Abstract
In this study, changes in metabolites during the fermentation of Agaricus bisporus compost under the Shanghai Lianzhong secondary fermentation method and Jiangsu Yuguan tertiary fermentation method were analysed by applying gas chromatography–mass spectrometry (GC–MS) to understand the differences in metabolites under different fermentation methods and find metabolic markers at different fermentation stages in different fermentation methods. The results showed that 1002 compounds were identified. Based on the differential metabolites from pathways of significant enrichment, it was found that L-aspartic acid and 5-aminobenzolevulinic acid could be used as potential metabolic markers to evaluate the phase 2 fermentation method of Shanghai Lianzhong and the phase 3 fermentation method of Jiangsu Yuguan, respectively. This study provides a reference for the preparation of quality-stable fermentation materials and further understanding of the cultivation of A. bisporus with fermentation materials.
1. Introduction
Agaricus bisporus is a large fungus belonging to the families Mycota, Eumycota, Basidiomycotina, Eubasidiomycetes, Agaricales, Agricaceae and Agaricus. It is an edible fungus that is most widely cultivated, with the highest yield and largest consumption worldwide.
As a key food source, over 2/3 of edible mushrooms worldwide are grown in mainland China. The mushroom industry in mainland China plays a more important role in food and medicine than in many Western countries, and it provides jobs for more than 25 million farmers [1]. The most important genus of edible fungi commercially produced in China is mushrooms (mainly Agaricus bisporus), accounting for approximately 30% of the global market [2].
The nutrients needed for the growth and development of A. bisporus mainly originate from composts, in which the components are mainly in four categories: carbon sources, nitrogen sources, mineral elements and auxins. Therefore, the fermentation quality of composts is closely related to the above four kinds of substances, and it is also one of the key factors affecting the yield of A. bisporus. A suitable total nitrogen content and C/N ratio are required for high-quality composts [3,4]. The humidity, total nitrogen content, pH and C/N ratio of the culture material should be in the ranges of 68–72%, 2.0–2.4%, 7.4–7.6, and 17–20:1, respectively [5,6,7]. In the second stage of producing edible fungi, where millet straw is used as compost, the physical and chemical indexes are the decisive factors in determining how much edible fungi will be yielded [8], and they need to be in certain ranges to obtain the highest yield. The fermentation of composts of A. bisporus is a process of decomposition and transformation of various chemical substrates by the action of microorganisms. The research mainly focused on the composition of composts [8,9,10,11,12], diversity and dynamics and functional characteristics of the microbial community [13,14], the application of spent mushroom substrate [15,16], the type of culture medium and supplement and the amount of addition [17].
In the past, studies on the composts of A. bisporus have mainly focused on nitrogen content and the C/N ratio and have not reported on metabolites during the fermentation process of composts. At present, the culture process of A. bisporus in China mainly adopts the secondary fermentation method and the tertiary fermentation method. However, due to the high technical requirements of the secondary fermentation method, it tends to cause low yield and quality fluctuations or even result in fermentation failure [18], but these shortcomings can be addressed by the tertiary fermentation method. However, the difference in metabolites in the fermentation process between these two methods has not been researched thus far.
Metabonomics is a science for the qualitative and quantitative analysis of all metabolites in life [19]. This technique is a new discipline that developed rapidly after genomics, transcriptomics and proteomics [20]. It has been reported that the application of the technology to the field of edible fungi has mainly covered the changes in volatile compounds during the ripening process of Volvariella volvacea and Pleurotus ostreatus [21], metabolic markers of damage to A. bisporus [22], and changes in extracellular metabolites in P. ostreatus mycelia under high temperature [23]. Therefore, it seems that the metabonomics technique can provide a way to identify metabolites [24] and study the differences in metabolites in composts under different fermentation methods.
In this study, metabonomic technology was used to study the changes in metabolites based on gas chromatography–mass spectrometry (GC–MS) in the fermentation process of the secondary fermentation method of Shanghai Lianzhong and the tertiary fermentation method of Jiangsu Yuguan. The main purpose of this study was to understand the differences in metabolites and to find the metabolic markers of different fermentation stages under different fermentation methods.
2. Materials and Methods
2.1. Secondary Fermentation Method
The compost was prepared via the tunnel fermentation method. Wheatgrass recipe: On the 1st to 3rd day, compost was created after mixing, and its temperature reached 75 °C. On the 4th to 15th day, the first fermentation in the tunnel was conducted. The compost was then turned three times, and the temperature exceeded 80 °C. On the 16th to 22nd day, the compost entered the second fermentation tunnel. The pasteurization temperature reached 60 °C, and the duration lasted 8 h. When the ammonia concentration was below 0.0005%, the fermented culture material was transported to a mushroom bed by the feeder with a feeding amount of 100–110 kg/m2, where it was sowed for factory fruiting management. After the mushroom was loaded, no soil was used for coverage. Soil was only used for coverage after 20 days when the mycelia budded. After 7 days of soil covering, the mycelia grew out of the surface of the soil. The air temperature in the growth room was gradually reduced to 17 °C. The carbon dioxide concentration was maintained at 1200 ppm, and the relative humidity was maintained at approximately 92% to induce the fruiting of mushroom bodies. After cooling for 11–13 days, mushrooms were picked. After picking, the material temperature was adjusted to 18–20 °C, and the relative humidity was adjusted to 90–92% to culture the strain for 3–5 days. The culture had a total of three mushroom flushes, and the production ended.
2.2. Tertiary Fermentation Method
The compost was prepared via the tunnel fermentation method. Wheatgrass recipe: On the 1st to 3rd day, the compost was prepared after being mixed with wheatgrass, and its temperature reached 75 °C. On the 4th to 15th day, the compost was moved to the first fermentation in the tunnel. The compost was then turned three times, and the temperature exceeded 80 °C. On the 16th to 22nd day, the second fermentation occurred in the tunnel; the pasteurization temperature was 60 °C, and the duration lasted 8 h. When the ammonia concentration reached 0.0005% or less and the temperature reached 25 °C, the strain was sown at a ratio of 0.55% (strain/wet compost: W/W). The wet nutrient material after sowing was transferred to a tunnel for the third fermentation (with the same specifications as the second fermentation in the tunnel) to cultivate the strain for 18–20 days, and the temperature was controlled at 25–26 °C. After the strain was germinated, it was transferred to a bed frame (length × width: 31.5 m × 1.4 m) with a feeding amount of 90–100 kg/m2 and covered with peat soil with a thickness of 4–5 cm. After 7 days of soil covering, the mycelia grew out of the soil surface. The air temperature in the mushroom room was gradually reduced to 17 °C. The carbon dioxide concentration was maintained at 1200 ppm, and the relative humidity was maintained at approximately 92% to induce the fruiting of mushroom bodies. After cooling for 11–13 days, mushrooms were ready for harvesting. After picking, the material temperature was adjusted to 18–20 °C, and the relative humidity was adjusted to 90–92% to culture the strain for 3–5 days, and 10 L of water was added at the same time. A total of three mushroom flushes were achieved, and the production ended.
The difference between the methods of the secondary and tertiary fermentations is as follows:
In the actual process, the yields under the secondary fermentation method were lower than those under the tertiary fermentation method, showing that it was not economically viable. The tertiary fermentation technique was introduced from the Netherlands. Its fermentation is automatically controlled in tunnel facilities. Automated equipment is used in most sections, such as prewetting, turning, filling, ventilating, and discharging. After the second fermentation is finished, the material directly enters the tunnel for a third closed germination. According to our surveys of production that applied the two approaches in China, we found that by applying the tertiary fermentation technique, the production of A. bisporus plants was stable in yields that were always higher than those of factories applying the secondary fermentation approach.
2.3. Experimental Design
In terms of the secondary fermentation method, we took samples at the Shanghai Lianzhong Edible Fungus Cooperative. One group of 6 samples from each of 6 sites in the cooperative was collected for each stage of fermentation. The samples from the first stage of fermentation were named N1F1, N1F2, N1F3, N1F4, N1F5 and N1F6, abbreviated as N1F, and the samples from the second stage of fermentation were named TS1, TS2, TS3, TS4, TS5, and TS6, abbreviated as TS. The samples are shown in Figure 1.

Figure 1.
Fermentation Samples: Samples in each tunnel of the tertiary fermentation method and the secondary fermentation method.
In the tertiary fermentation method, we took samples at the Jiangsu Yuguan Edible Fungus Cooperative. One group of 6 samples from each site in the cooperative was taken for each stage of fermentation. The 6 samples from the first stage of fermentation were named Phase I 1, Phase I 2, Phase I 3, Phase I 4, Phase I 5, and Phase I 6, abbreviated as Phase I; the 6 samples from the second stage of fermentation were named Phase II 1, Phase II 2, Phase II 3, Phase II 4, Phase II 5, and Phase II 6, abbreviated as Phase II; and the 6 samples from the third stage of fermentation were named Phase III 1, Phase III 2, Phase III 3, Phase III 4, Phase III 5, and Phase III 6, abbreviated as Phase III. The samples are shown in Figure 1.
The main materials used for fermentation were chicken manure and wheat straw for the secondary fermentation method and the tertiary fermentation method.
2.4. GC–MS Analysis
Headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME-GC–MS) was used in this experiment. Compost (3.0 g) was introduced into a 15 mL headspace-injection glass vial, and the vial was sealed tightly. At 100 °C, the SPME syringe was then inserted into the headspace of the vial with a 50/30 μm divinylbenzene/Carboxen/polydimethylsiloxane (DVB/CAR/PDMS)-coated fibre purchased from Supelco and shaken for 15 min at a speed of 450 rpm. The compounds were extracted for 40 min.
After extraction, the SPME fibre was placed into the injector of the GC–MS instrument and maintained for 5 min at 260 °C for desorption under splitless injection mode. A Pegasus BT GC-TOF Mass Spectrometer (LECO Corporation, St. Joseph, MI, USA) was utilized for this analysis with a DB-wax column (30 m × 0.25 mm × 0.25 µm, Agilent J&W Scientific, Folsom, CA, USA). The oven temperature program was as follows: 40 °C for 5 min, then increased to 250 °C at a rate of 5 °C/min and held for 5 min at 250 °C. High-purity helium (≥the purity of 99.999%) was used as the carrier gas at a flow rate of 1.0 mL/min. The mass spectrometer was operated in full scan mode from 20 to 650 m/z and with electron impact (EI) ionization at 70 eV and 230 °C at a quadrupole temperature of 150 °C.
2.5. Data Analysis
The raw GC–MS data were preprocessed by Chroma TOF (v 4.34, LECO, St Joseph, MI, USA) software. The response intensity of the spectral peak of the sample was normalized by the peak area normalization method, and the normalized data matrix was obtained and qualitatively compared to data in the NIST database (National Institute of Standards and Technology). Then, the data were analysed by principal component analysis (PCA) and (Orthogonal) partial least squares discrimination analysis ((O)PLS-DA). All data processing was conducted on the free online Majorbio Cloud Platform (www.majorbio.com accessed on 29 March 2019).
3. Results and Discussion
3.1. Analysis and Results
3.1.1. Identification of Metabolites (GC–MS Data Analysis)
Based on the nontargeted metabonomic method of GC–MS, the samples of secondary and tertiary fermentation of P. ostreatus from the industrial tunnel of Lianzhong Cooperative of Shanghai Province and Yuguan Company of Jiangsu, respectively, were collected after each stage of fermentation, and the metabolites were analysed. A total of 1002 compounds (Table S1) were identified during data preprocessing (removal of eigenvalues with missing values > 100%, filling method with minimum missing values, and data normalization method with summation).
All the metabolites were obtained after identification by MS and compared with the KEGG Database and Human Metabolome Database (HMDB). Then, the annotation information in the database was obtained, and the number of annotated metabolites in the database was counted. The metabolites identified were compared to the KEGG compound database to obtain the classification of the metabolites (Figure 2). All the compounds were mainly concentrated in compounds with biological roles (mainly amino acids and vitamins, etc.), phytochemical compounds (mainly alkaloids and terpenoids) and lipids (mainly PR01 isoprenoids).
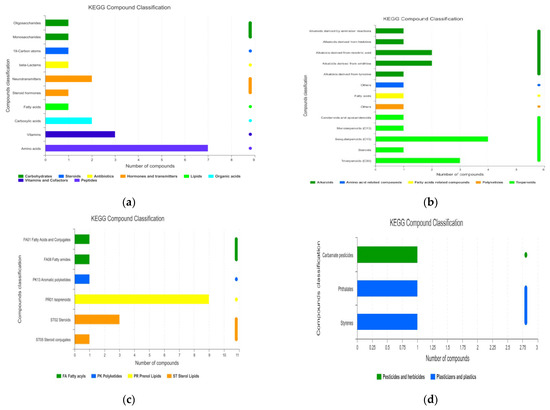
Figure 2.
Classification of metabolites based on Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation: (a) compounds with biological roles; (b) phytochemical compounds; (c) lipids; (d) pesticides; (e) endocrine disrupting compounds.
From Figure 2, the main metabolites obtained were terpenoids, alkaloids, fatty acid-related compounds, amino acid-related compounds, and polyketides. Of these, there were 10 and 7 terpenoids and alkaloids, respectively.
According to the information of metabolic pathways involved in metabolites (Figure 3), metabolites were mainly concentrated in such metabolism pathways, including amino acid metabolism, metabolism of cofactors and vitamins, carbohydrate metabolism, and metabolism of terpenoids and polyketides. The important pathways included ABC transporters (ATP-binding cassette transporter), aminoacyl-tRNA biosynthesis, and sesquiterpenoid and triterpenoid biosynthesis.
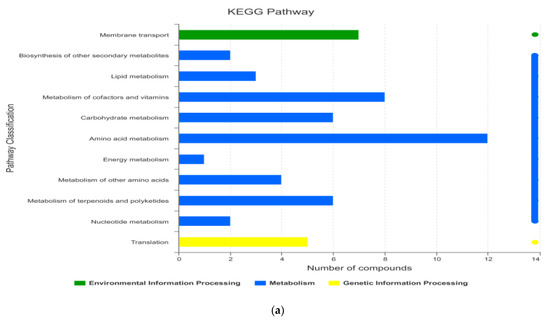
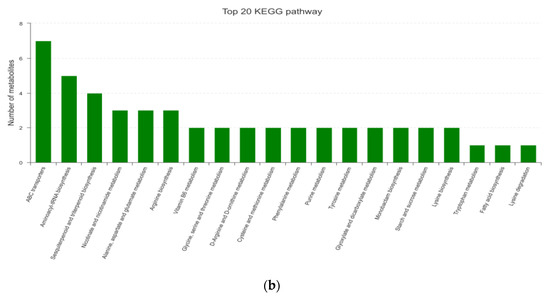
Figure 3.
Path analysis and functional annotation based on KEGG ((a) path analysis; (b) functional annotation).
3.1.2. PCA of Metabolites
From each group of samples, it was found that the contribution rates of PC1 and PC2 reached 27.1% and 21.5%, respectively (Figure 4a). There were high similarities and small differences between N1F and phase I and between TS and phase II. The difference between N1F and phase III was large (Figure 4a), showing a far distance from phase III. Further analysis of the contribution rates of PCA showed that PC1 and PC2 between N1F and phase I reached 39.0% and 25.3%, respectively (Figure 4b). The contribution rates of PC1 and PC2 between TS and phase II were 40.9% and 15.9%, respectively (Figure 4c).
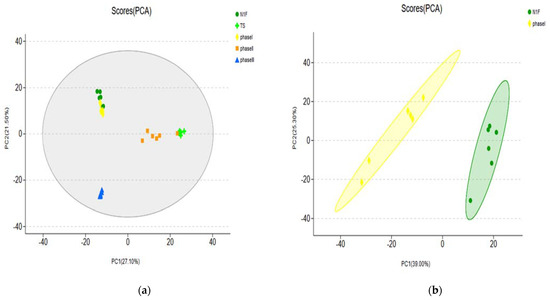
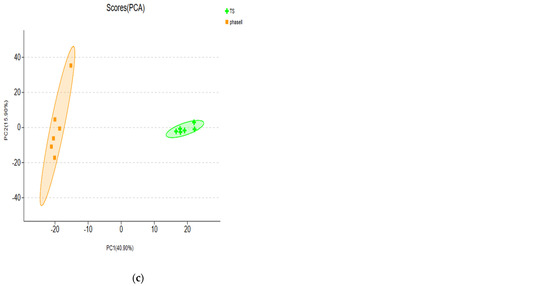
Figure 4.
Principal component analysis of samples. (a) PCA analysis of all groups (b) PCA analysis of the N1Fand Phase I (c) PCA analysis of the TS andPhase I.
3.1.3. Differential Metabolite Analysis
To further analyse the metabolites of different fermentation stages between the secondary fermentation method and the tertiary fermentation method, PLS-DA and OPLS-DA were carried out. According to the values of R2X (cum), R2Y (cum) and Q2 of each model, the PLS-DA model was selected for analysis. PLS-DA of each differential metabolic group showed that the separation degree of the two groups was higher, indicating that the classification effect was significant (Figure 5). The R2Y (cum) and Q2 of each differential metabolic group based on PLS-DA show that they have good fitting and satisfactory prediction ability (Table 1).
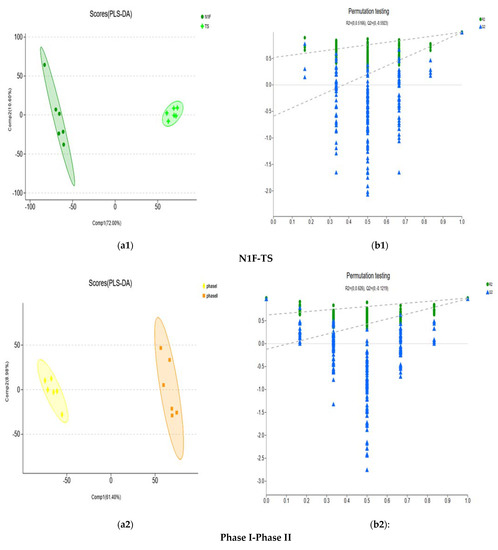
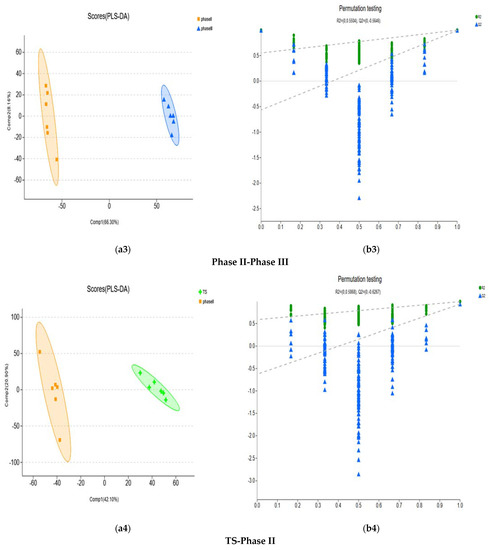
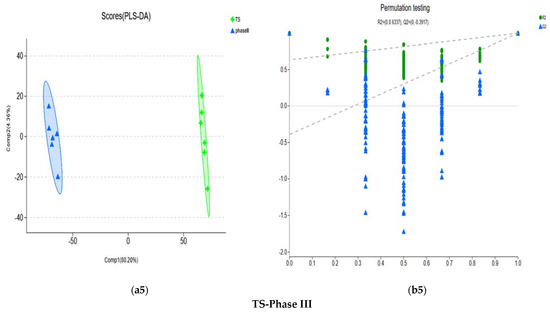
Figure 5.
Score plots and permutation testing under the PLS-DA model for compost metabolites of different groups. (a1,b1) represent the PLS-DA analysis of N1F-TS comparison groups. (a2,b2) represent PLS-DA analysis of Phase I-Phase II comparison groups. (a3,b3) represent PLS-DA analysis of Phase II–Phase III comparison groups. (a4,b4) represent PLS-DA analysis of the TS-Phase II comparison groups. (a5,b5) represent PLS-DA analysis of the TS-Phase III comparison groups. PLS-DA model verification: The X-axis represents the permutation retention of the permutation test (Keep the scale consistent with the original model Y variable order. The point with the permutation retention of 1 is the R2 and Q2 values of the original model). The Y-axis represent the values of the permutation test for R2 (green dots) and Q2 (blue triangles). The two dashed lines represent the regression lines of R2 and Q2, respectively.

Table 1.
Parameters of differential metabolites based on PLS-DA.
A 200-time permutation test was used to evaluate whether overfitting the PLS-DA would occur. The results showed that the PLS-DA model did not show overfitting, and it was credible based on the R2 intercept and Q2 intercept of each differential metabolic group (Figure 5).
Based on the VIP_PLS-DA > 1 and p value < 0.05 of the PLS-DA model, the differential metabolites of each sample group were screened and are listed in Table S2.
The differences in metabolites among treatments are shown in Figure 6. There were 92 differential metabolites between TS and N1F, 69 differential metabolites between Phase I and Phase II, and 48 differential metabolites between the two groups (Figure 6a). There were 123 differential metabolites between Phase II and Phase III and 39 differential metabolites between TS and N1F (Figure 6b). There were 48 common differential metabolites between TS-Phase II and Phase II–Phase III (Figure 6c).
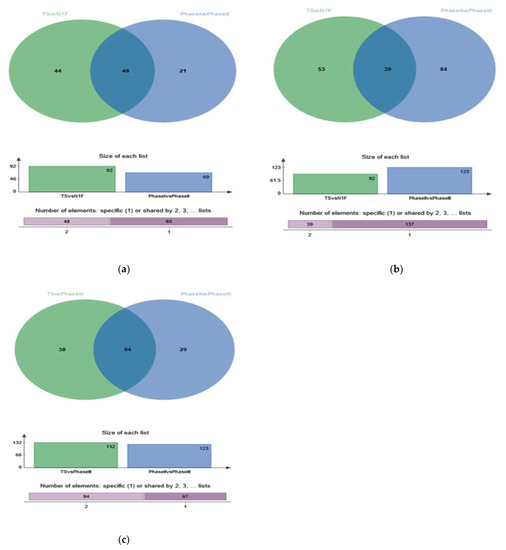
Figure 6.
VENN diagrams of differential metabolites among different metabolic groups. (a): Venn_ TS vs N1F, Phase I vs Phase II. (b) Venn-TS vs N1F, Phase II vs Phase III. (c): Venn-TS vs phase III, Phase II vs Phase III.
3.1.4. KEGG Enrichment Analysis and Main Differential Metabolites
The pathways of the differential metabolites between the N1F and TS stages in the Shanghai Lianzhong secondary fermentation method were mainly concentrated in carbohydrate metabolism, amino acid metabolism, membrane transport and other KEGG pathways (Figure 7a1). The enrichment analysis showed that the significantly enriched pathways were synthesis and degradation of ketone bodies (p < 0.01), arginine biosynthesis (p < 0.05), pantothenate and CoA biosynthesis (p < 0.05), alanine, aspartate, and glutamate metabolism (p < 0.05), beta-alanine metabolism (p < 0.05), phenylalanine, tyrosine and tryptophan biosynthesis (p < 0.05), and ABC transporters (Figure 7b1).
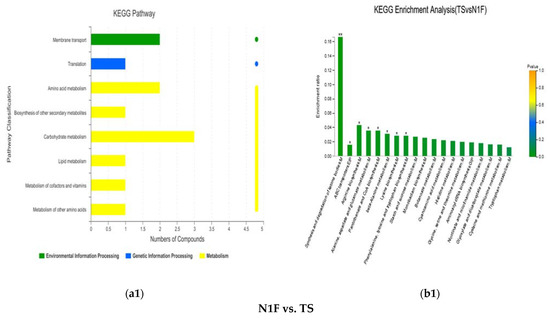
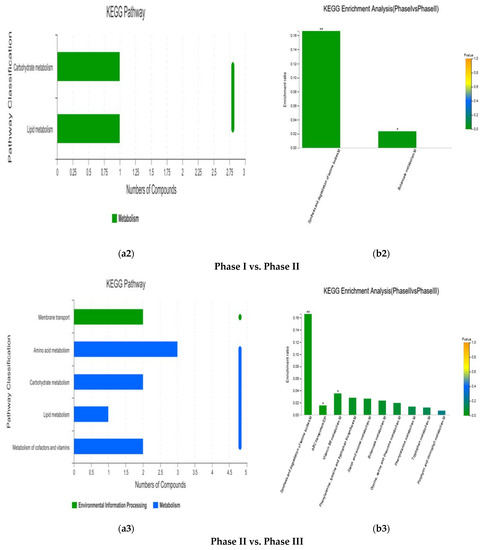
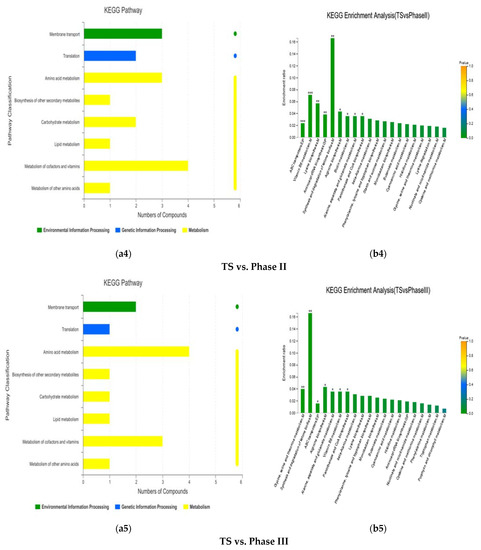
Figure 7.
KEGG pathway and enrichment analysis of differential metabolites of the metabolic set. (a1–a5) represent the path analysis between comparison groups at different stages, and (b1–b5) represent the functional annotations between comparison groups at different stages.
In the tertiary fermentation process of Jiangsu Yuguan, the pathways of differential metabolites between phase I and phase II were mainly concentrated in carbohydrate metabolism and lipid metabolism (Figure 7a2). The enrichment analysis showed that the significantly enriched pathways were synthesis and degradation of ketone bodies (p < 0.01) and butanoate metabolism (p < 0.05) (Figure 7b2). The main differential metabolite involved in the pathway was (R)-3-hydroxybutyric acid, which was significantly decreased from phase I to phase II (p < 0.01) (Table 2).

Table 2.
Significantly enriched pathways and differential metabolites.
The main differential metabolites involved in the pathway were (R)-3-hydroxybutyric acid, indole, L-aspartic acid, and maltose. (R)-3-Hydroxybutyric acid (p < 0.01), L-aspartic acid (p < 0.01), and indole (p < 0.01) were significantly higher at the TS stage than at the N1F stage for the secondary fermentation method in Shanghai lianzhong, while maltose (p < 0.01) was significantly lower (Table 2).
In the tertiary fermentation method of Jiangsu Yuguan, the pathways of the differential metabolites between phase II and phase III were mainly concentrated in membrane transport, amino acid metabolism, carbohydrate metabolism, lipid metabolism, metabolism of cofactors and vitamins and other KEGG pathways(Figure 7a3). Enrichment analysis showed that the significantly enriched pathways were synthesis and degradation of ketone bodies (p < 0.01), vitamin B6 metabolism (p < 0.05), and ABC transporters (p < 0.05) (Figure 7b3). (R)-3-Hydroxybutyric acid and 5-aminolevulinic acid had significantly higher contents (p < 0.01), while there were significantly lower contents in pyridoxal (p < 0.01) and maltose (p < 0.05) at phase III than at phase II (Table 2).
The pathways including the differential metabolites between the TS stage of the Shanghai Lianzhong secondary fermentation method and phase II of the Jiangsu Yuguan tertiary fermentation method were mainly concentrated in metabolism of cofactors and vitamins, amino acid metabolism, carbohydrate metabolism, membrane transport and other KEGG pathways (Figure 7a4). The enrichment analysis showed that the pathways of significant enrichment were synthesis and degradation of ketone bodies (p < 0.01), vitamin B6 metabolism (p < 0.001), lysine biosynthesis (p < 0.01), aminoacyl-tRNA biosynthesis (p < 0.01), arginine biosynthesis (p < 0.05), biotin metabolism (p < 0.05), alanine, aspartate, and glutamate metabolism (p < 0.05), pantothenate and CoA biosynthesis (p < 0.05), and ABC transporters (p < 0.001) (Figure 7b4). Compared to the Phase II stage, (R)-3-hydroxybutyric acid, pyridoxal, L-aspartic acid and L-lysine had significantly higher contents (p < 0.01), while pyridoxamine 5-phosphate (p < 0.01) and maltose (p < 0.05) had significantly lower contents at the TS stage (Table 2 and Table S2).
The pathways of the differential metabolites between the TS stage of the Shanghai Lianzhong secondary fermentation method and the Phase III stage of Jiangsu Yuguang tertiary fermentation method were mainly concentrated in amino acid metabolism, metabolism of cofactors and vitamins, and membrane transport(Figure 7a5). Enrichment analysis showed that the pathways of significant enrichment were synthesis and degradation of ketone bodies (p < 0.01); arginine biosynthesis (p < 0.05); glycine, serine and threonine metabolism (p < 0.01); alanine, aspartate and glutamate metabolism (p < 0.05); vitamin B6 metabolism (p < 0.05); pantothenate and CoA biosynthesis (p < 0.05); and ABC transporters (p < 0.05) (Figure 7b5). Compared to phase III, (R)-3-hydroxybutyric acid, L-aspartic acid and pyridoxal had significantly higher contents (p < 0.01), while 5-aminolevulinic acid had significantly lower contents at the TS stage (p < 0.01) (Table 2).
Some of the main and significantly KEGG enrichment pathways and differential metabolites between the above fermentation stages are shown in Figure 8. For the differential metabolites in synthesis and degradation of ketone bodies, (R)-3-hydroxybutyric acid, (See Figure 8a); for the differential metabolites of glycine, serine and threonine metabolism, L-Aspartate (See Figure 8b); for the differential metabolites of lysine biosynthesis, L-lysine, (See Figure 8c); for the differential metabolites of vitamin B6 metabolism, Pyridoxal, (See Figure 8d), and for the differential metabolite of arginine biosynthesis, Aspartate, (See Figure 8e))… these red-marked differential metabolites were significantly increased between different fermentation stages.
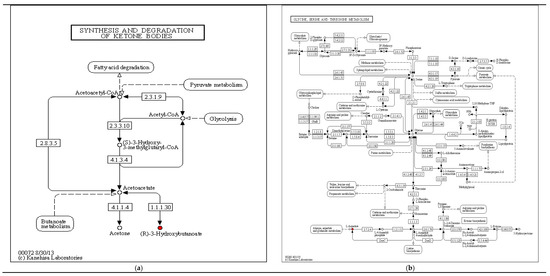
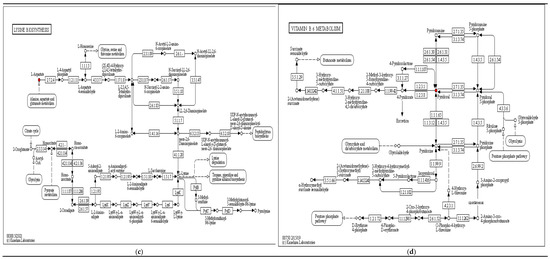
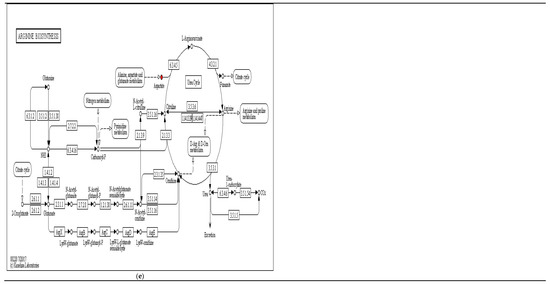
Figure 8.
Significant enrichment KEGG pathway ((a) synthesis and degradation of ketone bodies; (b) glycine, serine and threonine metabolism; (c) lysine biosynthesis; (d) vitamin B6 metabolism; (e) arginine biosynthesis).
3.2. Discussion
Currently, the factory cultivation of A. bisporus is developing rapidly, and the process mainly includes the secondary fermentation method and the tertiary fermentation method. The production of A. bisporus is a process of transforming raw materials into substrates that will promote the growth of A. bisporus (divided into two stages: PI and PII) [25]. It was found that the phase I (PI) stage lasted for 17 days in the fermentation process with millet straw as the culture material, which was approximately 2–3 times that of wheat straw and wheat–rice mixed straw, which is consistent with the observations of Zhang et al. [8], Vos et al. [10], Zhang et al. [11] and Wang et al. [12]. In the PI stage, we observed that thermophilic microorganisms proliferated and degraded carbohydrates and proteins when the temperature was up to 80 °C, which resulted in the release of heat and ammonia, consistent with the observations of Vieira et al. [13]. These reactions led to the softening of raw materials such as wheat straw and rice straw, which is consistent with the observations of Royse et al. [7,26]. In phase II, pasteurization was mainly used to eliminate insects, nematodes, competitive species and pathogens, while conditioning was mainly used to remove ammonia generated in the first stage, decompose carbohydrate sources (including hemicellulose and cellulose) and establish beneficial microflora that can feed and/or stimulate mushroom growth [13,27]. The activity and transcript levels decrease during mushroom formation, and therefore, laccase can only be used as a biomarker during phase III.
In the secondary fermentation method, the temperature and time during the fermentation process were not easy to control, and it tended to cause problems such as compost retting and a high free ammonia content, resulting in fermentation failure according to Zhang et al. [18]. These problems of the secondary fermentation method can be solved by continuous steam heating and heat preservation after secondary fermentation. According to the yield measurement, the average yield of A. bisporus in the Shanghai Lianzhong secondary fermentation method was 22.5 kg/m2 and that in the Jiangsu Yuguan tertiary fermentation method was 33.5 kg/m2 (Unreported), which may demonstrate that the tertiary fermentation method could significantly increase the yield of A. bisporus. The reason for the difference may be related to the change in metabolites in the fermentation process of the compost. Reports on changes in metabolites in the fermentation stage of secondary fermentation and tertiary fermentation method have not yet been published. We believe these metabolites may be important substances that reflect the quality of fermentation.
Based on the VIP > 1 and p value < 0.05 of PLS-DA analysis, 92 differential metabolites between the N1F and TS stages in the secondary fermentation method of Shanghai Lianzhong were identified, while 69 differential metabolites between phase I and phase II were determined in the tertiary fermentation method of Jiangsu Yutan, and 123 differential metabolites were found between phase II and phase III, indicating that more differential metabolites were produced by the tertiary fermentation method than the second fermentation process (Table S2). (R)-3-Hydroxybutyric acid was found at different stages. This compound mainly exists as a carbon source and energy storage material in organisms and is a commonly used material in solid-state fermentation. The difference in metabolites between the secondary fermentation method and Jiangsu Yuguan tertiary fermentation method was mainly involved in carbohydrate metabolism and amino acid metabolism. Enrichment analysis of KEGG pathways between the first stage and second stage in the secondary fermentation method of Shanghai Lianzhong and the tertiary fermentation method of Jiangsu Yuguan shows that the two methods have the same pathway of significant or extremely significant enrichment: synthesis and degradation of ketone bodies. In addition, the pathways of significant or extremely significant enrichment, including differential metabolites between the two stages of the secondary fermentation model of Shanghai Lianzhong, are also amino acid metabolic pathways, such as arginine, alanine, aspartate and glutamate metabolism; phenylalanine, tyrosine and tryptophan synthesis and metabolism; and vitamin B5 (pantothenate) and CoA biosynthesis, in addition to the pathway of synthesis and degradation of ketone bodies. Enrichment in metabolites along the two stages of the secondary fermentation method was higher than that of the Jiangsu Yuguan tertiary fermentation method.
The enriched pathways that were significant or extremely significant in phase II and phase III of the Jiangsu Yuguan tertiary fermentation method were the synthesis and degradation of ketone bodies, which was the same as that in phase I and phase II of the Shanghai Lianzhong fermentation method. The enriched pathways that were significant or extremely significant were also vitamin B6 metabolism and ABC transporters in phase II and phase III of the Jiangsu Yuguan tertiary fermentation method.
In both fermentation methods, the significantly enriched pathways, including differential metabolites between the NT stage and phase II were not only the synthesis and degradation of ketone bodies and amino acid metabolism, but also vitamin B6 metabolism, biotin metabolism, and pantothenic and CoA biosynthesis. Biotin is one of the few necessary sulfur cofactors involved in carbon dioxide transfer in carboxylation, decarboxylation and transcarboxylation in all organisms according to Knowles et al. [28,29,30]. The essential water-soluble vitamin B6 consists of six different vitamins, the alcohol pyridoxine (PN), theamine pyridoxamine (PM), thealdehyde pyridoxal (PL) and their respective 5′ phosphorylated forms (PNP, PMP, PLP) [31], in which the enzyme cofactor PLP is necessary for more than 140 enzyme reactions [32]. The main metabolic pathways for vitamin B6 to exert key coenzyme activities include (1) synthesis and catabolism of amino acids [33,34] and (2) conversion of amino acids to bioactive amines [35,36].
In the pathway of significant enrichment between the N1F and TS stages of the secondary fermentation method in Shanghai Lianzhong, differential metabolites with higher contents in the TS stage compared to those in the N1F stage were (R)-3-hydroxybutyric acid (p < 0. 01), L-aspartic acid (p < 0.01) and indole (p < 0.01). The results showed that the compost participated in carbon metabolism and amino acid metabolism in the process of fermentation, which accumulated carbon and nitrogen sources for the growth of P. ostreatus. In the secondary fermentation method, the content of L-aspartic acid in the TS stage was more than 10 times higher than that in the N1F stage. Therefore, it is speculated that L-aspartic acid can be used as a potential marker to identify the fermentation quality of composts, but we think the marker for the evaluation of the fermentation quality needs to be further verified.
The results may suggest that with the fermentation of the culture substrate, carbon and nitrogen metabolites accumulated, which provided nutrients for the cultivation of P. ostreatus. The content of 5-aminolevulinic acid was relatively low in phase I and phase II of the tertiary fermentation process but was extremely significantly increased in phase III (p < 0.01). It is speculated that 5-aminolevulinic acid can be used as a potential marker to measure the fermentation quality of composts under the tertiary fermentation method, but we think this compound needs to be further verified as a marker to evaluate fermentation quality. Previous studies have shown that A. bisporus, during vegetative growth, is able to modify lignin structures. It is proposed that the observed lignin degradation and modification increase the bioavailability of carbohydrates in wheat-based compost. However, a decrease in xylosyl residues could be observed in phase III 16 compared to phase II. In all phases, the main carbohydrate constituents were xylosyl (28–35 mol%) and glucosyl (52–56 mol%) residues, which was in agreement with previously published data. Recently, it was shown that during phase III composting, xylan is partly degraded, thereby increasing its water solubility.
5-Aminolevulinic acid is an important precursor of all tetrapyrrole compounds, such as chlorophyll and haem, in plants. As a plant growth regulator, this compound is also widely recommended to be used to promote the growth and yield of crops according to Xie et al. [37,38]. Pyridoxal is the existing form of vitamin B6, and its phosphorylated form PLP is a coenzyme of more than 100 cellular enzymes involved in various reactions of amino acid metabolism and many other metabolic processes, as per McCormick and Chen [39].
To compare the difference in metabolites between the secondary fermentation method and tertiary fermentation method, we analysed the metabolites at the TS stage in the secondary fermentation method and at phase II and phase III in the Jiangsu Yuguan tertiary fermentation method. At the TS stage of the secondary fermentation method, the contents of (R)-3-hydroxybutyric acid, pyridoxal, L-aspartic acid and L-lysine increased significantly (p < 0.01), while there was a significant decrease in the contents of pyridoxamine 5-phosphate (p < 0.01) and maltose (p < 0.05) compared to phase II of the tertiary fermentation method. Additionally, at the TS stage of the secondary fermentation method, the contents of (R)-3-hydroxybutyric acid, L-aspartic acid and pyridoxal increased significantly (p < 0.01), while the content of 5-aminobenzolevulinic acid decreased significantly (p < 0.01) compared to phase III of the tertiary fermentation method. This result may again prove that L-aspartic acid and 5-aminobenzolevulinic acid can be used as potential markers to evaluate the secondary fermentation method of Shanghai Lianzhong and the tertiary fermentation method of Jiangsu Yuguan, respectively. Carrasco and Preston [40] considered differential metabolites to be the main factors affecting fermentation quality. Tertiary fermentation was also studied to identify metabolites that promote mycelial growth. The compost utilized in the mushroom bed frame after phase II fermentation in the Shanghai Lianzhong Factory was not sampled and analysed. Therefore, there is no way to compare the small-molecule metabolites in the mycelial fermentation of the two factories. Further experimental analysis will be carried out in the future. The specific roles of these differential small molecules in the fermentation process need further verification. However, as few studies have been carried out by peers on such metabolites, we are limited to making comparisons.
4. Conclusions
In this study, we analysed the changes in metabolites during the fermentation process of A. bisporus composts under the secondary fermentation method and the Jiangsu Yuguan tertiary fermentation method employing GC–MS. We identified a total of 1002 compounds. Based on the differential metabolites from pathways of significant enrichment, it was found that L-aspartic acid and 5-aminobenzolevulinic acid can potentially be used as metabolic markers to evaluate the phase II fermentation method of Shanghai Lianzhong and the phase III fermentation method of Jiangsu Yuguan, respectively.
In the future, we will further verify the differential metabolites. Overall, our study provides further insights into the mechanics that promote mushroom growth and identified differential metabolites at different fermentation stages.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation8050222/s1, Table S1: Table of cationic results; Table S2: Differential metabolites at different fermentation stages of the two companies.
Author Contributions
Conceptualization, J.J., Q.W., Z.G., T.X. and J.H.; methodology, H.C. and X.S.; data curation, Q.W., J.Z. and J.H.; writing—original draft preparation, J.J., Z.G. and J.H.; writing—review and editing, J.H.; J.J. and Z.G., visualization, J.J. and J.H.; supervision, J.J. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Agriculture Research System of Shanghai, China [Grant No. 202209].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
We have obtained written informed consent from the patient for the publication of this article.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The data were analyzed on the online platform of Majorbio Cloud Platform (www.majorbio.com).The authors would like to thank zhiyong zhang (Shanghai Luming Biotech. Co. Ltd.) for assistance with the bioinformatics analysis of Data-Independent Acquisition(GC-MS metabolomics).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Y.Q.; Geng, W.; Shen, Y.Q.; Wang, Y.L.; Dai, Y.C. Edible mushroom cultivation for food security and rural development in China: Bio-innovation, technological dissemination and marketing. Sustainability 2014, 6, 2961–2973. [Google Scholar] [CrossRef]
- Royse, D.J.; Singh, M. A Global Perspective on the High Five: Agaricus, Pleurotus, Lentinula, Auricularia & Flammulina. In Proceedings of the 8th International Conference on Mushroom Biology & Mushroom Products, New Delhi, India, 19–22 November 2014; CABI: Wallingford, UK, 2014; pp. 1–6. [Google Scholar]
- Van Griensven, L.J.L.D. The cultivation of mushrooms. Nature 1988, 142, 416. [Google Scholar] [CrossRef]
- Sharma, H.S.; Kilpatrick, M. Mushroom (Agaricus bisporus) compost quality factors for predicting potential yield of fruiting bodies. Can. J. Microbiol. 2000, 46, 515–519. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, M.C.; de Jesus, J.P.; Vieira, F.R.; Viana, S.R.; Spoto, M.H.; de Almeida Minhoni, M.T. Dynamics of the chemical composition and productivity of composts for the cultivation of Agaricus bisporus strains. Braz. J. Microbiol. 2013, 44, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Zied, D.C.; Pardo-González, J.E.; Minhoni, M.T.A.; Pardo-Giménez, A. A reliable quality index for mushroom cultivation. J. Agric. Sci. 2011, 3, 50–61. [Google Scholar] [CrossRef]
- Royse, D.J.; Chalupa, W. Effects of spawn, supplement and phase II compost additions and time of re-casing second break compost on mushroom (Agaricus bisporus) yield and biological efficiency. Bioresour. Technol. 2009, 100, 5277–5282. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Wei, J.-K.; Wang, Q.-H.; Yang, R.; Gao, X.-J.; Sang, Y.-X.; Cai, P.-P.; Zhang, G.-Q.; Chen, Q.-J. Lignocellulose utilization and bacterial communities of millet straw based mushroom (Agaricus bisporus) production. Sci. Rep. 2019, 9, 1151. [Google Scholar] [CrossRef]
- Altieri, R.; Esposito, A.; Parati, F.; Lobianco, A.; Pepi, M. Performance of olive mill solid waste as a constituent of the substrate in commercial cultivation of Agaricus bisporus. Int. Biodeterior. Biodegrad. 2009, 63, 993–997. [Google Scholar] [CrossRef]
- Vos, A.M.; Heijboer, A.; Boschker, H.T.S.; Bonnet, B.; Lugones, L.G.; Wösten, H.A.B. Microbial biomass in compost during colonization of Agaricus bisporus. AMB Express 2017, 7, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Yang, S.; Zhang, W.; Xu, M.; Ma, A.; Zhuang, G.; Chen, G.; Liu, W. Diversity and dynamics of the microbial community on decomposing wheat straw during mushroom compost production. Bioresour. Technol. 2014, 170, 183–195. [Google Scholar] [CrossRef]
- Wang, L.; Mao, J.; Zhao, H.; Li, M.; Wei, Q.; Zhou, Y.; Shao, H. Comparison of characterization and microbial communities in rice straw- and wheat straw-based compost for Agaricus bisporus production. J. Ind. Microbiol. Biotechnol. 2016, 43, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.R.; Pecchia, J.A.; Segato, F.; Polikarpov, I. Exploring oyster mushroom (Pleurotus ostreatus) substrate preparation by varying phase I composting time: Changes in bacterial communities and physicochemical composition of biomass impacting mushroom yields. J. Appl. Microbiol. 2019, 126, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.A.; Thai, M. Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Appl. Microbiol. Biotechnol. 2018, 102, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yuan, X.; Wang, S.; Sun, F.; Hou, Z.; Hu, Q.; Zhai, L.; Cui, Z.; Zou, Y. Methane production and characteristics of the microbial community in the co-digestion of spent mushroom substrate with dairy manure. Bioresour. Technol. 2018, 250, 611–620. [Google Scholar] [CrossRef]
- Vieira, F.R.; de Andrade, M.C. Optimization of substrate preparation for oyster mushroom (Pleurotus ostreatus) cultivation by studying different raw materials and substrate preparation conditions (composting: Phases I and II). World J. Microbiol. Biotechnol. 2016, 32, 190. [Google Scholar] [CrossRef] [PubMed]
- Jeznabadi, E.K.; Jafarpour, M.; Eghbalsaied, S. King oyster mushroom production using various sources of agricultural wastes in Iran. Int. J. Recycl. Org. Waste Agric. 2016, 5, 17–24. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Gong, G.Z.; Li, Q.; Weng, H.D.; Cai, K.D. Stack fermentation technology of Agdcus bisporus compost. Edible Fungi China 2009, 28, 29–30, 35. [Google Scholar]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Hollywood, K.A.; Goodacre, R. Metabolomics for the masses: The future of metabolomics in a personalized world. New Horiz. Transl. Med. 2017, 3, 294–305. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Wu, W.-W.; Li, G.-K. A GC—MS study of the volatile organic composition of straw and oyster mushrooms during maturity and its relation to antioxidant activity. J. Chromatogr. Sci. 2008, 46, 690–696. [Google Scholar] [CrossRef]
- O’Gorman, A.; Barry-Ryan, C.; Frías, J. Evaluation and identification of markers of damage in mushrooms (Agaricus bisporus) postharvest using a GC/MS metabolic profiling approach. Metabolomics 2011, 8, 120–132. [Google Scholar] [CrossRef]
- Qiu, Z.; Wu, X.; Zhang, J.; Huang, C. High-temperature induced changes of extracellular metabolites in Pleurotus ostreatus and their positive effects on the growth of Trichoderma asperellum. Front. Microbiol. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, F.; Huang, F.; Xie, G.; Wei, R.; Chen, T.; Liu, J.; Zhao, A.; Jia, W. Metabolomics analysis reveals variation in Schisandra Chinensis cetabolites from different origins. J. Sep. Sci. 2014, 37, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.R.; Pecchia, J.A. An exploration into the bacterial community under different pasteurization conditions during substrate preparation (composting-phase II) for Agaricus bisporus cultivation. Microb. Ecol. 2018, 75, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Jurak, E.; Punt, A.M.; Arts, W.; Kabel, M.A.; Gruppen, H. Fate of carbohydrates and lignin during composting and mycelium growth of Agaricus bisporus on wheat straw based compost. PLoS ONE 2015, 10, e0138909. [Google Scholar] [CrossRef] [PubMed]
- Straatsma, G.; Gerrits, J.P.G.; Thissen, J.T.N.M.; Amsing, J.G.M.; Loeffen, H.; Van Griensven, L.J.L.D. Adjustment of the composting process for mushroom cultivation based on initial substrate composition. Bioresour. Technol. 2000, 72, 67–74. [Google Scholar] [CrossRef]
- Knowles, J.R. The mechanism of biotin-dependent enzymes. Annu. Rev. Biochem. 1989, 58, 195–221. [Google Scholar] [CrossRef]
- Hymes, J.; Wolf, B. Biotinidase and its roles in biotin metabolism. Clin. Chim. Acta 1996, 255, 1–11. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Zou, T.; Zhang, H.; Wang, Y.; Gao, R.; Li, Z.; He, J.; Feng, Y. Mycobacterium smegmatis BioQ defines a new regulatory network for biotin metabolism. Mol. Microbiol. 2014, 94, 1006–1023. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Amrhein, N.; Kappes, B.; Macheroux, P.; Tews, I.; Raschle, T. Two independent routes of de novo vitamin B6 biosynthesis: Not that different after all. Biochem. J. 2007, 407, 1–13. [Google Scholar] [CrossRef]
- Colinas, M.; Fitzpatrick, T.B. Interaction between vitamin B6 metabolism, nitrogen metabolism and autoimmunity. Plant Signal. Behav. 2016, 11, e1161876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Olson, B.; Bakun, P.; Dallal, G.E.; Selhub, J.; Rosenberg, I.H. Breakfast cereal fortified with folic acid, vitamin B-6, and vitamin B-12 increases vitamin concentrations and reduces homocysteine concentrations: A randomized trial. Am. J. Clin. Nutr. 2004, 79, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Toney, M.D. Reaction specificity in pyridoxal phosphate enzymes. Arch. Biochem. Biophys. 2005, 433, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Manegold, C.; Hoffmann, G.F.; Degen, I.; Ikonomidou, H.; Knust, A.; Laass, M.W.; Pritsch, M.; Wilichowski, E.; Hörster, F. Aromatic L-amino acid decarboxylase deficiency: Clinical features, drug therapy and follow-up. J. Inherit. Metab. Dis. 2009, 32, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, Z.; Cheng, X.; Gao, J.; Zhang, Z.; Wang, L. 5-Aminolevulinic acid promotes anthocyanin accumulation in Fuji apples. Plant Growth Regul. 2013, 69, 295–303. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M. Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J. Plant Growth Regul. 2013, 32, 663–679. [Google Scholar] [CrossRef]
- McCormick, D.B.; Chen, H. Update on interconversions of vitamin B-6 with its coenzyme. J. Nutr. 1999, 129, 325–327. [Google Scholar] [CrossRef]
- Carrasco, J.; Preston, G.M. Growing edible mushrooms: A conversation between bacteria and fungi. Environ. Microbiol. 2020, 22, 858–872. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).