of Three Different Siderophore Types in Streptomyces tricolor Strain HM10. Fermentation 2022, 8, 346. https://doi.org/10.3390/fermentation8080346
Rehan M, Barakat H, Almami IS, Qureshi KA, Alsohim AS. Production and Potential Genetic Pathways of Three Different Siderophore Types in Streptomyces tricolor Strain HM10. Fermentation. 2022; 8(8):346. https://doi.org/10.3390/fermentation8080346
Chicago/Turabian StyleRehan, Medhat, Hassan Barakat, Ibtesam S. Almami, Kamal A. Qureshi, and Abdullah S. Alsohim. 2022. "Production and Potential Genetic Pathways of Three Different Siderophore Types in Streptomyces tricolor Strain HM10" Fermentation 8, no. 8: 346. https://doi.org/10.3390/fermentation8080346
APA StyleRehan, M., Barakat, H., Almami, I. S., Qureshi, K. A., & Alsohim, A. S. (2022). Production and Potential Genetic Pathways of Three Different Siderophore Types in Streptomyces tricolor Strain HM10. Fermentation, 8(8), 346. https://doi.org/10.3390/fermentation8080346








